Abstract
Schistosomiasis mansoni is a fibrogenic liver disease that constitutes a major health problem in north–eastern Brazil. Although one common manifestation of the disease, periportal fibrosis (PPF), can be assessed by ultrasonography by well-trained physicians, the necessary equipment and personnel are not always readily available. Serum markers, including hyaluronic acid (HA), have been used as alternative means of measuring fibrosis. Recently serum concentrations of HA have been evaluated in 77 Brazilians (61 cases of schistosomiasis mansoni and 16 healthy controls) and compared against the ultrasound-evaluated PPF in the same subjects. The HA was measured using a non-competitive fluorescence-based assay, while the PPF was explored using a portable ultrasound scanner (SSD-500; Aloka, Tokyo) and graded, as patterns A–F, according to the World Health Organization’s ‘Niamey protocol’. In general, the serum concentrations of HA were found to be positively correlated with the severity of the PPF. The mean concentration of HA in the sera of the 16 controls was significantly lower than that recorded in the schistosomiasis cases who showed PPF of patterns D or E (P<0·001 for each). The cases who showed pattern-C PPF also had significantly less HA in their sera than the cases with PPF of patterns D or E (P<0·001 for each), and the cases with pattern-D fibrosis had significantly lower HA concentrations in their sera than the cases with PPF of pattern E (P<0·001).
In an analysis based on a receiver-operating-characteristic (ROC) curve, an HA concentration of 20·2 μg/litre of serum was identified as a threshold that could be used to distinguish moderate cases of PPF (i.e. patterns C or D) from the more advanced cases (i.e. patterns E or F), with a sensitivity of 60% and specificity of 65%.
In conclusion, it appears that serum concentrations of hyaluronic acid could be used as markers for periportal fibrosis in patients with schistosomiasis mansoni.
Although the human morbidity and mortality caused by schistosomiasis mansoni have been reduced over the last 25 years by chemotherapy, the disease still represents a significant health hazard, particularly in north–eastern Brazil (Katz and Peixoto, 2000; Araújo et al., 2007).
It is believed that between 2% and 7% of humans infected with Schistosoma mansoni go on to develop hepato–splenic disease, which is associated with portal hypertension and digestive bleeding brought on by the rupture of oesophageal varices or hypertensive gastropathy (Richter et al., 1992; Chitsulo et al., 2000; Ferraz et al., 2003).
Periportal fibrosis (PPF) or Symmers’ fibrosis is a pathognomonic hepato–splenic lesion characterised by broad fibrous bands that reach into the branches of the portal vein, spreading from the vein’s entry into the liver through to the divisions of the fourth-order branches (Andrade, 2008). The eggs of S. mansoni are deposited in the mesenteric veins and may subsequently become impacted in the portal venules, where they trigger a granulomatous inflammation that evolves into PPF (Andrade et al., 1997).
In the pathophysiology of portal hypertension in schistosomiasis mansoni, the primary outcomes are PPF-attributable resistance to blood flow in the pre-sinusoidal circulation of the branches of the portal vein and splenic-vein hyperflow resulting from an enlarged spleen (Raia et al., 1991; Vezozzo et al., 2006; Maia et al., 2007). As the pressure levels in the portal vein are correlated with severity of PPF, the assessment of such fibrosis — using liver biopsy, ultrasonography and/or serum markers (Santos et al., 2005; Parise et al., 2006; Rossi et al., 2007) — is of particular interest in clinical practice (Richter et al., 1992). In schistosomiasis mansoni, a liver wedge biopsy is the most accurate method of assessing PPF but requires major surgery. Unfortunately, a fine-needle biopsy does not always present a true histopathological picture because PPF, although diffuse, varies in intensity throughout the hepatic parenchyma (Dimmette, 1955). Ultrasonography is an important tool for assessing schistosomiasis mansoni because it allows enlargement of the left hepatic lobe, reduction of the right lobe, periportal thickening and splenomegaly to be detected (Domingues et al., 1993; Richter et al., 2001; Vezozzo et al., 2006; Maia et al., 2007). In 1996, a World Health Organization convention in the Nigerien capital city of Niamey proposed that the PPF seen in schistosomiasis mansoni be classified into six patterns (known as patterns A–F), with patterns A and F representing the least and most severe forms of the fibrosis, respectively (WHO, 1996). Although this ‘Niamey protocol’ is now employed in general practice, it requires ultrasound equipment and qualified examiners that are not readily available in all areas where schistosomiasis mansoni is endemic. The search for simpler but accurate methods for assessing PPF continues.
