Abstract
Although approximately 40% of all the people blinded by Onchocerca volvulus are Nigerians, almost nothing was known about the various cytospecies of the blackfly vectors present in Nigeria until 1981. The activation of the Nigerian National Onchocerciasis Control Programme in 1986 (and that programme’s initiation of mass distributions of ivermectin in 1991) provided a significant stimulus to understand the biology of the Nigerian vectors but the exploration of any possible differences between the cytospecies has been hampered by a lack of accessible taxonomic information. This review attempts to satisfy that need. There are nine different cytoforms reliably recorded from Nigeria (Simulium damnosum s.s. Nile form, S. damnosum s.s. Volta form, S. sirbanum Sirba form, S. sirbanum Sudanense form, S. soubrense Beffa form, S. squamosum A, S. squamosum B, S. squamosum C and S. yahense typical form), and three more are known from surrounding countries and might be reasonably expected to occur in Nigeria. All of these cytospecies are presumed to be vectors, although there have been almost no identifications of the vectors of O. volvulus in Nigeria. The biogeographical distribution of the cytoforms is broadly similar to that known in other parts of West Africa (although many of the cytoforms remain insufficiently studied). The physico–chemical hydrology of the Nigerian breeding sites of the cytospecies does not, however, correspond to that seen elsewhere in West Africa, and it is not clear whether this might be related to differences in the cytoforms. An illustrated cytotaxonomic key is presented to facilitate and encourage future studies.
Human onchocerciasis (river blindness), caused by the filarial parasite Onchocerca volvulus, is a debilitating tropical infection that can result in blindness and severe skin disease. Although onchocerciasis is also endemic to tropical America and the Yemen, 99% of all cases occur in Africa, and 95% of the African cases are infected via the bites of blackflies (Diptera: Simuliidae) of the Simulium (Edwardsellum) damnosum Theobald complex (WHO, 1995). Each year, the filariae transmitted by this complex are responsible for 250,000 cases of blindness, up to 500,000 more cases of severe visual impairment, and the loss of nearly 1 million disability-adjusted life-years (DALY; WHO, 1995; www.who.int/tdr/diseases/oncho/diseaseinfo.html). Compared with their healthy counterparts, individuals with symptomatic infection have been found to spend an additional 15% of their annual income on health, infected children have been found to be more likely to drop out of school, and symptomatic farmers have, on average, a third less land under cultivation — all differences that contribute to poverty (Fischer and Büttner, 2002). Although onchocerciasis has been named the third most important cause of preventable blindness in the tropics (Narita and Taylor, 1993), it is skin disease that is responsible for 60% of the DALY lost as the result of oncochercal infection. There is also some evidence that onchocerciasis may be associated with epilepsy and dwarfism (Basáñez et al., 2006) and the biting nuisance that blackflies represent also has a real but unmeasured effect on economic activities (Hougard et al., 1998).
On the basis of the banding sequences of the polytene chromosomes from the salivary glands of the larvae, Simulium damnosum s.l. has been shown to be made up of a complex of sibling species (Dunbar, 1966; Vajime and Dunbar, 1975; Quillévéré, 1975). Sibling species are real species that may differ in aspects of their biology but usually cannot be distinguished by morphology. Such species in the S. damnosum complex are often called cytospecies because they have been defined on the basis of their chromosomal variation. Cytotypes, on the other hand, are chromosomally distinctive populations of unknown specific status, although further research will probably show that some of them are actually cytospecies. Cytospecies and cytotypes are known together as cytoforms. There are 55 named cytoforms within the S. damnosum complex (Post et al., 2007), making it the largest known sibling species complex of any vector.
It is thought that all the West-African cytoforms of S. damnosum s.l. are vectors (although this has not yet been proven for some species, such as S. mengense, largely because of problems in identification), albeit of varying importance. As members of the damnosum subcomplex have greater longevity than members of the squamosum subcomplex (Millest et al., 1992), they tend to be more effective as vectors. Simulium damnosum s.s. and S. sirbanum are known to be capable of regular wind-assisted migrations of >400 km, and they can move in such large numbers that they can threaten the success of control efforts in the areas that they reach (Walsh et al., 1981; Baker et al., 1990). In contrast, S. squamosum seems to cross shorter distances (up to 125 km; Cheke and Garms, 1983), and S. yahense may only travel a few kilometres. The discrimination of the sibling species of S. damnosum s.l. was critical to the success of the World Health Organization’s Onchocerciasis Control Programme (OCP) in West Africa (Le Berre and Fiasorgbor, 1985; Yaméogo et al., 2004), which, through the insecticide treatment of vector breeding sites between 1974 and 2002, protected 40 million people in 11 countries against onchocerciasis and prevented 600,000 cases of blindness (WHO, 2002).
Nigeria has more people blinded by onchocerciasis than any other country — an estimated 100,000 cases of the 268,000 that occur worldwide — as well as approximately 3·2 million people infected with O. volvulus (WHO, 1995). The population of Nigeria exceeds that of all 11 countries covered by the old OCP combined. The Nigerian National Onchocerciasis Control Programme (NOCP) was established in 1982, became active in 1986 and started distribution of ivermectin in 1991 (Jiya, 1998). This programme’s aim is to eliminate onchocerciasis as a public-health problem, via annual distributions of ivermectin to communities where the incidence of the disease is high. Ivermectin conveys enormous clinical benefits but it is unclear whether it can normally interrupt transmission by S. damnosum s.l. in Africa (Borsboom et al., 2003). The development and application of alternative control strategies in Nigeria will benefit from a better understanding of the epidemiology of the disease, and this should take into account the differences between the vector cytospecies. Unfortunately, the number of publications on the S. damnosum complex in Nigeria remains small. Thirty years ago, Crosskey (1981) listed vector breeding sites throughout the country in relation to onchocerciasis foci but, at the time, the cytotaxonomic identities of the blackfly populations were very poorly known. Since then, although the advent of the NOCP was a significant stimulus to research into the epidemiology and transmission of onchocerciasis in Nigeria (Okonkwo et al., 1991; Adewale et al., 1999; Nwoke and Dozie, 2001; Ubachukwu and Anya, 2001; Oyibo and Fagbenro–Beyioko, 2003; Idowu et al., 2004; Ubachukwu, 2004; Opara et al., 2005), there has only been one direct published indication of which sibling species are vectors in Nigeria (Mafuyai et al., 1997) and this was based on only 14 infective flies [eight S. squamosum and six ‘savanna’ blackflies (i.e. S. damnosum s.s. or S. sirbanum)]. Even studies on the larval biology of Nigerian S. damnosum s.l. (Usip et al., 2003; Opara and Fagbemi, 2005; Ikpeama et al., 2006) have not always included identifications of the cytoforms under investigation.
It is surprising that so little is known about the role and importance of the different cytospecies in the transmission of O. volvulus in Nigeria, and the objective of this publication is to facilitate studies on these topics, by reviewing the members of the S. damnosum complex that have been reliably recorded from Nigeria, and providing the means for their cytotaxonomic identification. Similar guides have been published for other parts of West Africa (Boakye, 1993) and for East Africa (Krueger, 2006). The identification of the adult female vectors to cytospecies is outside the scope of this review and readers interested in this topic are referred to the publications by Wilson et al. (1993, 1994), Mafuyai et al. (1996b, 1997), Usip et al. (2003), Mank et al. (2004), Mustapha et al. (2004b), Ibeh et al. (2008) and Idowu et al. (2008). For an inventory of known cytotypes, with a list of their diagnostic inversions, see Post et al. (2007), and for descriptions of cytotaxonomic methods see, for example, Dunbar (1972), Quillévéré (1975), Vajime (1986), Boakye (1988) and Krüger (2003). Descriptions of cytotaxonomic nomenclature have been published by Vajime and Dunbar (1975), Quillévéré (1975), Vajime (1986), Boakye (1993) and Post et al. (2007).
REVIEW OF CYTOSPECIES OF THE S. damnosum COMPLEX IN NIGERIA
Vajime and Dunbar (1975) were the first to identify sibling species of the S. damnosum complex in West Africa. They recognised eight sibling species (S. squamosum, S. yahense, S. sanctipauli, S. soubrense, S. damnosum s.s., S. sirbanum, S. sudanense and S. dieguerense) and published an identification key based on chromosomal features. Quillévéré (1975) subsequently criticised the criteria for identifying S. soubrense and S. sanctipauli, arguing that the inversion 2L-7 was polymorphic within species and not diagnostic. Bedo (1977) also criticised the criteria given by Vajime and Dunbar (1975) for the identification of S. sirbanum and S. sudanese, for similar reasons. Although Post (1986) showed that S. sanctipauli and S. soubrense were separate species, he found the old diagnostic inversion 2L-7 [described by Vajime and Dunbar (1975)] to be intraspecific and proposed a new diagnostic inversion which he called 2L-A.
