Abstract
Healthy lifestyle behaviors are recommended to reduce cancer risk and overall mortality. Adherence to cancer-preventive health behaviors and subsequent cancer risk has not been evaluated in a diverse sample of postmenopausal women. We examined the association between the American Cancer Society (ACS) Nutrition and Physical Activity Cancer Prevention Guidelines score and risk of incident cancer, cancer-specific mortality, and all-cause mortality in 65,838 postmenopausal women enrolled in the Women’s Health Initiative Observational Study. ACS guidelines scores (0–8 points) were determined from a combined measure of diet, physical activity, body mass index (current and at age 18 years), and alcohol consumption. After a mean follow-up of 12.6 years, 8,632 incident cancers and 2,356 cancer deaths were identified. The highest ACS guidelines scores compared with the lowest were associated with a 17% lower risk of any cancer [HR, 0.83; 95% confidence interval (CI), 0.75–0.92], 22% lower risk of breast cancer (HR, 0.78; 95% CI, 0.67–0.92), 52% lower risk of colorectal cancer (HR, 0.48; 95% CI, 0.32–0.73), 27% lower risk of all-cause mortality, and 20% lower risk of cancer-specific mortality (HR, 0.80; 95% CI, 0.71–0.90). Associations with lower cancer incidence and mortality were generally strongest among Asian, black, and Hispanic women and weakest among non-Hispanic whites. Behaviors concordant with Nutrition and Physical Activity Cancer Prevention Guidelines were associated with lower risk of total, breast, and colorectal cancers and lower cancer-specific mortality in postmenopausal women.
Introduction
Nutrition and physical activity cancer prevention recommendations for lifestyle modification have been disseminated by leading cancer organizations and the U.S. Department of Health and Human Services for more than three decades (1–3). While it is well recognized that tobacco cessation is the lead behavioral change to reduce cancer risk, these published recommendations specifically target healthy diet, greater physical activity, moderation of alcohol consumption, and healthy body weight. Of note, Nutrition and Physical Activity Cancer Prevention Guidelines largely overlap with those aimed at overall chronic disease risk reduction (4) and, therefore, may hold potential in also reducing all-cause mortality.
Recent cohort analyses, including the Cancer Prevention Study (CPS) II (5) and the European Prospective Investigation of Cancer (EPIC) study (6), confirmed that behaviors consistent with Nutrition and Physical Activity Cancer Prevention Guidelines were associated with lower cancer incidence and mortality. Similarly, the Iowa Women’s Health Study reported that a lack of concordance with earlier cancer prevention guidelines was associated with a 35% greater cancer risk (7). Information on these relationships in older women and minority populations, however, is limited. To build upon available evidence, we applied the American Cancer Society (ACS) guidelines on nutrition and physical activity for cancer prevention (8) as a framework to evaluate associations with cancer outcomes in the Women’s Health Initiative Observational Study (WHI-OS) of postmenopausal women. This cohort affords a new opportunity to evaluate these associations in an older age group, within diverse racial/ethnic strata, and by smoking status. We hypothesized that behaviors most consistent with the nutrition and physical activity guidelines would be associated with lower cancer risk as well as all-cause and cancer-specific mortality.
Materials and Methods
Study population
The WHI-OS is a prospective study of health outcomes among 93,676 postmenopausal women enrolled between 1993 and 1998 in 40 U.S. clinical centers. Detailed study information has been reported (9). Briefly, women ages 50 to 79 years at enrollment completed questionnaires to characterize health habits, including physical activity, WHI food frequency questionnaire (10), medical history, and quality of life, and provided self-determined race/ethnicity information. Weight and height were measured at clinic visits by trained staff, and body mass index (BMI) was computed. Self-reported height and weight at age 18 years was used to estimate change in BMI during adulthood. The Institutional Review Boards at each institution approved the study and all participants provided written informed consent.
For this analysis, women were excluded if they had a personal history of cancer (n = 12,075) or cancer history was unknown (n = 752), had a cancer diagnosis (n = 2,246) or died (n = 710) within 2 years of study entry, were underweight (BMI < 18.5 kg/m2) at baseline (n = 1107), or had unknown BMI at baseline (n = 1,105) or at age 18 years (n = 2,703). Women with unreliable BMI data (change in height or weight between age 18 years and baseline >4 SDs beyond the mean; n = 4,948), those with unavailable (n = 96) or unreliable (<600 or >5,000 kcal/d, n = 3,571) dietary data, or those missing data for alcohol intake (n = 685), physical activity (n = 1,051), follow-up health status (n = 473), or any other covariates (n = 6,786) were also excluded. As a result, the analytical cohort comprised 65,838 women.
Outcome ascertainment
Details on the ascertainment of outcomes in the WHI-OS, including standardized operating procedures for local and centralized adjudication of cancer endpoints, have been described (11). Briefly, women completed a health status questionnaire annually. Medical records were collected for verification of self-reported study outcomes (cancer and death) and reviewed by trained WHI outcomes adjudicators. Vital status was determined through linkage with the National Death Index for women who were lost to followup. The underlying causes of death were coded according to the International Classification of Diseases (12). For this analysis, "any cancer" included all cancers other than nonmelanoma skin cancer, and cancer mortality was defined as death from any cancer other than non-melanoma skin cancer. Outcomes for specific cancers included breast, colorectal, endometrial, ovarian, and lung cancer; these cancer sites were selected on the basis of sufficiency in case numbers and/or evidence of an association with modifiable lifestyle behaviors included in the ACS guidelines for diet and physical activity and cancer prevention (1). All other types (except non-melanoma skin cancer) were combined into an "other cancer" group.
Nutrition and Physical Activity Cancer Prevention Guidelines score
The ACS cancer prevention guidelines score, based upon previously published work by McCullough and colleagues (5), included four behavior-associated components: body weight, physical activity, diet, and alcohol consumption. Specifically, the a priori score was derived from the individual components of the 2006 and more-recent 2012 ACS guidelines on nutrition and physical activity for cancer prevention (8, 13) using data collected at baseline and food and beverage line items on the food frequency questionnaire. Table 1 describes the ACS recommendations and how each component’s score was calculated. Behaviors least consistent with the recommendations were given a score of "0," mid-level concordance was given a score of "1," and behaviors that met the criteria were given a score of "2." Thus, women whose behaviors did not meet any recommendation had an overall nutrition and physical activity guidelines score of "0," whereas women whose behaviors conformed to all guidelines earned the maximum score of "8." While tobacco smoking is not included in the ACS diet and physical activity guidelines score (8, 13), it was considered in a stratified analysis, given its association with select cancers.
Table 1.
