Abstract
We have found that α-amino acid amide derivatives of 2-aminobenzothiazoles undergo a time-dependent, thermal rearrangement in which the amine group attacks the 2-position carbon of the thiazole ring to form a 5,5-spiro ring system. This is followed by sulfur leaving and air oxidation to the corresponding symmetrical disulfide. The isolated yields of such products are quite high (>70%) if there is conformational bias to further promote the intramolecular reaction such as for the 2-aminobenzothiazole amides derived from proline or 4-aminopiperidine-4-carboxylic acid. This rearrangement has not been described previously for α-amino acid amide derivatives of 2-aminobenzothiazoles. However, a related reaction involving 2-semicarbazido benzothiazoles has been recently reported.
Keywords: 2-aminobenzothiazole, rearrangement, riluzole amides, diphenyldisulfide, prodrugs
2-Aminobenzothiazole 1 is a well-represented substructure in medicinal and organic chemistry being found in agents to treat cancer and nerve degeneration.1-3 For example, riluzole 2 is approved by the US FDA for the treatment of amyotrophic lateral sclerosis (ALS), and is marketed under the trade name Rilutek®.4 The aromatic structure of 1 and the presence of endo- and exo-heteroatoms provide a stable framework for intermolecular interactions, such as π stacking and H-bond acceptors, that can elicit energetically favorable interactions with host proteins promoting desirable pharmacological responses. Compound 1 can undergo acylation of the exocyclic nitrogen, alkylation of the endocyclic nitrogen and oxidation of the sulfur.3 The thiazole ring of 1 is generally stable, but has been reported to cleave upon intramolecular attack of thiosemicarbazides, such as 3 to give 4, followed by disulfide formation between two equivalent thiophenol products (Scheme 1).5 This reaction presumably proceeds via formation of a spiro-5,5 intermediate, followed by formation of the 1,2,4-triazole and a free thiol, and then oxidative coupling of two thiols to the disulfide.
Scheme 1.
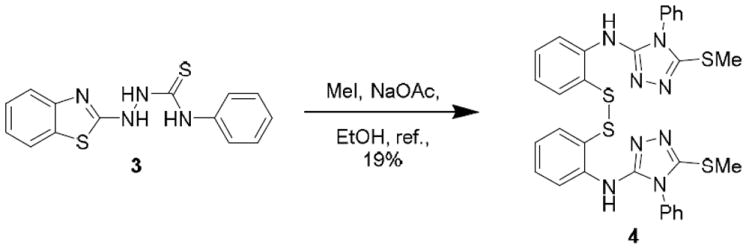
Rearrangement and reaction of a 2-thiosemicarbazide benzothiazole.5
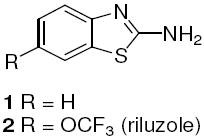
While preparing N-amino acid amide derivatives of 2, we discovered a similar reaction to the one shown in Scheme 1 involving thiazole ring opening. α-Amino acid derivatives of riluzole were prepared using the Mukaiyama coupling reagent followed by acid deprotection of the tert-butyloxycarbonyl (Boc) group (Scheme 2). These amide derivatives serve as prodrugs, being cleaved back to 2 in mouse plasma by the action of peptidases.6 However, in the absence of peptidase-mediated cleavage, an alternative, time-dependent chemical rearrangement and reaction process was observed. When l-proline riluzole amide 5 was treated diisopropylethylamine (DIPEA 40 °C, 72 hr), disulfide 6 was formed in high yield as identified via 1H-NMR and MS (Scheme 3, Table 1). A plausible mechanism for this rearrangement involves 5-membered ring attack of the nucleophilic amine nitrogen on the electrophilic 2-position of the benzothiazole providing strained spiro intermediate I that releases the sulfur as a leaving group to give thiol II, followed by S-oxidation to the isolated disulfide product 6. The reaction is most likely driven by disulfide formation that effectively removes the intermediate thiol from the mixture of equilibrating intermediates, as shown in Scheme 2, making the functionality unavailable to return to the 2-aminobenzothiazole via nucleophilic attack by the thiol on the imidazolium group. As expected, the trifluoromethoxy substituent of 5 does not affect the reaction, as the des-trifluoromethoxy compound 7 converted to 8 in the same way (Table 1). When sarcosine and primary amino acid conjugates of riluzole 9, 11 and 13 were exposed to these same conditions only trace amounts of rearrangement products 10, 12 and 14 were detected by LC/MS, although the starting material in each case was completely consumed after 72 hr. The product derived from the riluzole amide of 4-aminopiperidine-4-carboxylic acid, compound 15, formed 16 in high yield (75%). This is most likely due to the steric effects of the α,α-disubstitution pattern of the α-amino amide promoting intramolecular reaction via the Thorpe-Ingold effect.7 Morpholinyl amide 17 gave rearranged disulfide 18 in modest yield (14%), and the ring expanded proline derivative 19 provided disulfide product 20 also in modest isolated yield (35%).
Scheme 2.
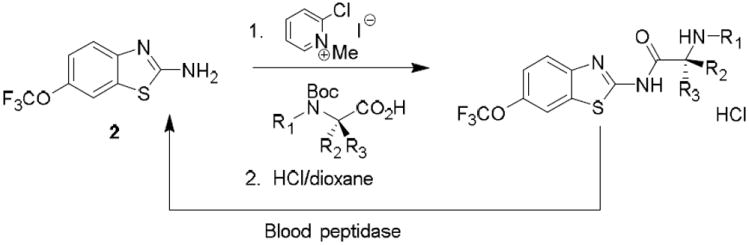
Preparation and peptidase mediated hydrolysis of riluzole-amino acid conjugates.
Scheme 3.