Only two cytospecies (S. damnosum s.s. and S. sirbanum) were recorded by Vajime and Dunbar (1975) from Nigeria, and the same two sibling species were also identified in Nigeria by Vajime and Quillévéré (1978), confirming the earlier work.
The Nigerian cytospecies of the S. damnosum complex were first reviewed by Crosskey (1981). Crosskey (1981) noted the presence of S. squamosum and S. sudanense in Nigeria, but did not cite any published work for these new identifications, referring instead to a personal communication from Dr R. W. Dunbar. Curiously, however, the observations made by Dunbar and Vajime (1981) partially contradicted Crosskey’s report. Dunbar and Vajime (1981) tabulated the distribution of cytospecies found in Africa and recognised four cytospecies from Nigeria (S. sudanense, S. damnosum s.s., ‘Volta form’ and S. sirbanum but not S. squamosum). The separate taxonomic status of S. damnosum s.s. and ‘Volta form’ remains controversial and has not been recognised by many authors. Most authors prefer to consider ‘Volta form’ as a cytotype within S. damnosum s.s. (Boakye, 1993), and this implies that there must also be a ‘typical’ cytotype. Post et al. (2007) referred to these two cytotypes as S. damnosum s.s. Volta form and S. damnosum s.s. Nile form.
Gregory (1982), in his brief review of the vectors of O. volvulus in Nigeria, reported that six cytospecies had been identified in the country: S. damnosum s.s., S. sirbanum, S. sudanense, S. soubrense, S. sanctipauli and S. squamosum. Of these, four had already been reported by Crosskey (1981), but Gregory (1982) was the first to report S. soubrense and S. sanctipauli from Nigeria. Gregory cited Vajime and Dunbar (1975) to support his statement that S. soubrense and S. sanctipauli occurred in Nigeria but — although they described eight species from West Africa, including the six cytospecies listed by Gregory — Vajime and Dunbar (1975) only reported two cytospecies (S. damnosum s.s. and S. sirbanum) from Nigeria (see above). It is unclear where Gregory (1982) obtained his information on Nigerian S. soubrense and S. sanctipauli but the source was most likely personal communications with Dr R. W. Dunbar and/or Dr C. G. Vajime, who were both working in Nigeria at the time.
Meredith et al. (1983) subsequently confirmed the presence of S. soubrense in Nigeria, although, at the time of their studies, the species status of S. soubrense and S. sanctipauli was unclear and the S. soubrense was simply called the ‘Beffa form’; the ‘Beffa form’ is now considered to be a cytotype of S. soubrense (Post, 1986; Boakye, 1993). Meredith et al. (1983) recorded the Beffa form from the Nigerian border with Benin, in the Okpara River. This finding was significant not only in confirming the presence of the cytospecies in Nigeria but also as a border report. The relevance of identifications on the borders of Nigeria, or from the surrounding countries, is that some members of the S. damnosum complex are known to migrate over very long distances (up to, and perhaps exceeding, 400 km) and the risk of such movement has been very important in planning strategies for onchocerciasis control (Garms et al., 1979) because immigrant flies can bring the human-infective larvae of O. volvulus with them. Roberts (1985) also reported the Beffa form of S. soubrense from Nigeria but did not state the criteria for his identification. It is probable that he was trying to use the morphological criteria described by Meredith et al. (1983), since he was not a cytotaxonomist. Outside of the OCP area, however, these morphological criteria should only be applied with great caution, otherwise incorrect identifications can occur. Meredith et al. (1983), for example, used the criteria to identify some Liberian blackflies as the Beffa form but this identification was probably incorrect because the Beffa form has never been identified chromosomally from rivers in the study area in Liberia. Post (1986) published the first reliable report of S. soubrense Beffa form from sites within Nigeria, identifying the form cytologically by the diagnostic inversion 2S-6b. Although Cheke et al. (1987) believed that Meredith et al. (1983) had recorded the same form from the Gurara River, near the Nigerian city of Abuja, the identification was morphological and needs to be confirmed cytologically.
Roberts and Irving–Bell (1987, 1996) reported identifications of adult S. squamosum and S. damnosum s.s. in a Nigerian valley but, again, these identifications were made (Roberts and Irving–Bell, 1987) or were probably made (Roberts and Irving–Bell, 1996) — no relevant detail is provided in the later publication — using the morphotaxonomic keys of Dang and Peterson (1980). Although these identifications are probably mostly correct [because, according to a personal communication from Dr R. W. Dunbar (Roberts and Irving–Bell, 1987), they correlate with cytotaxonomic identifications of larvae from the same valley], the morphotaxonomic separation of the adult females of S. squamosum and S. damnosum s.s. is known to be very difficult (Garms et al., 1982; Garms and Cheke, 1985; Wilson et al., 1993) and not always possible using the keys of Dang and Petersen (1980).
Akoh et al. (1987) examined new material from Nigeria and identified it cytotaxonomically, with stated criteria, as five cytospecies: S. squamosum, S. sanctipauli, S. damnosum s.s., S. sirbanum and (for the first time in the country) S. yahense. The identification of S. sanctipauli is surprising because the specimens came from the northern Guinea savannah, which is not typical of S. sanctipauli in other countries (Boakye, 1993).
Vajime (1989) synonymized S. sirbanum and S. sudanense under the name S. sirbanum and described them as ‘sub-siblings’, which was clearly meant to indicate their subspecies status. Vajime (1989) did not, however, designate subspecific trinomials and subsequent authors have treated S. sirbanum and S. sudanense as synonyms (Boakye, 1993; Adler and Crosskey, 2010) and referred to them as the Sirba form and the Sudanense form of S. sirbanum (Post et al., 2007).
Vajime and Gregory (1990) were the first to list eight cytospecies of the S. damnosum complex from Nigeria in a single work: S. damnosum s.s., Volta form, S. sirbanum, S. sanctipauli, S. soubrense, S. soubrense ‘Beffa form’, S. squamosum and S. yahense. They did not distinguish the two cytotypes described by Vajime (1989) within S. sirbanum. It is questionable if S. sanctipauli and S. soubrense (typical form) actually exist in Nigeria. This is because the identifications by Vajime and Gregory (1990) were based on inversion 2L-7 (Vajime and Dunbar, 1975), which was already known to be insufficient for separating S. soubrense from S. sanctipauli (see above). Post (1986) introduced a new inversion, 2L-A, for this purpose. Any identifications of S. sanctipauli and S. soubrense (typical form) without reference to 2L-A are questionable and are probably all misidentifications of specimens of the Beffa form of S. soubrense.
Mafuyai (1992) identified five cytospecies (S. damnosum s.s., S. yahense, S. sirbanum, S. sudanense and S. squamosum) in new Nigerian material, but also recognised the occurrence of the Volta form and S. sanctipauli in Nigeria. He did not recognise the different cytotypes within S. sirbanum, and found that, at two sites, male S. sirbanum lacked inversion IS-3, which means that they could not be of the Sirba form, Sudanense form or Type IV (Fiasorgbor and Cheke, 1992). Although this finding might indicate the presence of a fourth cytotype within S. sirbanum, this possibility needs further exploration and will not be considered further in this review.
Fiasorgbor and Cheke (1992) found S. sirbanum in the Okpara River on the Nigeria–Benin border, where Meredith et al. (1983) had already found the Beffa form of S. soubrense.
Working with mostly the same material as Mafuyai (1992), Wilson et al. (1994) confirmed the earlier findings by reporting four cytospecies (S. yahense, S. squamosum, S. damnosum s.s. and S. sirbanum). Mafuyai et al. (1996a, 1997) reported S. damnosum s.s. (without distinguishing the Volta form), S. sirbanum s.l., S. squamosum, S. yahense and S. soubrense (including the Beffa form). Bassey (1998) identified four species and reviewed seven cytospecies. The species she reported from new Nigerian samples were S. damnosum s.s. (without distinguishing the Volta form), S. sirbanum, S. squamosum and S. yahense. Boakye et al. (1998) summarized the cytotaxonomic identifications, during the period 1984–1993, for the old OCP area, which includes the whole of Benin and the Okpara River on the border with Nigeria. They confirmed the presence of S. damnosum, S. sirbanum and S. soubrense Beffa form in the Okpara River.
Vajime and Dunbar (1975) were the first to notice chromosomally distinct entities within S. squamosum but this variation was largely ignored until it was redescribed by Boakye (1993). In Nigeria, Traore–Lamizana et al. (2001) were the first to distinguish different named cytotypes within S. squamosum, identifying S. squamosum A and S. squamosum C. Significant new distribution records have been added for south–eastern Nigeria by Ibeh et al. (2006) (who also recorded S. squamosum B for the first time in Nigeria) and Onyenwe et al. (2007), who recorded the Sudanense form of S. sirbanum.