Hepatic stellate cells are the principal mediators of fibrosis and, when activated by tumour necrosis factor, platelet-derived growth factor and fibrogenic cytokines (tumour growth factor-β, angiotensin II and leptin), are transformed into the proliferating contractile cells that constitute the essence of the fibrotic response to hepatic aggression (Friedman, 2004; Bataller and Brenner, 2005; Morais et al., 2006). As well as the proteins responsible for fibrogenesis, other molecules originating from the degradation of the extracellular matrix [e.g. hyaluronic acid (HA), type-III pro-collagen, type-IV collagen and laminin] have been used to assess liver fibrosis (Santos et al., 2005; Rossi et al., 2007; Marinho et al., 2010).
Since, during fibrogenesis, HA synthesis in the stellate cells is increased and there is a reduced clearance of HA from the circulation, serum concentrations of HA are usually elevated in fibrotic liver diseases (Friedman, 2004; Bataller and Brenner, 2005), although there are few relevant data on HA concentrations in schistosomiasis mansoni. The main aim of the present study was to evaluate HA concentrations in the sera of Brazilians with schistosomiasis mansoni and see if they were good markers of the severity of PPF, which was assessed in the same subjects using ultrasonography and the ‘Niamey protocol’.
PATIENTS AND METHODS
The 61 cases of schistosomiasis mansoni included in the study had all attended the Gastro-enterology Clinic at the Hospital das Clínicas of the Universidade Federal de Pernambuco (UFPE), in Recife, in north–eastern Brazil. When first seen at the clinic, all 61 had a history of water contact in an endemic area for schistosomiasis mansoni, had ultrasonographic pictures compatible with PPF, and were found stool-positive for S. mansoni eggs. All had been treated with praziquantel at least 6 months before the blood samples investigated in the present study were collected. Subjects were excluded from the study if they presented markers for hepatitis B or C (see below), had a body mass index (BMI) of >30 kg/m2, consumed >210 ml ethanol/week, and/or suffered from diabetes, cirrhosis and/or a neoplastic disease.
As controls, blood samples were also collected from 16, apparently healthy employees of the Centro de Pesquisas Aggeu Magalhães (also in Recife). Shortly before blood samples were collected from them, all 16 had been found stool-negative for S. mansoni eggs and seronegative for the markers of hepatitis B and C (see below). All had BMI of <30 kg/m2 and were similar to the cases in terms of their socio–economic status.
Ethical Approval
The study protocol was approved by the Ethics Committee of the UFPE’s Health Sciences Centre, and all the cases and controls investigated gave their written informed consent.
Laboratory Tests
A blood sample of about 10 ml was collected, from a peripheral vein of each subject, into a Vacutainer® tube (BD Diagnostics, Franklin Lakes, NJ) and sent to the Central Laboratory at the UFPE’s Hospital das Clínicas. The sera were separated from the blood samples by centrifugation before a subsample of each serum was checked, for antibodies to the core antigen of the hepatitis B virus and antibodies to the hepatitis C virus, in commercial microparticle enzyme immuno-assays (Abbott Laboratories, Abbott Park, IL). A further, 1-ml subsample of each serum was frozen at −20°C and sent to the Department of Biochemistry (Discipline of Molecular Biology) of the Universidade Federal de São Paulo, in São Paulo, where each subsample was tested in a non-competitive fluorescence-based assay that can detect HA concentrations ranging from 0·2–500 μg/litre (Köpke–Aguiar et al., 2002; Martins et al., 2003). The assay used employed HA binding protein (isolated from bovine cartilage) immobilized in microwell ELISA plates (Martins et al., 2003).