Description of ACS cancer prevention guidelines scores
| ACS recommendation | Worst score (0) | Middle score (1) | Best score (2) |
|---|---|---|---|
| 1. "Maintain a healthy weight throughout life." | BMI at age 18 y: ≥30 kg/m2, or BMI at baseline: ≥30 kg/m2 | BMI at age 18 y: 25 to <30 kg/m2, or BMI at baseline: 25 to <30 kg/m2 | BMI at age 18 y: <25 kg/m2, and BMI at baseline: <25 kg/m2 |
| 2. "Adopt a physically active lifestyle with 30 min or more of moderate to vigorous intentional physical activity at least 5 d/wk; 45–60 min are preferable." | <8.75 MET h/wk | 8.75–17.5 MET h/wk | >17.5 MET h/wk |
| 3. "Consume a healthy diet with an emphasis on plant sources." | 0–2 diet points | 3–6 diet points | 7–9 diet points |
| 3A. "Eat 5 or more servings of a variety of vegetables and fruits each day." | 1 point for consuming ≥5 servings/d fruits + vegetables, plus 1 or 2 points for being in the second or third tertile of total carotenoids,a respectively. | ||
| 3B. "Choose whole grains in preference to processed (refined) grains)." | Percentage of grains consumed as whole grains divided into quartiles and assigned a score of 0–3 (lowest quartile = 0) | ||
| 3C. "Limit consumption of processed and red meats." | Intake of red + processed meat (servings/wk) divided into quartiles and assigned a score of 0–3 (lowest quartile = 3) | ||
| 4. "If you drink, limit consumption to 1 drink/d for women." | >1 drink/d | >0–≤1 drink/d | Nondrinker at baseline |
Tertiles of carotenoids consumed was used to capture quality of fruit and vegetables, modified from the original diversity score.
Statistical analysis
Descriptive statistics (means for continuous variables; proportions for categorical variables) summarizing participant characteristics were calculated and compared across collapsed levels of ACS cancer prevention guidelines scores. Associations between ACS guidelines scores and each cancer incidence or mortality outcome were tested using Cox proportional hazards regression. Models were adjusted for the following potential confounders: age (continuous), education (≤high school, some college, ≥college), smoking pack-years (never smoked, <5, 5–<20, 20+), nonsteroidal anti-inflammatory drug (NSAID) use (yes, no), aspirin use (yes, no), unopposed estrogen use (never, former, current), estrogen + progestin use (never, former, current), multivitamin use (yes, no), race/ethnicity (NHW, Hispanic, black, Asian/Pacific Islander, American Indian/Alaskan Native, other), total energy intake (continuous), parous (ever had a full-term pregnancy; yes, no, unknown), mammogram(ever, never; included only in models for any cancer incidence/ mortality, breast cancer incidence/mortality, and all-cause mortality), colonoscopy or sigmoidoscopy (ever, never; included only in models for any cancer incidence/mortality, colorectal cancer incidence/mortality, and all-cause mortality), family history (mother/father, full-biologic sister/brother, daughter/son, grandmother) of cancer (excluding non-melanoma skin cancer; yes, no, unknown), and having a current healthcare provider (yes, no). To remain consistent with the study by McCullough and colleagues (5), tertiles of carotenoids consumed were used to capture quality of fruit and vegetables, modified from the original diversity score.
For all outcomes, the ACS guidelines score was modeled as a categorical variable, with scores of 0 to 2 and 7 to 8 combined into separate collapsed categories due to low numbers of women with these scores. Tests for trend were conducted by modeling the ACS guidelines score as an ordinal variable (0–8). ACS guidelines were collapsed further into three categories (0–3, 4–5, 6–8) for cancer-specific mortality and analyses stratified by smoking or race/ethnicity due to the smaller numbers of events. Potential interactions between ACS guidelines score and smoking (pack-years), race/ethnicity, or family history were tested using likelihood ratio tests. Finally, the 4 individual components of the ACS guidelines score were examined in a model simultaneously, including the covariates listed above. The proportional hazards assumption was evaluated in each fully adjusted model by examining plots of the hazard for women with low (0–4) versus high (5– 8) ACS scores by follow-up time and verifying that the lines did not cross. All analyses were conducted using Stata 12.1 (StataCorp).
Results
The proportions of participants earning each ACS nutrition and physical activity cancer prevention guidelines score were as follows: 0 (0.4%), 1 (3.8%), 2 (12.2%), 3 (19.9%), 4 (22.9%), 5 (20.8%), 6 (13.7%), 7 (5.4%), and 8 (0.9%). Women with higher scores tended to be NHW or Asian, be more educated, be never-smokers, use multivitamins and estrogen + progestin, and report having had a colonoscopy. In contrast, lower scores were more common in women who were Hispanic or black, were obese, reported greater weight gain over adult life, and reported NSAID use (Table 2).
Table 2.
Characteristics of WHI-OS participants, by ACS cancer prevention guidelines score (n = 65,838)
| ACS cancer prevention guidelines score | |||
|---|---|---|---|
| Participant characteristic | 0–3 (n=23,885) | 4–5 (n=28,740) | 6–8 (n=13,213) |
| Demographics | |||
| Age, mean ± SD, y | 62.8 ± 7.2 | 63.5 ± 7.3 | 63.4 ± 7.4 |
| Race/ethnicity, % | |||
| Non-Hispanic white (NHW) | 84.0 | 85.7 | 87.1 |
| Hispanic | 3.79 | 3.17 | 2.26 |
| Black | 9.10 | 6.33 | 4.73 |
| Asian | 1.62 | 3.38 | 4.53 |
| Native American | 0.46 | 0.34 | 0.30 |
| Other | 1.08 | 1.08 | 1.07 |
| Education, % | |||
| ≤High school graduate | 25.5 | 19.0 | 14.4 |
| Some college | 39.2 | 35.7 | 32.6 |
| ≥College | 35.3 | 45.3 | 53.0 |
| Body size, mean ± SDa | |||
| BMI at age18 y, kg/m2 | 21.1 ± 3.1 | 20.4 ± 2.5 | 20.1 ± 2.0 |
| BMI at baseline, kg/m2 | 30.7 ± 5.7 | 25.8 ± 4.2 | 23.4 ± 2.7 |
| Weight change from age18 y to baseline, kg | 23.7 ± 14.1 | 13.1 ± 11.0 | 7.63 ± 7.9 |
| Height at baseline, cm | 161.8 ± 6.2 | 161.9 ± 6.3 | 162.2 ± 6.3 |
| Physical activity (MET h/wk), mean ± SD | 5.24 ± 6.3 | 14.7 ± 13.3 | 27.6 ± 15.3 |
| Diet, mean ± SD | |||
| Total energy, kcal/d | 1663 ± 649 | 1533 ± 567 | 1492 ± 525 |
| Fruit and vegetables, servings/d | 3.6 ± 1.9 | 4.5 ± 2.1 | 5.6 ± 2.3 |
| Total carotenoids, µg/d | 9540 ± 5278 | 11,305 ± 6070 | 13,949 ± 7011 |
| Red and processed meat, servings/d | 0.90 ± 0.64 | 0.62 ± 0.50 | 0.40 ± 0.38 |
| Whole grains, servings/d | 0.69 ± 0.62 | 0.86 ± 0.66 | 1.04 ± 0.71 |
| Proportion of grains consumed as whole grains | 0.31 ± 0.20 | 0.38 ± 0.21 | 0.45 ± 0.20 |
| Alcohol | |||
| Nondrinker at baseline, % | 20.3 | 29.9 | 41.5 |
| Intake among drinkers (drinks/wk), mean ± SD | 4.2 ± 6.8 | 3.5 ± 5.4 | 2.4 ± 3.3 |
| Smoking pack-years, % | |||
| 0 (never smoked) | 47.9 | 53.3 | 57.3 |
| <5 | 14.5 | 15.0 | 15.7 |
| 5–<19 | 14.7 | 14.7 | 13.8 |
| 20+ | 22.9 | 17.1 | 13.2 |
| Multivitamin use at baseline, % | 37.8 | 43.5 | 47.6 |
| Medication use, % | |||
| NSAIDb use at baseline | 23.0 | 17.9 | 13.8 |
| Aspirin use at baseline | 20.8 | 21.7 | 20.4 |
| Unopposed estrogen | |||
| Never used | 63.1 | 61.9 | 63.0 |
| Past user | 11.1 | 11.4 | 11.4 |
| Current user | 25.7 | 26.7 | 25.7 |
| Estrogen + progestin | |||
| Never used | 73.6 | 68.1 | 64.6 |
| Past user | 8.12 | 8.82 | 9.07 |
| Current user | 18.3 | 23.1 | 26.4 |
| Parous (ever had a full-term pregnancy), % | |||
| No | 10.4 | 10.2 | 10.1 |
| Yes | 79.5 | 79.5 | 79.7 |
| Unknown | 10.0 | 10.3 | 10.2 |
| Cancer screening history, % | |||
| Ever had a mammogram | 96.6 | 97.5 | 97.9 |
| Ever had a colonoscopy/sigmoidoscopy | 50.8 | 54.7 | 56.6 |
| Family history of cancer, % | |||
| No | 32.1 | 32.1 | 32.9 |
| Yes | 63.6 | 63.9 | 63.6 |
| Unknown | 4.35 | 4.03 | 3.47 |
| Have current healthcare provider, % | 94.5 | 95.5 | 95.3 |
Height/weight data were deemed unreliable if the difference between the 2 measurements (18 years and baseline) were >4 SDs beyond the mean.