The migratory habit noted in blackly sibling species makes cross-border identification very important, with the strong possibility of vector migration into Nigeria from the surrounding countries. Post et al. (2007) have listed the cytospecies present in Cameroon, Equatorial Guinea, Niger and Benin. In summary [with the data for countries bordering Nigeria updated from those provided by Post et al. (2007)], the cytospecies and cytotypes reliably recorded in Nigeria and her neighbours include:
Nigeria
S. damnosum subcomplex
S. damnosum s.s. Nile form
S. damnosum s.s. Volta form
S. sirbanum Sirba form
S. sirbanum Sudanense form
S. sanctipauli subcomplex
S. soubrense Beffa form
S. squamosum subcomplex
S. squamosum A (typical form)
S. squamosum B
S. squamosum C
S. yahense (typical form)
Cameroon
S. damnosum subcomplex
S. damnosum s.s. Nile form
S. damnosum s.s. Volta form
S. sirbanum s.l.
S. squamosum subcomplex
S. squamosum A (typical)
S. squamosum B
S. squamosum C
S. squamosum D
Kibwezi subcomplex
S. mengense
Benin
S. damnosum subcomplex
S. damnosum s.s. Nile form
S. damnosum s.s. Volta form
S. sirbanum Sudanense form
S. sirbanum Type IV
S. sanctipauli subcomplex
S. soubrense Beffa form
S. squamosum subcomplex
S. squamosum C
S. yahense (typical form)
Niger
S. damnosum subcomplex
S. damnosum s.s. Nile form
S. damnosum s.s. Volta form
S. sirbanum Sirba form
S. sirbanum Sudanense form
S. sirbanum Type IV
Equatorial Guinea (Bioko)
S. squamosum subcomplex
S. yahense Bioko form
REVIEW OF DISTRIBUTION OF THE CYTOSPECIES OF THE S. damnosum COMPLEX IN NIGERIA, WITH REFERENCE TO THE DISTRIBUTION OF ONCHOCERCIASIS
The distribution of human onchocerciasis in Nigeria was mapped by Crosskey (1981) from a review of all of the relevant data that had then been recorded. Subsequently (between 1994 and 1996), the disease in the whole country was re-surveyed by the NOCP, using a technique, called REMO (rapid epidemiological mapping of onchocerciasis), that is based upon the prevalence of nodules in selected communities. This yielded preliminary maps (Gemade et al., 1998; Noma et al., 2002) that were then refined and completed to give a detailed working picture of onchocerciasis across Nigeria (WHO, 2004; Basáñez et al., 2006). The maps have been used for the identification of areas of hyper- and meso-endemicity, which are the main targets for community-directed treatment with ivermectin (CDTI; Jiya, 1998). The NOCP has established 26 CDTI projects in four primary health zones, with the aim of reaching over 27 million people (WHO, 2004). Although this would not have been possible without the detail provided by REMO, the basic pattern of onchocerciasis described in Nigeria by Crosskey (1981) remains broadly true. Onchocerciasis is now known to be more widely distributed in Nigeria and this has resulted in the filling of gaps in the map that Crosskey (1981) created.
Onchocerciasis is mostly absent from the lowland areas of Nigeria, including the regions that geographers call the Coastlands (in the delta area and the coastal plain), the Niger–Benue Trough (through which the Niger and Benue Rivers flow), the Sokoto Plains (in the north–western corner of Nigeria) and the Chad Basin (in the north–eastern corner) [see figure 3.3 of Iloeje (1981)]. This absence of the disease largely reflects the absence of hard Precambrian rocks; rapids, which form the breeding sites for S. damnosum s.l., only generally occur where hard rocks form outcrops in river beds (Crosskey, 1981). The Nigerian lowlands do have some pockets of onchocerciasis, such as a focus near Nguru, in the Chad Basin close to the border with Niger, that has been mapped by REMO. Crosskey (1981) described a scattered series of small isolated foci associated with the Lower Niger Basin, and these are now known to be wider in extent, with onchocerciasis almost continuously distributed and associated with a number of geographical features, most notably the Udi Plateau and the Scarplands (Onyenwe et al., 2007). In the uplands, the three northern zones of onchocerciasis recognised by Crosskey (1981) — Zones 1, 2 and 3 — have been much better defined and are now known to more-or-less run into each other. Zone 1 (Borgu-Sokoto) is now considered to be more-or-less continuous with Zone 5 (south–western zone, associated with the mostly forested Western Uplands), which itself is continuous across the lower Niger Valley (see above) into Crosskey’s Zone 4 (southern Adamaya, associated with the uplands along the border with Cameroon, including the Oban Hills, Obudu Plateau, Adamawa Plateau, Shebshi Mountains and Alantika Mountains). Crosskey’s (mid-northern) Zone 2 is centred on the network of major rivers that originate as headwater streams around the edges of the Jos Plateau, and is mostly Guinea savanna. Crosskey’s (north–eastern) Zone 3 is mostly Sudan savanna and extends from the Mandara mountains (on the border with Cameroon) and Biu Plateau, in a south–easterly direction, across the Gongola valley and along the northern edge of the Benue Trough to meet the Jos Plateau, where it runs into Zone 2.
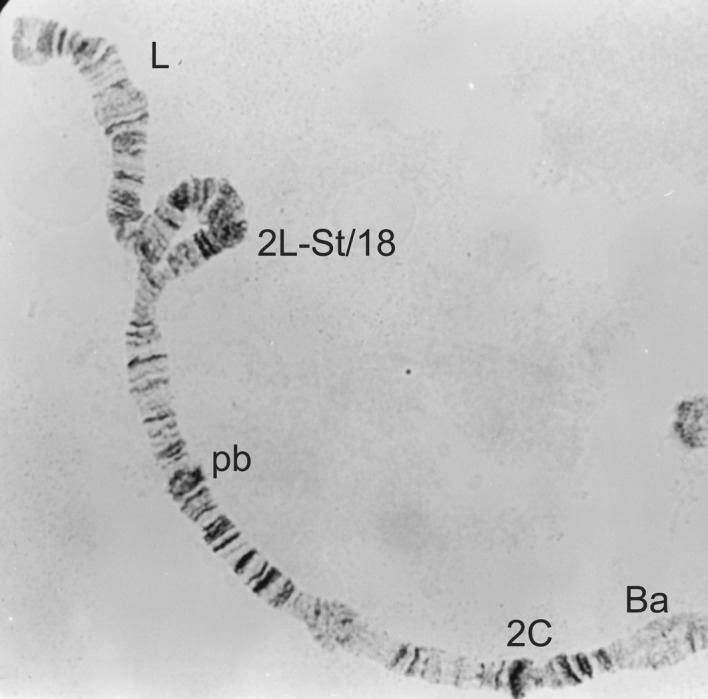
Figure 3.
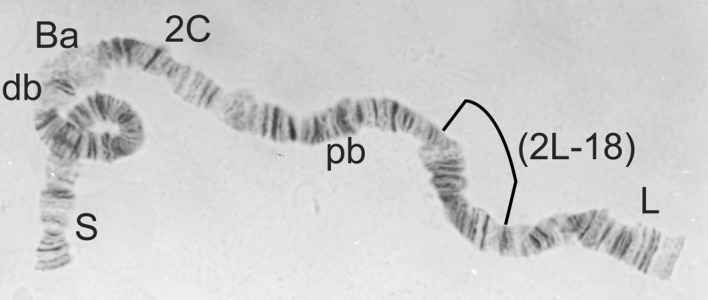
2L-St/St from Simulium squamosum, showing the relative position of inversion 2L-18.
Simulium squamosum is not known from Zones 1 (Borgu-Sokoto, Guinea savanna) or 3 (north–eastern, Sudan savanna) but has been reported to be widely spread in all the other zones (mostly Guinea savanna, savanna/forest mosaic, forest and/or montane; Mafuyai et al., 1996a; Bassey, 1998; Traore–Lamizana et al., 2001; Ibeh et al., 2006). It is the only cytospecies to have been confirmed as a vector of O. volvulus in Nigeria (Mafuyai et al., 1997), although further studies would be expected to incriminate additional cytospecies. Simulium squamosum C seems to occur throughout Nigeria but is joined by S. squamosum A and S. squamosum B in the south–east (Traore–Lamizana et al., 2001; Ibeh et al., 2006). Simulium squamosum D (Mustapha et al., 2004a) has not been recorded from Nigeria.
Simulium yahense has been reliably recorded from only eight sites in Nigeria, and these are all situated in the south–east (Akoh et al., 1987; Mafuyai et al., 1996; Bassey, 1998; Ibeh et al., 2006), in an onchocerciasis endemic area where Crosskey’s Zone 4 is now known to spread eastwards to join his scattered Zone-6 foci. Five of the eight sites are associated with the Udi Plateau (where it is forest or forest/savanna mosaic), one is on the Ibii River in the coastal lowland forest, and the other two are associated with the chain of mountains along the border with Cameroon at Agbokim Falls (on a tributary of Cross River) and the Obudu Plateau. The Bioko form of S. yahense (Post et al., 2003) has not been recorded from Nigeria and is probably extinct (Traoré et al., 2009).