Ultrasonography
Each case and control was given an abdominal scan, by the same examiner (A.L.D.), with an SSD-500 ultrasound scanner (Aloka, Tokyo) fitted with a 3·5-MHz convex transducer. During each scan, the characteristics of the liver parenchyma and the surfaces of the liver and spleen were explored and any PPF detected was classified according to the ‘Niamey protocol’ (WHO, 1996).
Statistical Analysis
Odds ratios (with their 95% confidence intervals), χ2 tests and logistic regression were used, as appropriate, to explore and compare the qualitative variables, while analysis of variance was used to investigate the mean values of the quantitative variables. The Levene test was used to assess the homogeneity of the variances, Tukey’s test being used subsequently when homogeneity was verified and the Tamhane test being used when the variances were not found to be homogeneous.
A receiver-operating-characteristic (ROC) curve was used to establish the cut-off point for the serum HA concentration that best differentiated cases with moderate PPF from those with severe fibrosis.
The Excel 2000 software package (Microsoft) and version 8.0 of the SPSS package (SPSS Inc, Chicago, IL) were used for the data storage and analysis. A P-value of ⩽0·05 was considered indicative of a statistically significant difference or association.
RESULTS
The 16 controls (10 women and six men) had a mean (s.d.) age of 34·3 (9·5) years (range = 21–57 years). The 61 cases of schistosomiasis mansoni (36 women and 25 men) were, in general, slightly older, with a mean (s.d.) age of 46·7 (12·3) years (range = 23–65 years). Forty-two (69%) of the cases had experienced previous episodes of upper gastro-intestinal bleeding, and 27 (44%) had each had a splenectomy.
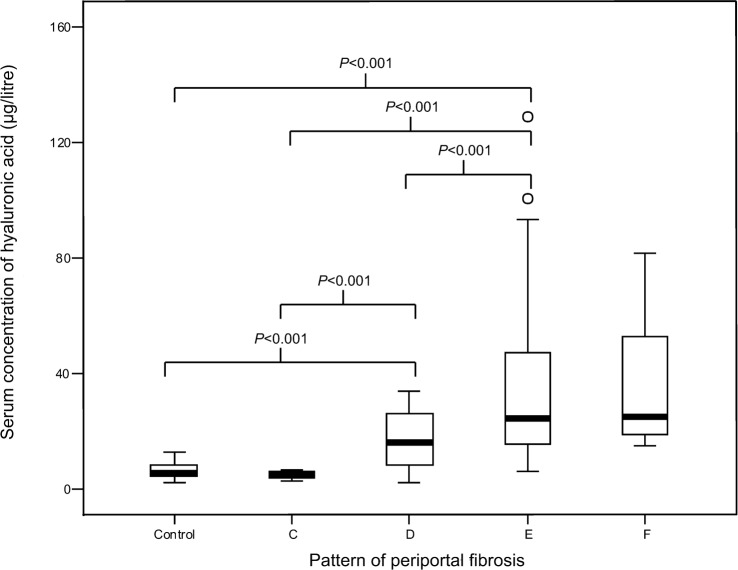
The serum concentrations of HA found in the 16 controls and 61 cases are summarized in Table 1 and the Figure. The mean concentration of HA in the sera of the controls was significantly lower than that recorded in the schistosomiasis cases who showed PPF of patterns D or E (P<0·001 for each) (but similar to that seen in the cases with pattern-C or pattern-F PPF). The cases who showed pattern-C PPF also had significantly less HA in their sera than the cases with PPF of patterns D or E (P<0·001 for each), and the cases with pattern-D fibrosis had significantly lower HA concentrations in their sera than the cases with PPF of pattern E (P<0·001). No examples of pattern-A or pattern-B fibrosis were observed.