Includes NSAID combinations.
Over a mean follow-up period of 12.6 years, 8,632 (13.1%) women were diagnosed with cancer. Among the specific cancers investigated, invasive breast cancer was the most common (n = 3,549), followed by lung and colorectal cancers. A total of 7,106 women (10.8%) died during the follow-up period; 2,357 had a cancer-specific cause of death, including 192 from breast cancer, 285 from lung cancer, 190 from colorectal cancer, 43 from endometrial cancer, and 182 from ovarian cancer.
Higher ACS nutrition and physical activity guidelines scores were associated with lower risk of incident cancer overall (Table 3). In the fully adjusted model, risk for any cancer was 17% lower in women with a score of 7 or 8compared with those with the lowest scores (0–2). Furthermore, the highest scores were associated with a 22% lower breast cancer incidence and 52% lower colorectal cancer incidence. A 27% lower risk of endometrial cancer was shown for women with the highest scores (although the estimate from the categorical analysis was not significant, the overall trend was significant). ACS scores were not associated with lower incidence of lung, ovarian, or other cancers.
Table 3.
Associations between ACS guidelines score and cancer incidence (n = 65,838)
| Cancer outcome | ACS score | Person-years, (millions) |
Events n (%) |
Age-adjusted HR (95% CI) |
Fully adjusteda HR (95% CI) |
|---|---|---|---|---|---|
| Any cancer (other than non-melanoma skin cancer) | |||||
| 0—2 | 1,341 | 1,475 (13.7) | 1.00 | 1.00 | |
| 3 | 2,011 | 1,768 (13.5) | 0.95 (0.89–1.02) | 0.97 (0.91–1.04) | |
| 4 | 2,711 | 1,985 (13.2) | 0.90 (0.84–0.96) | 0.93 (0.86–0.99) | |
| 5 | 2,261 | 1,777 (13.0) | 0.87 (0.81–0.93) | 0.90 (0.84–0.97) | |
| 6 | 1,008 | 1,120 (12.4) | 0.82 (0.75–0.88) | 0.85 (0.78–0.92) | |
| 7—8 | 216 | 507 (12.1) | 0.79 (0.72–0.88) | 0.83 (0.75–0.92) | |
| Ptrendb | <0.001 | <0.001 | |||
| Trendb | 0.96 (0.94–0.97) | 0.96 (0.95–0.98) | |||
| Breast | |||||
| 0—2 | 13,76 | 619 (5.75) | 1.00 | 1.00 | |
| 3 | 2,065 | 724 (5.51) | 0.95 (0.85–1.05) | 0.94 (0.84–1.04) | |
| 4 | 2,779 | 813 (5.39) | 0.90 (0.81–1.00) | 0.88 (0.79–0.98) | |
| 5 | 2,318 | 732 (5.36) | 0.88 (0.79–0.98) | 0.85 (0.76–0.95) | |
| 6 | 1,034 | 449 (4.97) | 0.80 (0.71–0.91) | 0.76 (0.67–0.86) | |
| 7—8 | 221 | 212 (5.07) | 0.82 (0.70–0.96) | 0.78 (0.67–0.92) | |
| Ptrendb | <0.001 | <0.001 | |||
| Trendb | 0.96 (0.94–0.98) | 0.95 (0.93–0.97) | |||
| Colorectal | |||||
| 0—2 | 1410 | 145 (1.35) | 1.00 | 1.00 | |
| 3 | 2114 | 185 (1.41) | 0.99 (0.80–1.23) | 1.03 (0.83–1.28) | |
| 4 | 2845 | 169 (1.12) | 0.76 (0.61–0.95) | 0.81 (0.65–1.02) | |
| 5 | 2376 | 145 (1.06) | 0.70 (0.56–0.87) | 0.77 (0.61–0.98) | |
| 6 | 1057 | 79 (0.87) | 0.57 (0.44–0.76) | 0.64 (0.49–0.85) | |
| 7—8 | 226 | 28 (0.67) | 0.43 (0.29–0.65) | 0.48 (0.32–0.73) | |
| Ptrendb | <0.001 | <0.001 | |||
| Trendb | 0.87 (0.83–0.91) | 0.89 (0.85–0.94) | |||
| Endometrial | |||||
| 0—2 | 1,411 | 97 (0.90) | 1.00 | 1.00 | |
| 3 | 2,118 | 103 (0.78) | 0.86 (0.65–1.13) | 0.85 (0.65–1.13) | |
| 4 | 2,846 | 112 (0.74) | 0.79 (0.60–1.04) | 0.77 (0.58–1.01) | |
| 5 | 2,378 | 96 (0.70) | 0.74 (0.56–0.95) | 0.69 (0.52–0.92) | |
| 6 | 1,057 | 68 (0.75) | 0.78 (0.57–1.11) | 0.71 (0.51–0.97) | |
| 7—8 | 226 | 33 (0.79) | 0.82 (0.55–1.21) | 0.73 (0.49–1.09) | |
| Ptrendb | 0.048 | 0.008 | |||
| Trendb | 0.95 (0.89–1.00) | 0.93 (0.87–0.98) | |||
| Ovarian | |||||
| 0—2 | 1,416 | 51 (0.47) | 1.00 | 1.00 | |
| 3 | 2,124 | 58 (0.44) | 0.92 (0.63–1.34) | 0.93 (0.64–1.36) | |
| 4 | 2,853 | 82 (0.54) | 1.10 (0.78–1.56) | 1.13 (0.79–1.61) | |
| 5 | 2,384 | 60 (0.44) | 0.87 (0.60–1.27) | 0.90 (0.61–1.31) | |
| 6 | 1,059 | 52 (0.58) | 1.12 (0.76–1.65) | 1.16 (0.78–1.72) | |
| 7—8 | 227 | 23 (0.55) | 1.07 (0.66–1.75) | 1.13 (0.68–1.87) | |
| Ptrendb | 0.634 | 0.494 | |||
| Trendb | 1.02 (0.95–1.09) | 1.03 (0.95–1.10) | |||
| Lung | |||||
| 0—2 | 1,413 | 143 (1.33) | 1.00 | 1.00 | |
| 3 | 2,120 | 185 (1.41) | 1.01 (0.81–1.25) | 1.15 (0.93–1.44) | |
| 4 | 2,848 | 173 (1.15) | 0.79 (0.63–0.98) | 1.00 (0.80–1.25) | |
| 5 | 2,380 | 181 (1.32) | 0.88 (0.71–1.10) | 1.22 (0.97–1.52) | |
| 6 | 1,058 | 107 | 0.78 (0.60–1.00) | 1.17 (0.91–1.52) | |
| 7—8 | 227 | 44 | 0.68 (0.49–0.96) | 1.14 (0.81–1.60) | |
| Ptrendb | 0.004 | 0.163 | |||
| Trendb | 0.94 (0.90–0.98) | 1.03 (0.99–1.08) | |||
| Otherc | |||||
| 0—2 | 1,398 | 445 (4.14) | 1.00 | 1.00 | |
| 3 | 2,096 | 526 (4.01) | 0.93 (0.82–1.06) | 0.95 (0.84–1.08) | |
| 4 | 2.815 | 652 (4.33) | 0.97 (0.86–1.10) | 1.00 (0.88–1.13) | |
| 5 | 2.352 | 580 (4.24) | 0.93 (0.82–1.05) | 0.96 (0.84–1.09) | |
| 6 | 1,046 | 387 (4.29) | 0.93 (0.81–1.06) | 0.96 (0.83–1.10) | |
| 7—8 | 224 | 172 (4.11) | 0.88 (0.74–1.05) | 0.92 (0.77–1.10) | |
| Ptrendb | 0.128 | 0.383 | |||
| Trendb | 0.98 (0.96–1.01) | 0.99 (0.96–1.01) | |||