It is probable that all records of S. soubrense and S. sanctipauli in Nigeria refer to the Beffa form of S. soubrense (see above). Cytological identifications of S. soubrense Beffa form were reported by Meredith et al. (1983) from the Okpara River on the border with Benin, in an area of Guinea savanna, where REMO has filled in the gap between Crosskey’s Zones 1 (Borgu-Sokoto) and 5. Simulium soubrense Beffa form is also known to occur in Zone 5 (south–western rainforest; Post, 1986). It has also been identified cytotaxonomically by Post (1986) and Akoh et al. (1987) (as S. sanctipauli) around the Jos Plateau in Guinea savanna, in Crosskey’s Zone 2. Cheke et al. (1987) believed that Meredith et al. (1983) had recorded the Beffa form of S. soubrense from the Gurara River, near Abuja (also in Zone 2). This identification was morphological and, whilst it needs to be confirmed cytologically, is consistent with the other records from the area.
Simulium damnosum s.s. and S. sirbanum have been frequently recorded in Nigeria but without any attempt to distinguish the cytotypes within the two cytospecies (i.e. the Volta form and Nile form of S. damnosum s.s., and the Sirba form and Sudanense form of S. sirbanum). Published reports for Nigeria show that S. sirbanum s.l. has a more northerly distribution than S. damnosum s.s. (Mafuyai et al., 1996a; Bassey, 1998; Ibeh et al., 2006; Onyenwe et al., 2007). Of these two cytospecies, only S. damnosum s.s. has been recorded from the onchocerciasis focus in the rainforest of south–western Nigeria (Crosskey’s Zone 5) (although S. sirbanum has been reported from the Okpara River where it forms the border with Benin), and S. damnosum s.s. is also common in south–eastern Nigeria (Crosskey’s Zones 6 and 4), where S. sirbanum has only been recorded from five sites (including forest sites). Both species have been recorded from Crosskey’s Zones 1 and 2, which are both situated in the Guinea savanna, but only S. sirbanum is known from the north–eastern onchocerciasis zone (Crosskey’s Zone 3), which is situated in the Sudan savanna.
Vajime (1989) split S. sirbanum into two cytotypes (the Sirba form and Sudanense form) from analysis of material from eight countries, including one site in Nigeria (near Zaria, on the edge of Crosskey’s Zone 2) where he found both cytotypes. Onyenwe et al. (2007) identified the Sudanense form of S. sirbanum breeding at a Guinea-savanna site in south–eastern Nigeria, in an onchocerciasis focus in the Scarplands (Ubachukwu, 2004). Vajime and Dunbar (1975) considered that S. sudanense [sensu Vajime and Dunbar (1975)] was mostly sympatric with S. sirbanum [sensu Vajime and Dunbar (1975)], and analysis of the proportions represented by the Sirba and Sudanense forms, using data from Vajime (1989) and Onyenwe et al. (2007), showed no correlation with latitude (Spearman’s rs = 0·0838; P>0·25), and hence no evidence for a north–south separation (unpubl. obs.).
There is very little information available concerning the distribution of the Nile and Volta forms of S. damnosum s.s.. Dunbar and Vajime (1981) did not present any collection data but simply stated that the Nile form occurred in the rivers draining the Jos Plateau and in the rivers in eastern Nigeria, whereas the Volta form occurred elsewhere in Nigeria. When, however, Vajime and Gregory (1990) reassessed the situation, they found that both forms could be found across the full width of the savanna belt, from Guinea in the west to Uganda in the east. Both forms were common in the Guinea savanna, but the Nile form tended to extend its range into the Sudan savanna and the Volta form into the forest. Vajime and Gregory (1990) presented collection data from 12 sites in Nigeria, which showed the Volta form in sites in the forest onchocerciasis zones of south–eastern and south–western Nigeria and in the Borgu-Sokoto onchocerciasis zone (Guinea savanna). The Nile form was generally much more common and also found in the forest onchocerciasis zones of south–eastern and south–western Nigeria and in the Borgu-Sokoto onchocerciasis zone, as well as in the southern Adamawa onchocerciasis zone (Crosskey’s Zone 4) and in the rivers draining the Jos Plateau (Crosskey’s Zone 2).
THE PHYSICO–CHEMICAL CHARACTERISTICS OF THE BREEDING SITES
The physico–chemical characteristics of rivers are known to be correlated with the distribution of different species of blackflies (Crosskey, 1990). Simulium damnosum s.l. is traditionally considered to be a species of fast-flowing, broken ‘white-water’ in rapids of rivers, but also medium-sized streams, especially in upland areas (Crosskey, 1990). Grunewald (1976, 1981) noted that members of the S. damnosum complex in West Africa have not been found in rivers with water velocities of <0·4 or >2·4 m/s, nor from rivers with water temperatures lower than 16·8°C or greater than 33°C (most being found in water at 18·3–31·8°C in West Africa; Ocran et al., 1982). Members of the complex have also not been found breeding in rivers with high levels of ammonia (>0·5 mg NH4-N/litre), a sign of organic pollution, although some other Simulium species, such as S. hargreavesi and S. adersi, are found in such rivers (Grunewald, 1976, 1981). Simulium damnosum s.l. only breeds in water with (a wide range of) relatively high oxygen saturations (75%–>105%), whereas other species, such as S. alcocki and S. unicornutum, have been recorded in Nigerian rivers with only 21·5% oxygen saturation (Grunewald, 1976, 1981). The physico–chemical characteristics of Nigerian rivers occupied by members of the S. damnosum complex have been published by Mafuyai et al. (1996), Bassey (1998), Nwoke and Uwazie (1991), Opara and Fagbemi (2005), Ikpeama et al. (2006), Ibeh et al. (2007) and Onyenwe et al. (2007) [although Nwoke and Uwazie (1991), Opara and Fagbemi (2005) and Ikpeama et al. (2006) did not identify the cytospecies involved]. In Nigeria, members of the complex have been mostly found breeding in water with velocities of 0·55–2·2 m/s, but Ibeh et al. (2007) reported a mean maximum of 2·8 m/s, and Opara and Fagbemi (2005) reported a range of 0·1–5·6 m/s. The latter observation is remarkable because it not only extends both the upper and lower limits for the velocities of water supporting breeding S. damnosum s.l., but also the upper limit for Simuliidae as a whole, which was previously recorded at 0·05–3·5 m/s (Crosskey, 1990). It is important that these exceptional findings are confirmed. Nigerian breeding sites have been found to have water temperatures of 18–33°C and pH values of 5·6–8·7, which are within the limits recorded elsewhere for S. damnosum s.l. (Grunewald, 1976, 1981). Opara and Fagbemi (2005) recorded S. damnosum s.l. breeding in rivers with unusually high levels of ammonia in Nigeria (but always <1 mg/litre, except on one occasion when an unexplained measurement of approximately 6 mg/litre was recorded).
With respect to the different sibling species within the S. damnosum complex, Quillévéré et al. (1977) concluded that the only physico–chemical factor that separated the different cytospecies throughout the whole year was pH. Although Grunewald (1976, 1981) considered that conductivity was also important, dividing the West-African members of the complex into Group I [S. yahense, S. sanctipauli and S. mengense, all supposedly found in acidic waters (pH 5·7–6·2) with low conductivity (<50 μS/m)] and Group II [S. squamosum, S. soubrense, S. damnosum s.s. and S. sirbanum, all supposedly found in nearly pH-neutral rivers with higher conductivity (50–150 μS/m)], the results from Nigeria do not support this separation. Mafuyai et al. (1996), Bassey (1998) and Opara and Fagbemi (2005), for example, recorded low conductivity (0·5–20 μS/m) at all breeding sites used by members of the S. damnosum complex in Nigeria, including those used by sibling species in Grunewald’s Group II. Onyenwe et al. (2007) also recorded S. sirbanum (Sudanense form) breeding throughout the entire year in fairly acidic water (pH 5·6–5·9), although Mafuyai et al. (1996) and Bassey (1998) recorded higher pH values (range 6·5–7·9) at all the breeding sites for the S. damnosum complex that they investigated in Nigeria. Ibeh et al. (2007) found S. yahense in neutral to alkaline rivers (pH 6·5–8·6) and S. squamosum in water showing an even wider range of pH values (6·4–8·7).
The differences seen in the physico–chemical characteristics of breeding sites used by a particular cytospecies, between the different Nigerian studies and between Nigeria and other parts of West Africa, could be related to cytoform differences. Onyenwe et al. (2007), for example, were dealing with the Sudanense form of S. sirbanum, and it is not known whether other related studies involved the Sirba form or Type IV. Similarly, it is not clear whether studies of S. damnosum s.s. in Nigeria refer to the Nile form, which is probably relatively uncommon to the west of the country (Boakye, 1993). Simulium squamosum in Nigeria is represented by S. squamosum A, B and C, whereas further west only S. squamosum C and E are found.