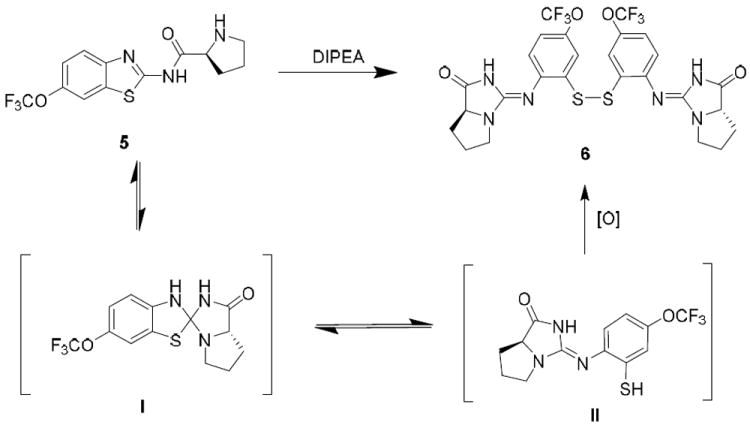
Intramolecular rearrangement of an α-amino acid 2-aminobenzothiazole derivative.
Table 1.
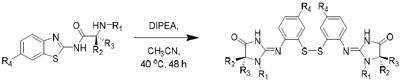
| |||||||
|---|---|---|---|---|---|---|---|
| Starting material | R1 | R2 | R3 | R4 | Product | Conversion (%)c | Isolated Yield (%) |
| 5 | -CH2CH2CH2- | H | CF3O | 6 | 100 | 80 | |
| 7 | -CH2CH2CH2- | H | H | 8 | 100 | 78 | |
| 9 | H | H | H | CF3O | 10e | trace | - |
| 11 | H | CH3 | H | CF3O | 12e | trace | - |
| 13 | Me | H | H | CF3O | 14e | trace | - |
| 15 | H | -CH2CH2NHCH2CH2- | CF3O | 16 | 90 | 75d | |
| 17 | -CH2CH2OCH2- | H | CF3O | 18 | 20 | 14 | |
| 19 | -CH2CH2CH2CH2- | H | CF3O | 20 | - | 35 | |
Reaction conditions: 0.10 M starting material in 5% DIPEA/acetonitrile, 40 °C, 72 hr.
All starting materials and products were characterized by 1H-NMR, ESI pos. LC/MS and HRMS.
Determined by LC/MS (ESI pos).
Isolated as the trifluoroacetate salt following preparative reversed phase HPLC.
Starting material consumed leading to a complex product mixture.
This type of rearrangement was unexpected due to the thiazole ring converting under mild conditions to a nonaromatic substance. However, the recent description of a similar rearrangement involving 2-thiosemicarbazide benzothiazoles is quite similar, showing that suitably-disposed nucleophiles that can form a 6-membered ring spirocycle onto the 2-carbon of benzothiazole will react followed by sulfur leaving, and formation of the corresponding symmetrical disulfide.5 In the case described in this report, the presence of N-acyl substitution causes the thiazole carbon to be more electrophilic and the amide provides an sp2 planar steric constraint helping the nucleophilic amine attack in an intramolecular manner. The substitution pattern of the α-amino acid is important since amides 5, 7 and 15 worked best while many standard primary α-amino acid and sarcosine amides (viz. 9, 11 and 13) produce the rearranged disulfide products transiently, precluding their isolation.
In summary, we have described a rearrangement of α-amino acid amide derivatives of 2-aminobenzothiazoles that leads to imidazolium substituted phenyl disulfides. The reaction works well with cyclic amino acids and poorly with standard primary amino acid and sarcosine derivatives. The rearrangement should be noted by researchers seeking to prepare amides of 2-aminobenzothiazoles in which there is a nucleophilic atom suitably positioned for forming a 5-membered ring.
Supplementary Material
Acknowledgments
We thank the National Institutes of Health for financial support (grant R44 CA156781-03). We also thank Prof. Suzie Chen of Rutgers University and Dr. Garry Smith of FCCDC for support and encouragement, and Dr. Chris Creighton for helpful advice.
Footnotes
Supplementary Material Available. Representative synthetic procedures for 17 and 18 and spectral data for all new compounds described.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Malik JK, Manvi FV, Nanjwade BK, Singh S, Purohit P. Review of the 2-amino substituted benzothiazoles: different methods of the synthesis. Pharmacia Lettre. 2010;2:347–359. [Google Scholar]
- 2.Malik JK, Manvi FV, Nanjwade BK, Purohit P. New 2-amino substituted benzothiazoles: a new profile of biological activities. J Pharmacy Res. 2009;2:1687–1690. [Google Scholar]
- 3.Bondock S, Fadaly W, Metwally MA. Recent trends in the chemistry of 2-aminobenzothiazoles. J Sulf Chem. 2009;30:74–107. [Google Scholar]
- 4.Doble A. Neurology. 1996;47:233S. doi: 10.1212/wnl.47.6_suppl_4.233s. [DOI] [PubMed] [Google Scholar]
- 5.Savitskii PV, Vas’kevich RI, Zborovskii YuL, Staninets VI, But SA, Chernega AN. Heterocyclizations of 1-(Benzothiazol-2-yl)-4-phenylthiosemicarbazide. Russ J Org Chem. 2008;44:402–406. [Google Scholar]
- 6.McDonnell ME, Vera MD, Blass BE, Pelletier JC, King RC, Fernandez-Metzler C, Smith GR, Wrobel JE, Chen S, Wall BA, Reitz AB. Bioorg Med Chem. 2012;20:5642–5648. doi: 10.1016/j.bmc.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jung ME, Piizzi G. Chem Rev. 2005;105:1735. doi: 10.1021/cr940337h. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