Adjusted for age (continuous), education (≤high school, some college,≥college), smoking pack-years (never smoking, <5, 5–19, 20+), NSAID use at baseline (yes, no), aspirin use at baseline (yes, no), unopposed estrogen use (never, former, current), estrogen + progestin use (never, former, current), multivitamin use at baseline (yes, no), race/ethnicity (NHW, Hispanic, black, Asian, Native American, other), total energy intake (continuous), parous (yes, no, unknown), mammogram (ever, never; included only in models for any cancer and breast cancer), colonoscopy/sigmoidoscopy (ever, never; included only in models for any cancer and colorectal cancer), family history (mother/father, full-blooded sister/brother, daughter/son, grandmother) of cancer (yes, no, unknown), and having a current healthcare provider (yes, no).
Trend tested by modeling ACS score as an ordinal variable (0–8).
Any cancer other than breast, colorectal, endometrial, ovarian, lung, and non-melanoma skin cancer.
All-cause mortality risk was 27% lower in women scoring 6 to 8 compared with those scoring 0 to 3 (Table 4). Also, death from any cancer was 20% lower among women with the highest scores. Furthermore, women with the highest scores showed a striking 61% lower risk of colorectal cancer death. Risk of death from breast cancer was 33% lower in women with the highest scores (although the estimate from the categorical analysis was not significant, the overall trend was significant). Higher ACS guidelines scores were nonsignificantly associated with lower risk of death from endometrial (Ptrend = 0.085), ovarian (Ptrend = 0.373), or other (Ptrend = 0.083) cancers. ACS scores were not associated with lung cancer mortality.
Table 4.
Associations between ACS guidelines score and all-cause and cancer-specific mortality (n = 65,838)
| Cause of death | ACS score | Person-years, millions | Events n (%) |
Age-adjusted HR (95% CI) |
Fully adjusteda HR (95% CI) |
|---|---|---|---|---|---|
| Any cause | |||||
| 0—3 | 7,015 | 2,862 (12.0) | 1.00 | 1.00 | |
| 4–5 | 10,464 | 3,074 (10.7) | 0.78 (0.74–0.82) | 0.86 (0.82–0.91) | |
| 6–8 | 2,270 | 1,170 (8.85) | 0.62 (0.58–0.66) | 0.73 (0.68–0.78) | |
| Ptrendb | <0.001 | <0.001 | |||
| Trendb | 0.89 (0.88–0.90) | 0.93 (0.92–0.95) | |||
| Any cancer (other than non-melanoma skin cancer) | |||||
| 0–3 | 7,015 | 945 (4.00) | 1.00 | 1.00 | |
| 4–5 | 10,464 | 1,003 (3.53) | 0.80 (0.73–0.88) | 0.88 (0.81–0.97) | |
| 6–8 | 2,270 | 408 (3.12) | 0.68 (0.61–0.77) | 0.80 (0.71–0.90) | |
| Ptrendb | <0.001 | <0.001 | |||
| Trendb | 0.91 (0.89–0.94) | 0.95 (0.93–0.98) | |||
| Breast cancer | |||||
| 0—3 | 7,015 | 78 (0.33) | 1.00 | 1.00 | |
| 4—5 | 10,464 | 85 (0.30) | 0.83 (0.61–1.13) | 0.90 (0.65–1.23) | |
| 6—8 | 2,270 | 29 (0.22) | 0.59 (0.39–0.91) | 0.67 (0.43–1.03) | |
| Ptrendb | 0.008 | 0.049 | |||
| Trendb | 0.88 (0.81–0.97) | 0.91 (0.83–1.00) | |||
| Colorectal cancer | |||||
| 0—3 | 7,015 | 94 (0.40) | 1.00 | 1.00 | |
| 4—5 | 10,464 | 76 (0.27) | 0.61 (0.45–0.83) | 0.66 (0.49–0.90) | |
| 6—8 | 2,270 | 20 (0.15) | 0.34 (0.21–0.55) | 0.39 (0.24–0.63) | |
| Ptrendb | <0.001 | <0.001 | |||
| Trendb | 0.80 (0.73–0.88) | 0.83 (0.75–0.91) | |||
| Endometrial cancer | |||||
| 0–3 | 7,015 | 21 (0.09) | 1.00 | 1.00 | |
| 4–5 | 10,464 | 14 (0.05) | 0.50 (0.26–0.99) | 0.49 (0.25–0.98) | |
| 6–8 | 2,270 | 8 (0.06) | 0.60 (0.27–1.36) | 0.59 (0.26–1.37) | |
| Ptrendb | 0.086 | 0.085 | |||
| Trendb | 0.84 (0.69–1.02) | 0.84 (0.68–1.02) | |||
| Ovarian cancer | |||||
| 0–3 | 7,015 | 63 (0.27) | 1.00 | 1.00 | |
| 4–5 | 10,464 | 86 (0.30) | 1.06 (0.76–1.47) | 1.10 (0.79–1.53) | |
| 6–8 | 2,270 | 33 (0.25) | 0.86 (0.56–1.30) | 0.90 (0.58–1.39) | |
| Ptrendb | 0.230 | 0.373 | |||
| Trendb | 0.94 (0.86–1.04) | 0.96 (0.87–1.05) | |||
| Lung cancer | |||||
| 0–3 | 7,015 | 110 (0.47) | 1.00 | 1.00 | |
| 4–5 | 10,464 | 130 (0.46) | 0.92 (0.71–1.18) | 1.18 (0.91–1.52) | |
| 6–8 | 2,270 | 45 (0.34) | 0.68 (0.48–0.96) | 1.07 (0.75–1.52) | |
| Ptrendb | 0.018 | 0.505 | |||
| Trendb | 0.91 (0.85–0.98) | 1.03 (0.95–1.11) | |||
| Other cancerc | |||||
| 0–3 | 7,015 | 534 (2.26) | 1.00 | 1.00 | |
| 4–5 | 10,464 | 561 (1.97) | 0.79 (0.70–0.89) | 0.85 (0.76–0.96) | |
| 6–8 | 2,270 | 257 (1.96) | 0.75 (0.65–0.87) | 0.86 (0.74–1.00) | |
| Ptrendb | <0.001 | 0.083 | |||
| Trendb | 0.93 (0.90–0.97) | 0.97 (0.94–1.00) | |||
Adjusted for age (continuous), education (≤high school, some college, ≥college), smoking pack-years (never smoking, <5, 5–19, 20+), NSAID use baseline (yes, no), aspirin use baseline (yes, no), unopposed estrogen use (never, former, current), estrogen + progestin use (never, former, current), multivitamin use baseline (yes, no), race/ethnicity (NHW, Hispanic, black, Asian, Native American, other), energy intake (continuous), parous (yes, no, unknown), mammogram (ever, never; included only in models for any cause, any cancer, breast cancer), colonoscopy/sigmoidoscopy (ever, never; included only in models for any cause, any cancer, colorectal cancer), family history of cancer (yes, no, unknown), and having a current healthcare provider (yes, no).