CYTOTAXONOMIC KEY FOR THE IDENTIFICATION OF MEMBERS OF THE S. damnosum COMPLEX IN NIGERIA AND THE SURROUNDING COUNTRIES, USING POLYTENE CHROMOSOMES FROM THE LARVAE
The key is based upon preparation and examination of polytene chromosomes from the larval silk glands (see above), and scoring the banding pattern of the three pairs of chromosomes (Fig. 1). Many of the diagnostic criteria in this key refer to the characteristics of populations rather than individual specimens. For example, the determination of sex linkage of inversion 2L-18 in couplet 3 requires the cytotaxonomic analysis of sufficient numbers of larvae from the population being studied. Taxa known to occur in Nigeria are indicated in bold type.
Figure 1.
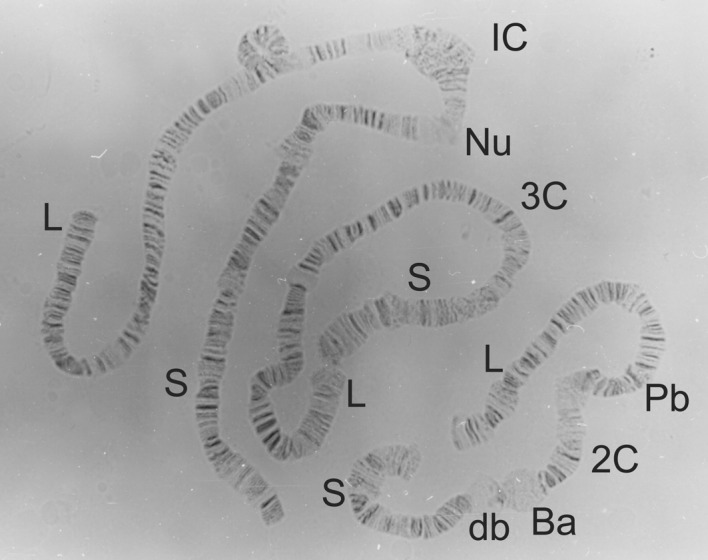
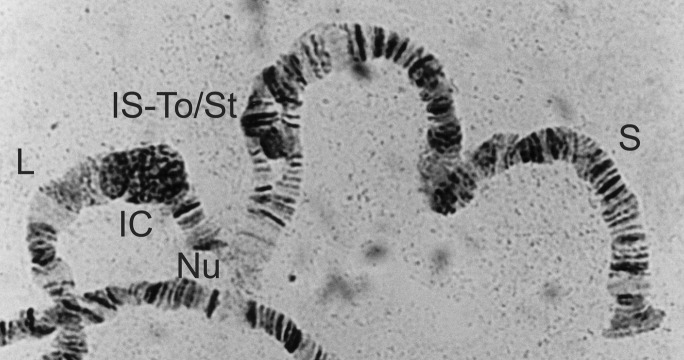
Full karyotype of Simulium squamosum, showing the standard sequence (St/St) in all chromosome arms and normal centromeres. Chromosomes 1, 2 and 3 are labelled at the centromere (as 1C, 2C and 3C, respectively) and the short and long arms are labelled S and L, respectively. Other chromosomal landmarks include the nucleolus organizing region (Nu), the Ring of Balbiani (Ba), the double bubble (db) and the parabalbiani (Pb).
| 1. | |
| 1C expanded (Fig. 2) | S. mengense |
| 1C normal (Figures 1 and 14) | Other cytospecies (2) |
| 2. | |
| 2L-St/St, St/18 or 18/18 (Figures 3, 4 and 5) | S. squamosum subcomplex (3) |
| 2L-C/C, C/C.8 or C.8/C.8 (Figures 6, 7 and 8) | S. damnosum subcomplex (7) |
| 2L-4·6/4·6 or 4·6.A/4·6.A (Figures 9 and 10) | S. sanctipauli subcomplex (10) |
| 3. | |
| 2L-18 sex linked (Figures 3, 4 and 5) | S. yahense (4) |
| 2L-18 absent or not sex-linked | S. squamosum (5) |
| 4. | |
| Males 2L-St/18; females 2L-18/18 (Figures 4 and 5) | S. yahense typical form |
| Males 2L-St/18; females 2L-St/St (Figures 3 and 4) | S. yahense Bioko form |
| 5. | |
| Males with 1C long split (ICa) (Fig. 11) | S. squamosum ‘A’ |
| Males with 1C short split (ICb) (Fig. 12) | S. squamosum ‘B’ |
| Males without 1Ca or ICb | Other S. squamosum cytotypes (6) |
| 6. | |
| Males 1S-To/St (Fig. 13) | S. squamosum ‘D’ |
| Males without 1S-To (Fig. 14) | S. squamosum ‘C’ |
| 7. | |
| 2L-C/C or 2L-C/C.8 (Figures 6 and 7) | S. damnosum s.s. (8) |
| 2L-C.8/C.8 (Fig 8) | S. sirbanum (9) |
| 8. | |
| Males 2L-C/C.8; females 2L-C/C (Figures 6 and 7) | S. damnosum s.s. Volta form |
| Males 2L-C/C; females 2L-C/C (Fig. 6) | S. damnosum s.s. Nile form |
| 9. | |
| Males 1S-St/3 or St/St; females 1S-St/St (Figures 14 and 15) | S. sirbanum Sirba form |
| Males 1S-St/3; females 1S-3/3 (Figures 15 and 16) | S. sirbanum Sudanense form |
| Males 1S-3/3; females 1S-3/3 (Fig. 16) | S. sirbanum Type IV |
| 10. | |
| 2L-4·6/4·6 (Fig. 9) | S. soubrense (11) |
| 2L-4·6.A/4·6.A (Fig. 10) | S. sanctipauli |
| 11. | |
| Males 2S-St/St (Fig. 17) | S. soubrense typical form |
| Males 2S-St/6b (Fig. 18) | S. soubrense Beffa form |
Figure 2.
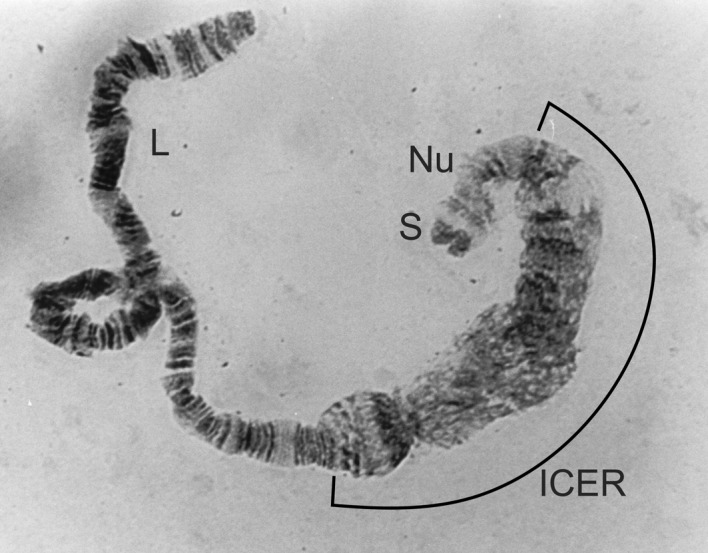
1C expanded centromere (1CER) from Simulium mengense.
Figure 4.
2L-St/18 heterozygote from a male Simulium yahense.
Figure 5.
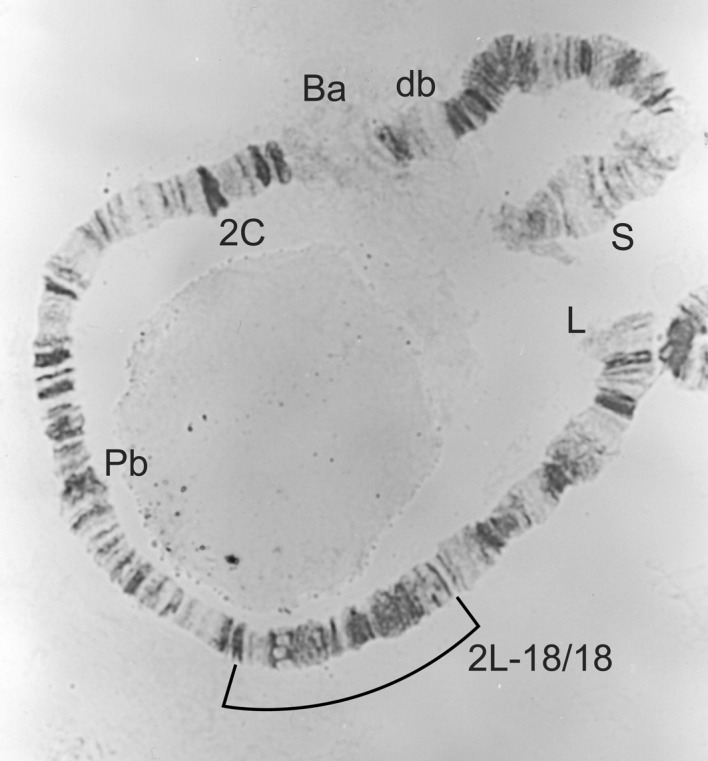
2L-18/18 homozygote from a female Simulium yahense.