Table 1. Serum concentrations of hyaluronic acid in the 77 adult Brazilians investigated in the present study.
| Hyaluronic acid (μg/litre) | |||
| Pattern of periportal fibrosis (WHO, 1996) | No. of subjects | Mean and (s.d.) | Range |
| None (healthy controls) | 16 | 6·2 (2·8) | 2·2–12·8 |
| C (peripheral fibrosis) | 4 | 4·8 (1·5) | 3·0–6·0 |
| D (central fibrosis) | 22 | 17·0 (9·9) | 2·0–34·0 |
| E (advanced fibrosis) | 27 | 37·3 (31·0) | 6·0–129·0 |
| F (very advanced fibrosis) | 8 | 36·4 (23·6) | 15·0–82·0 |
| C or D | 26 | 15·1 (10·1) | 2·0–34·0 |
| E or F | 35 | 37·1 (29·2) | 6·0–129·0 |
A ‘box-and-whisker’ plot of the concentrations of hyaluronic acid detected in the sera of 16 healthy controls and 61 cases of schistosomiasis mansoni with periportal fibrosis of patterns C, D, E or F.
When the subjects were considered as three groups — healthy controls, cases with moderate fibrosis (patterns C and D) and cases with severe fibrosis (patterns E and F) — it became clear that mean serum HA concentrations increased significantly (P<0·001) as the PPF became more severe (Table 1). Exploration of an ROC curve indicated that the use of a threshold of 20·2 μg HA/litre of serum gave the best differentiation between the cases with moderate PPF and those with more severe PPF (Table 2), achieving a sensitivity of 60% [with a 95% confidence interval (CI) of 43·8%–76·2%], a specificity of 65% (CI = 47·1%–83·7%), a positive predictive value of 70% (CI = 53·6%–86·4%), and a negative predictive value of 55% (CI = 37·3%–72·4%).
Table 2. The patterns of periportal fibrosis (WHO, 1996) seen in the cases of schistosomiasis mansoni found to have >20·2 or ⩽20·2 μg hyaluronic acid/litre of serum.
| Hyaluronic acid | No. of subjects showing pattern: | |||
| (μg/litre) | E or F | C or D | Odds ratio and (95% confidence interval) | P |
| >20·2 | 21 | 9 | 2·8 (1·0–8·1) | 0·053 |
| ⩽20·2 | 14 | 17 | 1 | |
DISCUSSION
In many areas where schistosomiasis mansoni is endemic, liver biopsies carry too much risk and the equipment and personnel required for accurate ultrasonography are not readily available. The main aim of the present study, in which ultrasonography was used as the ‘gold standard’ for classifying PPF, was to explore the value of serum HA as a marker for PPF.
Ricard–Blum et al. (1999) found that serum concentrations of HA (but not those of type-I collagen or type-III pro-collagen) were markedly higher in (ultrasonography-evaluated) cases of PPF than in healthy controls, and suggested that serum concentrations of HA could be used to assess morbidity in schistosomiasis mansoni. More recently, Marinho et al. (2010) reported how serum concentrations of HA (but not those of type-IV collagen) could be used to separate patients with light PPF from those with intense fibrosis. They also reported a positive correlation between serum HA concentrations and the prevalence of portal hypertension (as indicated by relatively large portal- and splenic-vein diameters) in patients with PPF caused by schistosomiasis mansoni. Although these results contrast with those of Burchard et al. (1998), who found no correlation between serum concentrations of HA (or type-III pro-collagen or laminin) in cases of schistosomiasis mansoni and the results of ultrasound scans, most of the cases investigated in this earlier study only had mild fibrosis.
In other studies of Brazilian cases of schistosomiasis mansoni, the severity of PPF (as assessed with ultrasound) has been found to correlate with ‘aspartate aminotransferase to platelet ratio indexes’ (APRI; Lambertucci et al., 2007) and with serum concentrations of immunoglobulin G (Correia et al., 2009).