Trend tested by modeling ACS score as an ordinal variable (0–8).
Any cancer other than breast, colorectal, endometrial, ovarian, lung, and non-melanoma skin cancer.
There were no significant interactions between smoking and ACS Nutrition and Physical Activity Cancer Prevention Guidelines score for the outcomes assessed (Table 5). In both smokers and nonsmokers, higher ACS guidelines scores were associated with favorable clinical outcomes. The combined influence of smoking and ACS guidelines score was examined further in an analysis comparing never-smokers with ACS scores of 7 to 8 to current smokers with ACS scores of 0 to 2. The never-smoking, high-scoring women showed significantly lower risk than smoking, low-scoring women of any cancer [hazard ratio (HR), 0.41; 95% confidence interval (CI), 0.32–0.51], cancer mortality (HR, 0.20; 95% CI, 0.13–0.30), and all-cause mortality (HR, 0.19; 95% CI, 0.15–0.24). There were no significant interactions between family history of cancer and ACS guidelines score for any of these 3 outcomes (data not shown).
Table 5.
Associations between ACS guidelines score and outcomes, stratified by smoking history
| Any incident cancer | All-cause mortality | Cancer-specific mortality | |||||
|---|---|---|---|---|---|---|---|
| Smoking history | ACS score | Events n (%) |
HR (95% CI)a | Events n (%) |
HR (95% CI)1 | Events n (%) |
HR (95% CI)1 |
| Never | |||||||
| 0–3 | 1,358 (11.9) | 1.00 | 1,139 (9.96) | 1.00 | 341 (3.01) | 1.00 | |
| 4–5 | 1,789 (11.7) | 0.92 (0.86–0.99) | 1,417 (9.26) | 0.84 (0.78–0.91) | 437 (2.88) | 0.89 (0.77–1.03) | |
| 6–8 | 845 (11.2) | 0.84 (0.77–0.92) | 603 (7.97) | 0.71 (0.64–0.78) | 189 (2.52) | 0.76 (0.63–0.91) | |
| Ptrendb | <0.001 | <0.001 | 0.004 | ||||
| Trendb | 0.96 (0.94–0.98) | 0.92 (0.90–0.94) | 0.94 (0.90–0.98) | ||||
| <5 pack-years | |||||||
| 0–3 | 425 (12.3) | 1.00 | 315 (9.11) | 1.00 | 96 (2.80) | 1.00 | |
| 4–5 | 500 (11.6) | 0.87 (0.76–0.99) | 359 (8.35) | 0.88 (0.75–1.02) | 111 (2.61) | 0.90 (0.68–1.19) | |
| 6–8 | 251 (12.1) | 0.89 (0.76–1.05) | 156 (7.53) | 0.80 (0.65–0.97) | 63 (3.06) | 1.09 (0.79–1.52) | |
| Ptrendb | 0.130 | 0.015 | 0.607 | ||||
| Trendb | 0.97 (0.94–1.01) | 0.94 (0.90–0.99) | 1.02 (0.94–1.11) | ||||
| 5–19 pack-years | |||||||
| 0–3 | 472 (13.4) | 1.00 | 351 (9.99) | 1.00 | 119 (3.42) | 1.00 | |
| 4–5 | 607 (14.4) | 1.01 (0.90–1.15) | 443 (10.5) | 0.97 (0.84–1.12) | 154 (3.69) | 1.02 (0.80–1.30) | |
| 6–8 | 234 (12.8) | 0.86 (0.73–1.01) | 152 (8.33) | 0.78 (0.64–0.94) | 50 (2.76) | 0.75 (0.53–1.05) | |
| Ptrendb | 0.248 | 0.033 | 0.197 | ||||
| Trendb | 0.98 (0.94–1.02) | 0.95 (0.91–1.00) | 0.95 (0.89–1.03) | ||||
| 20+ pack-years | |||||||
| 0–3 | 988 (18.0) | 1.00 | 1,057 (19.3) | 1.00 | 389 (7.24) | 1.00 | |
| 4–5 | 866 (17.7) | 0.93 (0.85–1.02) | 855 (17.4) | 0.84 (0.77–0.92) | 301 (6.25) | 0.82 (0.70–0.95) | |
| 6–8 | 297 (17.0) | 0.87 (0.76–0.99) | 259 (14.8) | 0.71 (0.62–0.82) | 106 (6.18) | 0.80 (0.64–1.00) | |
| Ptrendb | 0.010 | <0.001 | 0.022 | ||||
| Trendb | 0.96 (0.94–0.99) | 0.93 (0.90–0.95) | 0.95 (0.90–0.99) | ||||
| Test for interactionc | P = 0.805 | P = 0.602 | P = 0.488 | ||||
Adjusted for age (continuous), education (≤high school, some college, ≥college), NSAID use at baseline (yes, no), aspirin use at baseline (yes, no), unopposed estrogen use (never, former, current), estrogen + progestin use (never, former, current), multivitamin use at baseline (yes, no), race/ethnicity (NHW, Hispanic, black, Asian, Native American, other), total energy intake (continuous), parous (yes, no, unknown), mammogram (ever, never), colonoscopy/sigmoidoscopy (ever, never), family history (mother/father, full-blooded sister/brother, daughter/son, grandmother) of cancer (yes, no, unknown), and having a current healthcare provider (yes, no).