Figure 6.
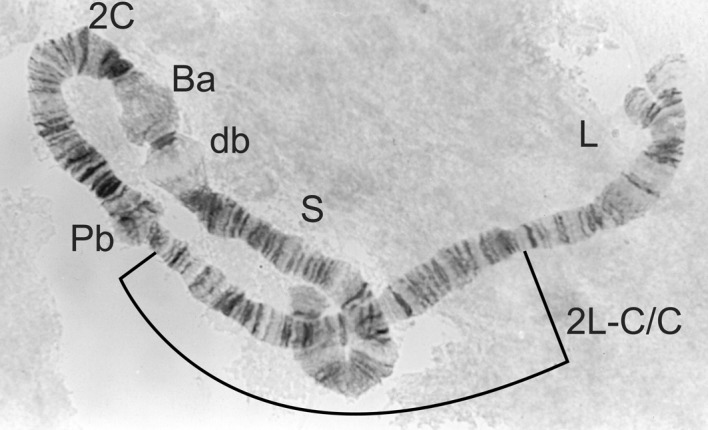
2L-C/C homozygote from Simulium damnosum s.s.
Figure 7.
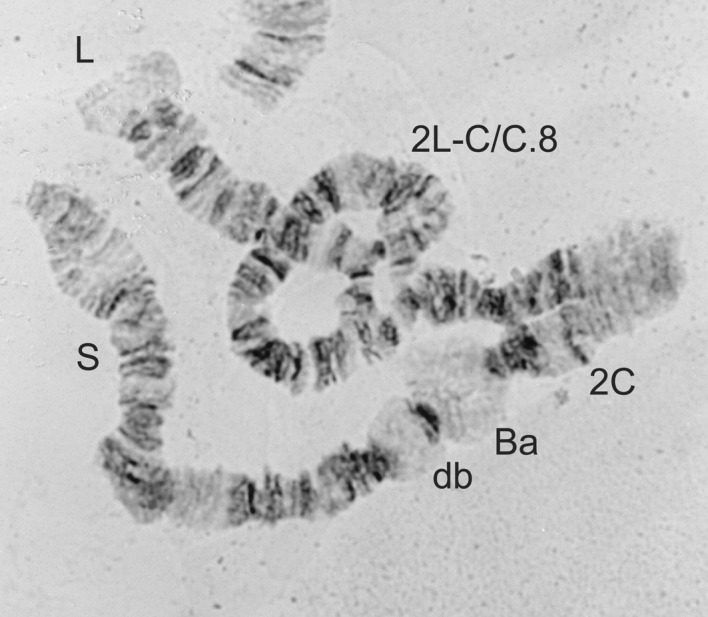
2L-C/C.8 heterozygote from a male Simulium damnosum s.s.
Figure 8.
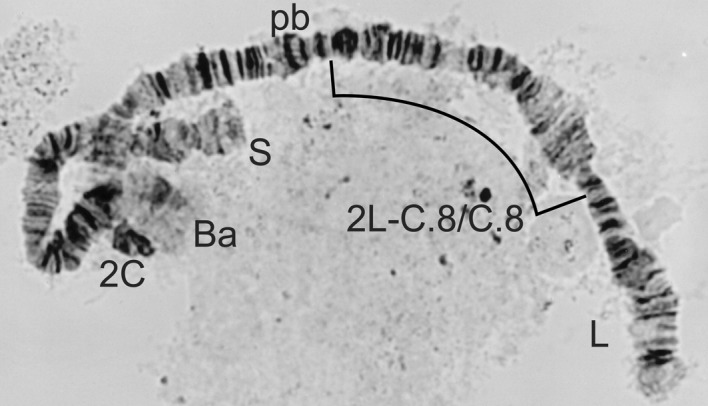
2L-C.8/C.8 homozygote from Simulium sirbanum.
Figure 9.
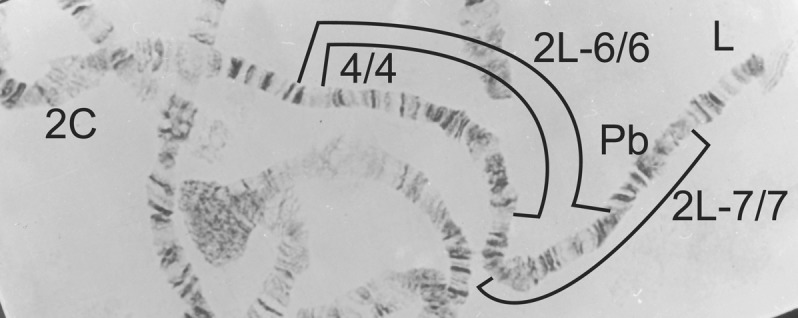
2L-4·6/4·6 from Simulium soubrense (also homozygous for 2L-7/7).
Figure 10.
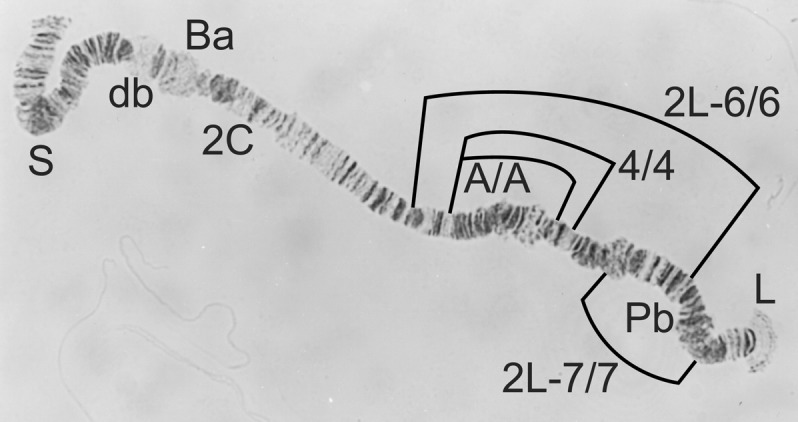
2L-4·6.A/4·6.A from Simulium sanctipauli (also homozygous for 2L-7/7).
Figure 13.
1S-St/To from a male Simulium squamosum ‘D’.
Figure 14.
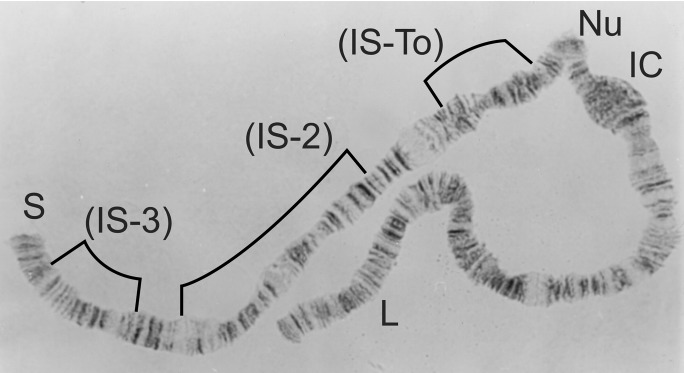
1S-St/St and 1C normal from a male Simulium squamosum ‘C’ (also showing the relative position of inversions 1S-To, 1S-2 and 1S-3).
Figure 15.
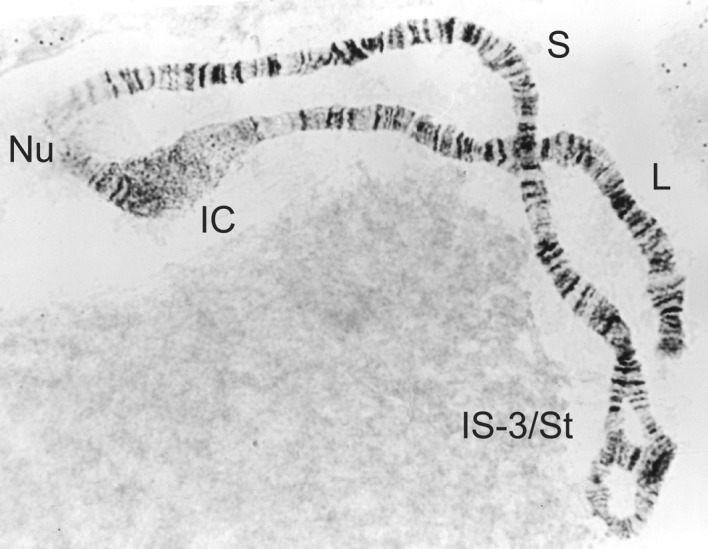
1S-St/3 heterozygote from a male Simulium sirbanum.
Figure 16.