In the present study, a serum HA concentration of about 20·2 μg/litre was found to be the best HA threshold for distinguishing patients with moderate PPF from those with severe fibrosis. In their earlier study, Köpke–Aguiar et al. (2002) found that a similar HA threshold (20 μg/litre of serum) could be used to separate schistosomiasis patients with and without portal hypertension, while a much higher threshold (80 μg/litre) could be used to differentiate patients with cirrhosis from those with portal hypertension caused by schistosomiasis mansoni. Among Sudanese cases of schistosomiasis mansoni, Pascal et al. (2000) found that serum concentrations of HA increased as the severity of PPF increased, and Eboumbou et al. (2005) found that cases had about 22·4 μg HA/litre of serum if they did not have PPF and up to 91·2 μg HA/litre if they had severe PPF. Curiously, the HA concentrations detected by Pascal et al. (2000) were generally higher than the corresponding values reported by Eboumbou et al. (2005) or observed in the present study. It seems possible that the cases of schistosomiasis mansoni investigated in the present study had even higher serum concentrations of HA before they were treated (the blood samples investigated in the present study were collected at least 6 months after the cases had been given praziquantel). The concentrations of HA in the sera of patients with schistosomiasis mansoni tend to fall a few weeks after treatment with praziquantel (Hassanein et al., 1997; Ricard–Blum et al., 1999). Many of the cases investigated in the present study had also had their spleens removed and it is possible that the splenectomies led to reductions in serum HA. In a study of patients with schistosomiasis mansoni in a tertiary-care hospital in Brazil, Wyszomirska et al. (2005) observed post-splenectomy reductions in the serum concentrations of type-IV collagen.
The measurement of PPF via serum markers, such as HA, would seem to have a wide application, especially in endemic areas where ultrasonography is difficult or impossible. In schistosomiasis mansoni, the early detection of hepatic involvement is crucial, not only to impede the development of more severe morbidity but also to guide the epidemiological control of S. mansoni. The measurement of serum HA concentrations may also be useful during post-treatment follow-up, to monitor the regression of any PPF.
Further studies involving many more patients and other areas of endemicity will be necessary in order to confirm the present results and see if they are applicable world-wide.
REFERENCES
- Andrade ZA.(2008)Schistosomiasis and hepatic fibrosis regression. Acta Tropica 10879–82. [DOI] [PubMed] [Google Scholar]
- Andrade ZA, Silva LM, Souza MM.(1997)An experimental approach to the pathogenesis of ‘pipestem’ fibrosis (Symmers’ fibrosis of the liver). Memórias do Instituto Oswaldo Cruz 92699–706. [DOI] [PubMed] [Google Scholar]
- Araújo KCGM, Resendes APC, Souza-Santos R, Silveira JC, Jr, Barbosa CS.(2007)Análise espacial dos focos de Biomphalaria glabrata e de casos humanos de esquistossomose mansônica em Porto de Galinhas, Pernambuco, Brasil, no ano 2000. Cadernos de Saúde Pública 23409–417. [DOI] [PubMed] [Google Scholar]
- Bataller R, Brenner DA.(2005)Liver fibrosis. Journal of Clinical Investigation 115209–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burchard GD, Guissé-Sow F, Diop M, Ly A, Lanuit R, Gryssels B, Gressner AM.(1998)Schistosoma mansoni infection in a recently exposed community in Senegal: lack of correlation between liver morphology in ultrasound and connective tissue metabolites in serum. Tropical Medicine and International Health 3234–241. [DOI] [PubMed] [Google Scholar]