Trend tested by modeling ACS score as an ordinal variable (0–8).
Likelihood ratio test for interaction between smoking and ACS guidelines score (continuous) on each outcome.
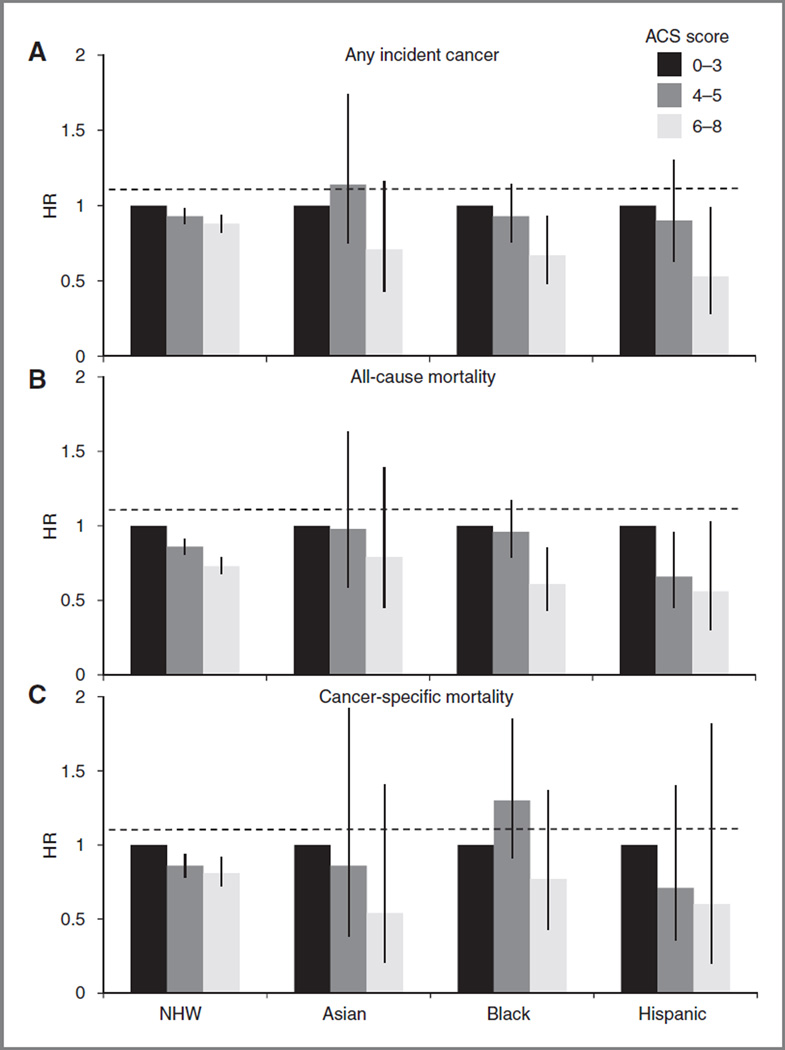
Race/ethnicity modified some of the associations examined (Fig. 1). Risk of any incident cancer was lowest for Hispanic and black women with the highest ACS guidelines scores (HR, 0.53; 95% CI, 0.28–0.99 and HR, 0.67; 95% CI, 0.48–0.93; respectively), whereas NHW and Asian women experienced modestly lower risk (HR, 0.88; 95% CI, 0.82– 0.94 and HR, 0.00371;; 95% CI, 0.43–1.16; respectively; likelihood ratio test, P=0.05). Similarly, for all-cause mortality risk, Hispanic and black women with the highest scores showed 44% and 39% lower risk, respectively (HR, 0.56; 95% CI, 0.03–1.03 and HR, 0.61; 95% CI, 0.43–0.85; respectively), whereas NHW and Asian women had more modestly lower risk (HR, 0.73; 95% CI, 0.68–0.79 and HR, 0.79; 95% CI, 0.45–1.39; respectively). Finally, although only NHW women with the highest scores showed significantly lower cancer-specific mortality (HR, 0.81; 95% CI, 0.72–0.92), nonsignificant point estimates for risk in all 3 minority groups were below that for NHWs: HR = 0.54, 0.77, and 0.60 for Asian, black, and Hispanic women, respectively (likelihood ratio test for race/ethnicity-by-score interaction, P = 0.56).
Figure 1.
Associations between ACS cancer prevention guidelines score and (A) any incident cancer, (B) all-cause mortality, and (C) cancer-specific mortality. HRs were calculated with the lowest scores (0–3) as the reference group (HR = 1.0), stratified by race/ethnicity, and adjusted for the following: age (continuous), education (≤high school, some college, ≥college), smoking pack-years (never smoking, <5, 5–19, 20+), NSAID use at baseline (yes, no), aspirin use at baseline (yes, no), unopposed estrogen use (never, former, current), estrogen + progestin use (never, former, current), multivitamin use at baseline (yes, no), total energy intake (continuous), parous (yes, no, unknown), mammogram (ever, never), colonoscopy/sigmoidoscopy (ever, never), family history (mother/father, full-blooded sister/brother, daughter/son, grandmother) of cancer (yes, no, unknown), and having a current healthcare provider (yes, no). Vertical bars represent 95% CIs. Horizontal dashed lines represent the null HR of 1.0. Likelihood ratio test for interaction between race/ethnicity and ACS guidelines score (continuous) on each outcome: any incident cancer, P = 0.050; allcause mortality, P = 0.116; and cancer-specific mortality, P = 0.555.
The 4 components of the ACS nutrition and physical activity cancer prevention guidelines score, adjusted for each other, were associated to varying degrees with incidence of specific cancers and all-cause mortality (data not shown). For breast cancer incidence, alcohol avoidance and BMI < 25 kg/m2 at age 18 years and baseline were associated with lower risk. For colorectal cancer incidence, BMI was strongly associated with lower risk, followed by physical activity and diet; alcohol intake showed no association with risk. Physical activity was the health behavior most strongly associated with lower all-cause mortality, followed by BMI and diet, whereas alcohol abstinence showed a positive association (HR, 1.31; 95%; CI, 1.21–1.42). However, moderate alcohol consumers (0– ≤1 drink/d) did not havealtered all-cause mortality compared with women with greater intake (>1 drink/d).
Discussion
General lifestyle behavioral guidance to promote health and prevent disease is a cornerstone of the nation’s public health policy (4). To this end, the ACS and others have developed nutrition and physical activity guidelines specific to cancer prevention that are largely consistent with recommendations for the prevention of chronic diseases (1, 2). Few of these guidelines have been tested prospectively to determine whether these recommendations are associated with lower cancer risk, cancer mortality, or overall mortality. Here, we present compelling evidence from the WHI-OS to indicate that concordance with the ACS Guidelines on Nutrition and Physical Activity for Cancer Prevention is associated with lower risk of any cancer, including breast and colorectal cancer, as well as death from any cause in postmenopausal women.