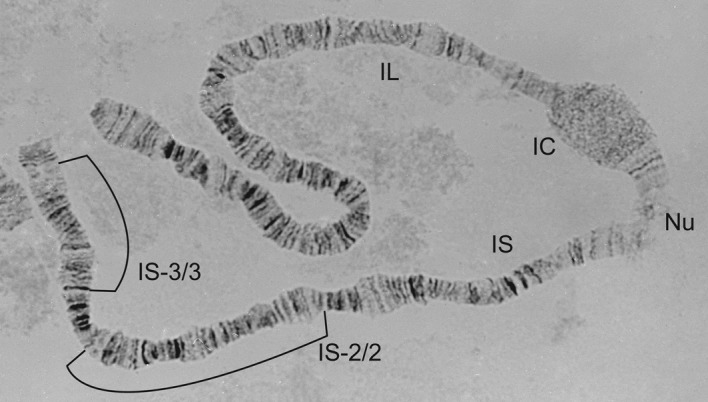
1S-3/3 homozygote from a female Simulium sirbanum (also homozygous for 1S-2/2).
Figure 17.
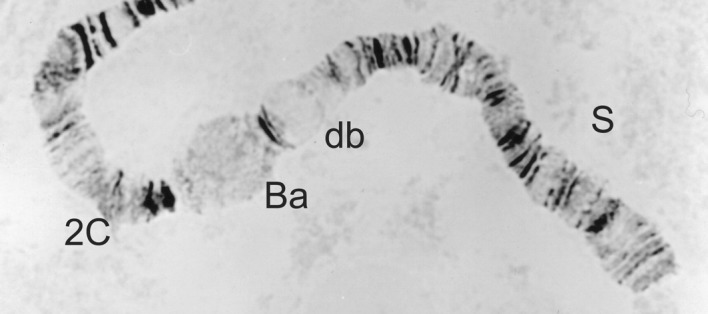
2S-St/St homozygote, as seen in a female Simulium soubrense of the Beffa form.
Figure 18.
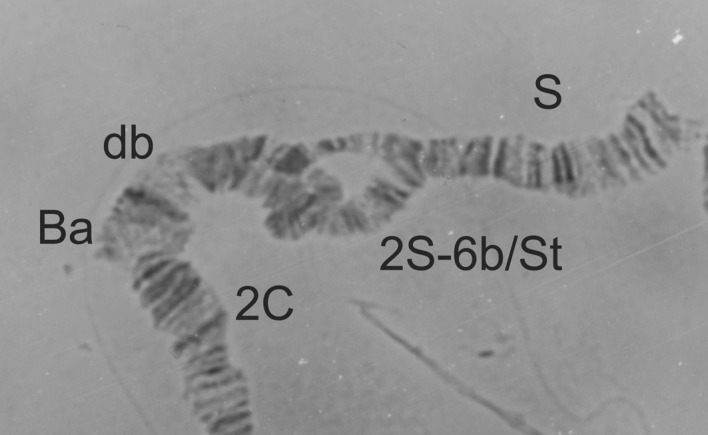
2S-St/6b heterozygote from a male Simulium soubrense of the Beffa form.
Acknowledgments
This work was partly funded by the British Medical Research Council (via project grant 77615) and partly by TDR — the United Nations Children’s Fund/United Nations Development Programme/World Bank/World Health Organization’s Special Programme for Research and Training in Tropical Diseases — via a research institute strengthening grant (ID900314), a research training grant (ID880037), a re-entry grant (ID930332) and a research project grant (ID950612). The authors thank Dr M. Traore-Lamizana, for Figures 11 and 12, and Z. Adams, for her technical and statistical assistance.
Figure 11.
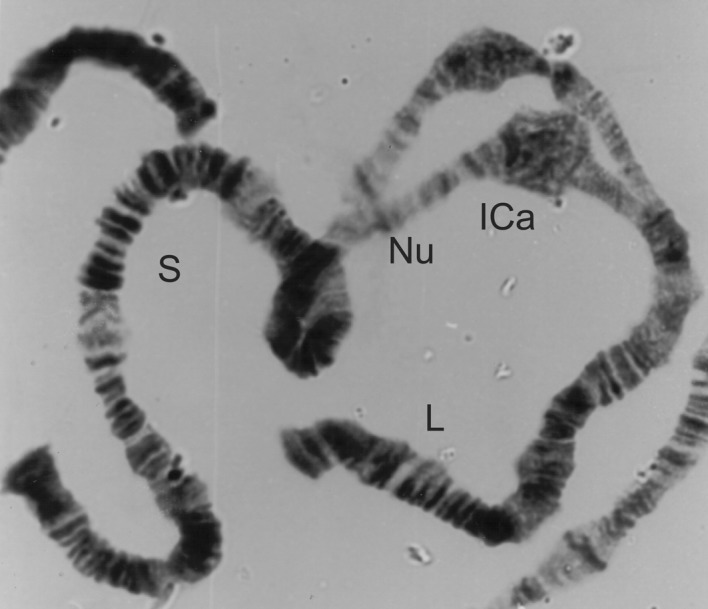
1C long split from a male Simulium squamosum ‘A’.
Figure 12.
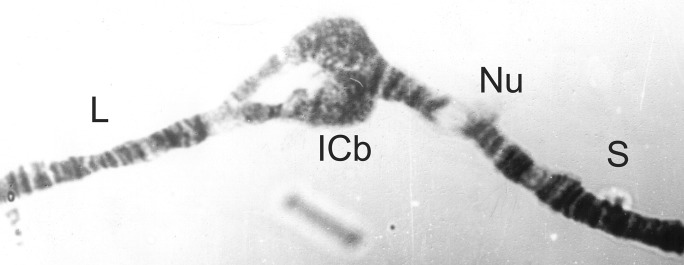
1C short split from a male Simulium squamosum ‘B’.
REFERENCES
- Adewale B, Mafe MA.&Oyerinde JPO.(1999)Infectivity and transmission dynamics of Simulium damnosum s.l. around Owena Dam (Ondo state). West African Journal of Medicine 18257–260. [PubMed] [Google Scholar]
- Adler PH.&Crosskey RW.(2010)World Blackflies (Diptera: Simuliidae): a Comprehensive Revision of the Taxonomic and Geographical Inventory [2010] Clemson, SC: Department of Entomology, Soils and Plant Sciences, Clemson University [Google Scholar]
- Akoh JI, Tada I, Uchida A, Sato Y.&Hirai H.(1987)Cytotaxonomic appraisal of the black flies, Simulium damnosum s.l. Theobald (Diptera: Simuliidae) from different ecological zones of Nigeria Proceeding of Nigeria/Japan Joint Conference on Trace Metals, Diarrhoea, Goitre and Medical Entomologypp.195–197.Jos, Nigeria: University of Jos [Google Scholar]
- Baker RHA, Guillet P, Sékétéli A, Poudiougo P, Boakye D, Wilson MD.&Bissan Y.(1990)Progress in controlling the reinvasion of windborne vectors into the western area of the Onchocerciasis Control Programme in West Africa. Philosophical Transactions of the Royal Society of London, Series B 328731–750. [DOI] [PubMed] [Google Scholar]
- Basáñez M.-G, Pion SDS, Churcher TS, Breitling LP, Little MP.&Boussinesq M.(2006)River blindness: a success story under threat? PLoS Medicine 31454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassey SAE.(1998)Cytological studies and the distribution of Simulium damnosum complex in Nigeria, Cameroon and Equatorial Guinea. PhD thesisAhmadu Bello University; Zaria, Nigeria [Google Scholar]
- Bedo DG.(1977)Cytogenetics and evolution of Simulium ornatipes Skuse (Diptera: Simuliidae). I. Sibling speciation. Chromosoma 6437–65. [Google Scholar]
- Boakye D.(1988)Sexing simuliid larvae during cytological examinations. Transactions of the Royal Society of Tropical Medicine and Hygiene 82144. [DOI] [PubMed] [Google Scholar]
- Boakye D.(1993)A pictorial guide to the chromosomal identification of members of the Simulium damnosum Theobald complex in West Africa with particular reference to onchocerciasis control programme Area. Tropical Medicine and Parasitology 44223–244. [PubMed] [Google Scholar]
- Boakye DA, Back C, Fiasorgbor GK, Sib APP.&Coulibaly Y.(1998)Sibling species distributions of the Simulium damnosum complex in the West African Onchocerciasis Control Programme area during the decade 1984–93, following intensive larviciding since 1974. Medical and Veterinary Entomology 12345–358. [DOI] [PubMed] [Google Scholar]
- Borsboom GJJM, Boatin BA, Nico JD, Nagelkerke NJD, Agoua H, Akpoboua KLB, Soumbey Alley EW, Bissan Y, Renz A, Yameogo L, Remme JHF.&Habbema DF.(2003)Impact of ivermectin on onchocerciasis transmission: assessing the empirical evidence that repeated ivermectin mass treatments may lead to elimination/eradication in West Africa. Filaria Journal 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheke RA.&Garms R.(1983)Reinfestations of the southeastern flank of the Onchocerciasis Control Programme area by windborne vectors. Philosophical Transactions of the Royal Society of London, Series B 302471–484. [Google Scholar]
- Cheke RA, Garms R, Ouedraogo J, Somé A.&Sowah S(1987)The Beffa form of Simulium soubrense of the S. damnosum complex in Togo and Benin. Medical and Veterinary Entomology 129–35. [DOI] [PubMed] [Google Scholar]
- Crosskey RW.(1981)A review of Simulium damnosum s.l. and human onchocerciasis in Nigeria, with special reference to cytographical distribution and development of a Nigerian national control campaign. Tropenmedizin und Parasitologie 322–16. [PubMed] [Google Scholar]
- Crosskey RW.(1990)The Natural History of Blackflies. Colchester, U.K: John Wiley & Sons [Google Scholar]
- Dang PT.&Petertson BV.(1980)Pictorial keys to the main species groups within the Simulium damnosum Theobald complex occurring in West Africa. Tropenmedizin und Parasitologie 31117–120. [PubMed] [Google Scholar]
- Dunbar RW.(1966)Four sibling species included in Simulium damnosum Theobald (Diptera: Simuliidae) from Uganda. Nature 209597–599. [Google Scholar]
- Dunbar RW.(1972)Polytene Chromosome Preparations from Tropical Simuliidae Document WHO/ONCHO/172·95Geneva: World Health Organization [Google Scholar]
- Dunbar RW.&Vajime CG.(1981)Cytotaxonomy of the Simulium damnosum complex Black flies: the Future for Biological Methods in Integrated Controled, Laird M.ed pp.31–43.London: Academic Press [Google Scholar]
- Fiasorgbor GK.&Cheke RA.(1992)Cytotaxonomic confirmation of two forms of Simulium sirbanum in the eastern part of the Onchocerciasis Control Programme in West Africa. Medical and Veterinary Entomology 6139–142. [DOI] [PubMed] [Google Scholar]
- Fischer P.&Büttner DW.(2002)The epidemiology of onchocerciasis and the long term impact of existing control strategies on this infection The Filariaeds Klei T R.ed & Rajan T V.ed pp.43–57.London: Kluwer Academic [Google Scholar]