- Chitsulo L, Engels D, Montresor A, Savioli L.(2000)The global status of schistosomiasis and its control. Acta Tropica 7741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia HST, Domingues ALC, Lopes EP, Morais CNL, Sarteschi C, Moura IMF.(2009)Níveis séricos de globulinas e a intensidade da fibrose hepática em pacientes com esquistossomose mansônica. Arquivos de Gastroenterologia 46194–198. [DOI] [PubMed] [Google Scholar]
- Dimmette RM.(1955)Liver biopsy in clinical schistosomiasis; comparison of wedge and needle types. Gastroenterology 29219–234. [PubMed] [Google Scholar]
- Domingues ALC, Lima ARF, Dias HS, Leão GC, Coutinho A.(1993)An ultrasonographic study of liver fibrosis in patients infected with Schistosoma mansoni in north–east Brazil. Transactions of the Royal Society of Tropical Medicine and Hygiene 87555–558. [DOI] [PubMed] [Google Scholar]
- Eboumbou C, Steghens JP, Abdallahi SOM, Adil M, Pierre G, van Kappel A, Quarashi A, Bouchra G, de Reggi M.(2005)Circulating markers of oxidative stress and liver fibrosis in Sudanese subjects at risk of schistosomiasis and hepatitis. Acta Tropica 9499–106. [DOI] [PubMed] [Google Scholar]
- Ferraz AAB, Albuquerque PC, Lopes EP, Araújo JGC, Jr, Carvalho AHF, Ferraz EM.(2003)The influence of periportal (pipestem) fibrosis on long term results of surgical treatment for schistosomotic portal hypertension. Arquivos de Gastroenterologia 104–10. [DOI] [PubMed] [Google Scholar]
- Friedman SL.(2004)Stellate cells: a moving target in hepatic fibrogenesis. Hepatology 401041–1043. [DOI] [PubMed] [Google Scholar]
- Hassanein HI, Zoheiry MM, Voss B, El Attar GM, Hassan SI, Demerdash ZA, El Baz HM, Yousif OA.(1997)Effect of early treatment with praziquantel on serum connective tissue metabolite markers in children and adolescents with intestinal schistosomiasis mansoni. Arzneimittel Forschung 4784–87. [PubMed] [Google Scholar]
- Katz N, Peixoto SV.(2000)Análise crítica da estimativa do número de portadores de esquistossomose mansoni no Brasil. Revista da Sociedade Brasileira de Medicina Tropical 33303–308. [DOI] [PubMed] [Google Scholar]
- Köpke-Aguiar LA, Martins JR, Passerotti CC, Toledo CF, Nader HB, Borges DR.(2002)Serum hyaluronic acid as a comprehensive marker to assess severity of liver disease in schistosomiasis. Acta Tropica 84117–126. [DOI] [PubMed] [Google Scholar]
- Lambertucci JR, Silva LCS, Antunes CM.(2007)Aspartate aminotransferase to platelet ratio index and blood platelet count are good markers for fibrosis evaluation in schistosomiasis mansoni. Revista da Sociedade Brasileira de Medicina Tropical 40599. [DOI] [PubMed] [Google Scholar]
- Maia MD, Lopes EP, Ferraz AAB, Barros FMR, Domingues ALC, Ferraz EM.(2007)Evaluation of splenomegaly in the hepatosplenic form of mansoni schistosomiasis. Acta Tropica 101183–186. [DOI] [PubMed] [Google Scholar]
- Marinho CC, Bretas T, Voieta I, Queiroz LC, Ruiz-Guevara R, Teixeira AL, Antunes CM, Prata A, Lambertucci JR.(2010)Serum hyaluronan and collagen IV as non-invasive markers of liver fibrosis in patients from an endemic area for schistosomiasis mansoni: a field-based study in Brazil. Memórias do Instituto Oswaldo Cruz 105471–478. [DOI] [PubMed] [Google Scholar]