Overall, WHI women were concordant with several of the nutrition and physical activity prevention guidelines, with 32.9% having at least mid-level scores for all 4 components and 32.3% fully adherent to at least 2 of the 4 components. The variance in scores within this large cohort of women permitted robust evaluation of the associations of interest. Similar to our findings, in the Iowa Women’s Health Study, lower cancer risk was associated with higher fruit and vegetable intake, lower red meat intake, lower fat intake, lower BMI, and higher physical activity (7). In addition, the EPIC cohort showed a 19% lower overall cancer incidence in women who met criteria for at least 5 of the 7 health behaviors evaluated (6) and also reported protective associations for incident breast and colorectal cancer, consistent with our findings. In relation to specific cancer sites, our results showed a 22% lower risk for breast cancer, similar to, but not as strong as, that observed in an analysis from the VITAL study in which adherence to the WCRF/AICR guidelines resulted in a 60% lower risk (14), although coding for alcohol differed between the 2 studies.
Mortality prevention associated with guideline adherence in our study aligned with that reported by McCullough and colleagues (5), with reductions in overall mortality of 29% and 42% for WHI-OS and CPS II women, respectively. For cancer-specific mortality, risk reductions were 12% and 24% in WHI-OS and CPS II women (5), respectively. Furthermore, in a U.K. study (15), a 2.5-fold greater cancer mortality and a 3.4-fold greater overall mortality risk were seen with nonconcordance to guidelines regarding smoking, physical activity, alcohol intake, and vitamin C levels (as an indicator of fruit and vegetable consumption).
An important contribution of the current study was our ability to evaluate these relationships in different racial/ethnic strata. Interestingly, NHW women generally showed modest risk reductions, whereas Hispanic and black women with the highest scores had a significant 33% and 47% lower risk of cancer, respectively. Similarly, striking risk reductions were shown for all-cause mortality, although associations with cancer-specific mortality were significant only in NHW. The significant association between ACS score and all-cause mortality but not cancer-specific mortality in Hispanic and black postmenopausal women may reflect a more advanced stage of disease at diagnosis or, perhaps, disease that is more resistant to treatment (16, 17). Overall, behaviors consistent with the ACS nutrition and physical activity cancer prevention guidelines may be associated with reduced cancer risk and lower overall mortality in older U.S. women, particularly Hispanic and black postmenopausal women.
Our findings that a combination of lifestyle behaviors is associated with reduced cancer outcomes more so than any one specific behavior is supported by other research (5, 6, 15, 18). A study from the U.K. showed an overall increase in cancer risk for low concordance with healthy lifestyle behaviors in sum (15), but not for any of the individual behaviors other than current smoking. Alternately, some studies have shown that select lifestyle behaviors may drive the association with lower cancer risk more than others. In a U.K. cohort of males and females, lower leisure time physical activity was associated with higher risk of cancer, but a healthy diet (specifically, fruit and vegetable intake) alone was not significantly protective (19). In our study, avoidance of alcohol was associated with a lower incidence of breast cancer but was not associated with the other cancer sites evaluated. We also found a 24% lower risk of all-cause mortality for women who were fully concordant with the physical activity guideline, in line with other research (20). Of note, higher BMI, regardless of other behaviors, was associated with higher overall mortality, as previously shown (20–23). Also, stable adult BMI was associated with lower risk of cancer. These results were supported byanother study in which having a stable BMI (between 18.5 and 24.9 kg/m2) over a 10-year period in adulthood was associated with lower cancer and all-cause mortality (5). Overall, when the data were dichotomized for those reporting a combination of healthy behaviors, diet, activity, and never smoking, as compared with those reporting low ACS (diet, activity) in current smokers, we found striking reductions in cancer incidence (59%), cancer specific mortality (80%), and all-cause mortality (81%) in never smokers with higher guideline score. This suggests that greater adherence to cancer-preventive behaviors afforded the greatest reduction in cancer risk as well as a survival benefit. Of note, the ACS cohort analysis showed similar associations in never and former smokers but did not include current smokers in the analysis for comparison (5). The primary limitation of our study is the multicollinearity of health behaviors. While we controlled for numerous confounding variables, multiple regression methods are inherently compromising, as these exposures are difficult to tease apart analytically. In addition, we applied 2 time points to define adult weight gain and thus assess the stability of BMI in adulthood. This may not be optimal given that 92% of these women gained weight after the age of 18 years. In addition, the ACS nutrition and physical activity guidelines score used here did not consider weight another study in which having a stable BMI (between 18.5 and 24.9 kg/m2) over a 10-year period in adulthood was associated with lower cancer and all-cause mortality (5). Overall, when the data were dichotomized for those reporting a combination of healthy behaviors, diet, activity, and never smoking, as compared with those reporting low ACS (diet, activity) in current smokers, we found striking reductions in cancer incidence (%), cancer specific mortality (80%), and all-cause mortality (81%) in never smokers with higher guideline score. This suggests that greater adherence to cancer-preventive behaviors afforded the greatest reduction in cancer risk as well as a survival benefit. Of note, the ACS cohort analysis showed similar associations in never and former smokers but did not include current smokers in the analysis for comparison (5).
The primary limitation of our study is the multicollinearity of health behaviors. While we controlled for numerous confounding variables, multiple regression methods are inherently compromising, as these exposures are difficult to tease apart analytically. In addition, we applied 2 time points to define adult weight gain and thus assess the stability of BMI in adulthood. This may not be optimal given that 92% of these women gained weight after the age of 18 years. In addition, the ACS nutrition and physical activity guidelines score used here did not consider weight distribution by including measures such as waist circumference, yet these measures have been associated with overall mortality in older people as well (24). Furthermore, dietary intake, physical activity, body weight, and alcohol intake may be misrepresented if women are ill at baseline. We attempted to reduce potential reverse causation or misclassification by excluding women who received a cancer diagnosis or died within the first 2 years of enrollment. We did not, however, exclude women with recent myocardial infarction or stroke, as this would have substantially attenuated our sample size and markedly reduced statistical power. Diet and physical activity measures were self-reported and, as such, subject to measurement error; however, body weight and height were measured, improving the precision of this exposure. In addition, this study assessed concordance with ACS guidelines at baseline only and did not consider changes in behavioral exposures over time, with the exception of BMI. Nevertheless, these findings suggest that healthy behaviors, many of which cluster in individuals, may reduce the risk of incident cancer, cancer-specific mortality, and all-cause mortality.
Conclusions
Our results suggest that healthy lifestyle behaviors recommended for nutrition and physical activity behavior may be associated with lower risk of cancer and death in postmenopausal women. The lower cancer incidence and all-cause mortality risk showed in Hispanic and black postmenopausal women, in relation to nutrition and physical activity behaviors, warrant further study.
Acknowledgments
Grant Support
The WHI program is funded by the National Heart, Lung, and Blood Institute; NIH; and U.S. Department of Health and Human Services through contracts HHSN268201100046C, HHSN268201100001C, HHSN268201100002C, HHSN268201100003C, HHSN268201100004C, and HHSN271201100004C.
Program Office: (National Heart, Lung, and Blood Institute, Bethesda, MD) Jacques Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller.
Clinical Coordinating Center Clinical Coordinating Center: (Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg.
Investigators and Academic Centers: (Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; and (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker.
Women’s Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker. For a list of all investigators who have contributed to WHI science, please visit: https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx.
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Authors' Contributions
Conception and design: C.A. Thomson, M.L. Stefanick, M.Z. Vitolins, J. Wactawski-Wende, M.L. Neuhouser
Development of methodology: C.A. Thomson, M. McCullough, R.T. Chlebowski, J.E. Manson, H. Tindle
Acquisition of data (provided animals, acquired and managed patients, provided facilities, etc.): R.T. Chlebowski, M.L. Stefanick, J.E. Manson, J. Ockene, M.Z. Vitolins, J. Wactawski-Wende, G.E. Sarto, D.S. Lane, M.L. Neuhouser
Analysis and interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): B.C. Wertheim, R.T. Chlebowski, J.E. Manson, H. Tindle, M.Z. Vitolins, M.L. Neuhouser
Writing, review, and/or revision of the manuscript: C.A. Thomson, M. McCullough, B.C. Wertheim, R.T. Chlebowski, M.E. Martinez, M.L. Stefanick, T.E. Rohan, J.E. Manson, H. Tindle, J. Ockene, M.Z. Vitolins, J. Wactawski-Wende, G.E. Sarto, D.S. Lane, M.L. Neuhouser
Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): J.E. Manson
Study supervision: J. Ockene, J. Wactawski-Wende, D.S. Lane
References
- 1.American Cancer Society (ACS) Cancer facts and Figures. [Accessed July 5, 2013];2012 www.cancer.org. [Google Scholar]
- 2.World Cancer Research Fund (WCRF) and American Institute for Cancer Research (AICR) Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. [Accessed July 5, 2013]; www.dietand cancer report.org. [Google Scholar]
- 3.World Cancer Research Fund (WCRF) and American Institute for Cancer Research (AICR) Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. [Accessed July 5, 2013]; www.dietand cancer report.org. [Google Scholar]
- 4.World Cancer Research Fund (WCRF) and American Institute for Cancer Research (AICR) Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. [Accessed July 5, 2013]; www.dietand cancer report.org. [Google Scholar]
- 5.McCullough ML, Patel AV, Kushi LH, Patel R, Willett WC, Doyle C, et al. Following cancer prevention guidelines reduces risk of cancer, cardiovascular disease, and all-cause mortality. Cancer Epidemiol Biomarkers Prev. 2011;20:1089–1097. doi: 10.1158/1055-9965.EPI-10-1173. [DOI] [PubMed] [Google Scholar]
- 6.Romaguera D, Vergnaud AC, Peeters PH, van Gils CH, Chan DS, Ferrari P, et al. Is concordance with World Cancer Research Fund/American Institute for Cancer Research guidelines for cancer prevention related to subsequent risk of cancer? Results from the EPIC study. Am J Clin Nutr. 2012;96:150–163. doi: 10.3945/ajcn.111.031674. [DOI] [PubMed] [Google Scholar]
- 7.Cerhan JR, Potter JD, Gilmore JM, Janney CA, Kushi LH, Lazovich D, et al. Adherence to the AICR cancer prevention recommendations and subsequent morbidity and mortality in the Iowa Women's Health Study cohort. Cancer Epidemiol Biomarkers Prev. 2004;13:1114–1120. [PubMed] [Google Scholar]
- 8.Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62:30–67. doi: 10.3322/caac.20140. [DOI] [PubMed] [Google Scholar]
- 9.Design of the Women's Health Initiative clinical trial and observational study. The Women's Health Initiative Study Group. Control Clin Trials. 1998;19:61–109. doi: 10.1016/s0197-2456(97)00078-0. [DOI] [PubMed] [Google Scholar]
- 10.Patterson RE, Kristal AR, Tinker LF, Carter RA, Bolton MP, Agurs-Collins T. Measurement characteristics of the Women's Health Initiative food frequency questionnaire. Ann Epidemiol. 1999;9:178–187. doi: 10.1016/s1047-2797(98)00055-6. [DOI] [PubMed] [Google Scholar]
- 11.Curb JD, McTiernan A, Heckbert SR, Kooperberg C, Stanford J, Nevitt M, et al. Outcomes ascertainment and adjudication methods in the Women's Health Initiative. Ann Epidemiol. 2003;13(9 Suppl):S122–S128. doi: 10.1016/s1047-2797(03)00048-6. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control and prevention (CDC) Classification of Diseases, Functioning, and Disability. [Accessed July 5, 2013]; www.cdc.gov/nchs/icd.htm.
- 13.Kushi LH, Byers T, Doyle C, Bandera EV, McCullough M, McTiernan A, et al. American Cancer Society Guidelines on Nutrition and Physical Activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2006;56:254–281. doi: 10.3322/canjclin.56.5.254. quiz 313–14. [DOI] [PubMed] [Google Scholar]
- 14.Hastert TA, Beresford SA, Patterson RE, Kristal AR, White E. Adherence to WCRF/AICR cancer prevention recommendations and risk of postmenopausal breast cancer. Cancer Epidemiol Biomarkers Prev. 2013;22:1498–1508. doi: 10.1158/1055-9965.EPI-13-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khaw KT, Wareham N, Bingham S, Welch A, Luben R, Day N. Combined impact of health behaviours and mortality in men and women: the EPIC-Norfolk prospective population study. PLoS Med. 2008;5:e12. doi: 10.1371/journal.pmed.0050012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morris GJ, Mitchell EP. Higher incidence of aggressive breast cancers in African-American women: a review. J Natl Med Assoc. 2008;100:698–702. doi: 10.1016/s0027-9684(15)31344-4. [DOI] [PubMed] [Google Scholar]
- 17.Esnaola NF, Ford ME. Racial differences and disparities in cancer care and outcomes: where's the rub? Surg Oncol Clin N Am. 2012;21:417–437. viii. doi: 10.1016/j.soc.2012.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harnack L, Nicodemus K, Jacobs DR, Jr, Folsom AR. An evaluation of the Dietary Guidelines for Americans in relation to cancer occurrence. Am J Clin Nutr. 2002;76:889–896. doi: 10.1093/ajcn/76.4.889. [DOI] [PubMed] [Google Scholar]
- 19.Kvaavik E, Batty GD, Ursin G, Huxley R, Gale CR. Influence of individual and combined health behaviors on total and cause-specific mortality in men and women: the United Kingdom health and lifestyle survey. Arch Intern Med. 2010;170:711–718. doi: 10.1001/archinternmed.2010.76. [DOI] [PubMed] [Google Scholar]
- 20.van Dam RM, Li T, Spiegelman D, Franco OH, Hu FB. Combined impact of lifestyle factors on mortality: prospective cohort study in U.S. women. BMJ. 2008;337:a1440. doi: 10.1136/bmj.a1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Teucher B, Rohrmann S, Kaaks R. Obesity: focus on all-cause mortality and cancer. Maturitas. 2010;65:112–116. doi: 10.1016/j.maturitas.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 22.Flegal KM, Graubard BI, Williamson DF, Gail MH. Cause-specific excess deaths associated with underweight, overweight, and obesity. JAMA. 2007;298:2028–2037. doi: 10.1001/jama.298.17.2028. [DOI] [PubMed] [Google Scholar]
- 23.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 24.Price GM, Uauy R, Breeze E, Bulpitt CJ, Fletcher AE. Weight, shape, and mortality risk in older persons: elevated waist-hip ratio, not high body mass index, is associated with a greater risk of death. Am J Clin Nutr. 2006;84:449–460. doi: 10.1093/ajcn/84.1.449. [DOI] [PubMed] [Google Scholar]