- Martins JR, Passerotti CC, Maciel RM, Sampaio LO, Dietrich CP, Nader HB.(2003)Practical determination of hyaluronan by a new noncompetitive fluorescence-based assay on serum of normal and cirrhotic patients. Analytical Biochemistry 31965–72. [DOI] [PubMed] [Google Scholar]
- Morais CNL, Carvalho BM, Melo WG, Lopes EP, Domingues AL, Jucá NT, Souza W, Abath FG, Montenegro SM.(2006)Preliminary evaluation of cytokines in the hepatitis C–schistosomiasis co-infection. Memórias do Instituto Oswaldo Cruz 101(Suppl. 1)353–354. [DOI] [PubMed] [Google Scholar]
- Parise ER, Rosa H.(1992)Serum laminin in hepatic schistosomiasis. Transactions of the Royal Society of Tropical Medicine and Hygiene 86179–181. [DOI] [PubMed] [Google Scholar]
- Parise ER, Oliveira AC, Figueiredo-Mendes C, Lanzoni V, Martins JR, Nader HB, Ferraz ML.(2006)Noninvasive serum markers in the diagnosis of structural liver damage in chronic hepatitis C virus infection. Liver International 261095–1099. [DOI] [PubMed] [Google Scholar]
- Pascal M, Abdallahi OM, Elwali NE, Mergani A, Quarashi A, Magzoub M, de Reggi M, Gharib B.(2000)Hyaluronate levels and markers of oxidative stress in the serum of Sudanese subjects at risk of infection with Schistosoma mansoni. Transactions of the Royal Society of Tropical Medicine and Hygiene 9466–70. [DOI] [PubMed] [Google Scholar]
- Raia S, Mies S, Alfiere F.(1991)Portal hypertension in mansonic schistosomiasis. World Journal of Surgery 15176–187. [DOI] [PubMed] [Google Scholar]
- Ricard-Blum S, Hartmann DJ, Grenard P, Ravaoalimalala VE, Boisier P, Esterre P.(1999)Relationships between several markers of extracellular matrix turn-over and ultrasonography in human schistosomiasis mansoni. American Journal of Tropical Medicine and Hygiene 60658–663. [DOI] [PubMed] [Google Scholar]
- Richter J, Monteiro ES, Braz RM, Abdalla MI, Abdel-Rahim M, Fano U, Huntgeburth U, Feldmeier H.(1992)Sonographic organometry in Brazilian and Sudanese patients with hepatosplenic schistosomiasis mansoni and its relation to the risk of bleeding from oesophageal varices. Acta Tropica 51281–290. [DOI] [PubMed] [Google Scholar]
- Richter J, Domingues ALC, Barata CH, Prata AR, Lambertucci JR.(2001) Report on the second satellite symposium on ultrasound in schistosomiasis. Memórias do Instituto Oswaldo Cruz 96(Suppl.)151–156. [DOI] [PubMed] [Google Scholar]
- Rossi E, Adams LA, Bulsara M, Jeffrey GP.(2007)Assessing liver fibrosis with serum marker models. Clinical Biochemist Reviews 283–10. [PMC free article] [PubMed] [Google Scholar]
- Santos VN, Leite-Mór MM, Kondo M, Martins JR, Nader HB, Lanzoni V, Parise ER.(2005)Serum laminin, type IV collagen and hyaluronan as fibrosis markers in non-alcoholic fatty liver disease. Brazilian Journal of Medical and Biological Research 38747–753. [DOI] [PubMed] [Google Scholar]
- Vezozzo DC, Farias AQ, Cerri GG, da Silva LC, Carrilho FJ.(2006)Assessment of portal hemodynamics by Doppler ultrasound and of liver morphology in the hepatosplenic and hepatointestinal forms of schistosomiasis mansoni. Digestive Disease and Science 511413–1419. [DOI] [PubMed] [Google Scholar]
- World Health Organization(1996)Ultrasound in Schistosomiasis. A Practical Guide to the Standardized Use of Ultrasonography for the Assessment of Schistosomiasis-related Morbidity Niamey: WHO [Google Scholar]
- Wyszomirska RMAF, Nishimura NF, Almeida JR, Yamanaka A, Soares EC.(2005)High serum laminin and type IV collagen levels in schistosomiasis mansoni. Arquivos de Gastroenterologia 42221–225. [DOI] [PubMed] [Google Scholar]