- Garms R.&Cheke RA.(1985)Infections with Onchocerca volvulus in different members of the Simulium damnosum complex in Togo and Benin. Zeitschrift für Angewandte Zoologie 72479–495. [Google Scholar]
- Garms R, Walsh JF.&Davies JB.(1979)Studies on the re-invasions of the onchocerciasis control programme in the Volta River basin by Simulium damnosum s.l. with emphasis on the south–western areas. Tropenmedizin und Parasitologie 30345–362. [PubMed] [Google Scholar]
- Garms R, Cheke RA, Vajime CG.&Sowah S.(1982)The occurrence and movements of different members of the Simulium damnosum complex in Togo and Benin. Zeitschrift für Angewandte Zoologie 69219–236. [Google Scholar]
- Gemade EII, Jiya JY, Nwoke BEB, Ogunba EO, Edeghere H, Akoh JI.&Omojola A.(1998)Human onchocerciasis: current assessment of disease burden in Nigeria by rapid epidemiological mapping. Annals of Tropical Medicine and Parasitology 92(Suppl. 1)S79–S83. [DOI] [PubMed] [Google Scholar]
- Gregory WG.(1982)A brief review of the control of vectors of onchocerciasis in Nigeria. Proceedings of the First National Congress on Onchocerciasis, 1–4 September 1982pp.15–19.Zaria, Nigeria: Ahmadu Bello University [Google Scholar]
- Grunewald J.(1976)The hydro–chemical and physical conditions of the environment of the immature stages of some species of the Simulium (Edwardsellum) damnosum complex (Diptera). Tropenmedizin und Parasitologie 27438–454. [PubMed] [Google Scholar]
- Grunewald J.(1981)Hydro–chemical and physical characteristics of the larval sites of species of the Simulium damnosum complex Blackflies: the Future for Biological Methods of Integrated Controled.Laird M.ed pp.227–235.London: Academic Press [Google Scholar]
- Hougard J.-M, Agoua H, Yaméogo L, Akpoboua KLB, Sékétéli A.&Dadzie KY.(1998)Blackfly control: what choices after onchocerciasis? World Health Forum 19281–284. [PubMed] [Google Scholar]
- Ibeh OO, Nwoke BEB, Adegoke JA.&Mafuyai HB.(2006)Cytospecies identifications of vectors of human onchocerciasis in south eastern Nigeria. African Journal of Biotechnology 51813–1818. [Google Scholar]
- Ibeh OO, Nwoke BEB.&Adegoke JA.(2007)Distribution and ecology of breeding sites of Simulium damnosum s.l. in south–eastern primary health zone of Nigeria. Nigerian Journal of Parasitology 2832–38. [Google Scholar]
- Ibeh OO, Nwoke BEB.&Adegoke JA.(2008)Morphological differentiation of vectors of onchocerciasis, Simulium damnosum complex, in south–east Nigeria. Nigerian Journal of Parasitology 2961–66. [Google Scholar]
- Idowu ET, Adewale B, Mafe MA, Appelt B.&Bamgbose A.(2004)Endemicity of onchocerciasis in some local government areas of Niger state. African Journal of Medical Sciences 3331–34. [PubMed] [Google Scholar]
- Idowu ET, Awolola TS, Mafe MA.&Otubanjo OA.(2008)Identification of three members of the Simulium damnosum (Diptera: Simuliidae) group in south western Nigeria. African Journal of Medicine and Medical Sciences 3771–76. [PubMed] [Google Scholar]
- Ikpeama CA, Nwoke BEB.&Anosike JC.(2006)Studies on the ecology and distribution of blackflies (Diptera: Simuliidae) in Imo state, Nigeria. International Journal of Natural and Applied Sciences 2412–416. [Google Scholar]
- Iloeje NP.(1981)A New Geography of Nigeria Ikeja, Nigeria: Longman Nigeria [Google Scholar]
- Jiya JJ.(1998)Problems and perspective in programme management: the case of the National Onchocerciasis Control Programme in Nigeria. Annals of Tropical Medicine and Parasitology 92(Suppl. 1)S167–S168. [DOI] [PubMed] [Google Scholar]
- Krüger A.(2003)An alternative protocol for the preparation of the polytene chromosomes of larval Simulium damnosum s.l. (Diptera: Simuliidae). Annals of Tropical Medicine and Parasitology 97657–660. [DOI] [PubMed] [Google Scholar]
- Krueger A.(2006)Guide to blackflies of the Simulium damnosum complex in eastern and southern Africa. Medical and Veterinary Entomology 2060–75. [DOI] [PubMed] [Google Scholar]
- Le Berre R.&Fiasorgbor GK.(1985)Importance de l’identification cytotaxonomique des espèces du complexe Simulium damnosum dans la lutte contre l’onchocercose. Parassitologia 27179–185. [PubMed] [Google Scholar]
- Mafuyai HB.(1992)Studies on the taxonomy and distribution of the Simulium damnosum complex in Nigeria, in relation to human onchocerciasis PhD thesisUniversity of Salford; Salford, U.K [Google Scholar]
- Mafuyai HB, Post RJ, Vajime CG.&Molyneux DH.(1996a)Cytotaxonomic identifications of the Simulium dimnosum complex (Diptera: Simuliidae) from Nigeria. Tropical Medicine and International Health 1779–785. [DOI] [PubMed] [Google Scholar]
- Mafuyai HB, Wilson MD.&Post RJ.(1996b)Morphological differentiation of adult females of the Simulium damnosum complex from Nigeria. Medical and Veterinary Entomology 10190–192. [DOI] [PubMed] [Google Scholar]
- Mafuyai HB, Post RJ, Molyneux DH.&Davies DH.(1997)First sibling species identifications of Nigerian onchocerciasis vectors. Transactions of the Royal Society of Tropical Medicine and Hygiene 9190–91. [DOI] [PubMed] [Google Scholar]
- Mank R, Wilson MD, Rubio JM.&Post RJ.(2004)A molecular marker for the identification of Simulium squamosum (Diptera: Simuliidae). Annals of Tropical Medicine and Parasitology 98197–208. [DOI] [PubMed] [Google Scholar]
- Meredith SEO, Cheke RA.&Garms R.(1983)Variation and distribution of forms of Simulium soubrense and S. sanctipauli in West Africa. Annals of Tropical Medicine and Parasitology 77627–640. [DOI] [PubMed] [Google Scholar]
- Millest AL, Cheke RA, Howe MA, Lehane MJ.&Garms R.(1992)Determining the ages of adult females of the Simulium damnosum complex (Diptera: Simuliidae) by the pteridine accumulation method. Bulletin of Entomological Research 82219–226. [Google Scholar]
- Mustapha M, Post RJ, Enyong P.&Lines J.(2004a)A new cytotype of Simulium squamosum (Diptera: Simuliidae) from southwest Cameroon. Medical and Veterinary Entomology 18296–300. [DOI] [PubMed] [Google Scholar]
- Mustapha M, Post RJ.&Krüger A.(2004b)The cytotaxonomy and morphotaxonomy of Simulium mengense (Diptera: Simuliidae). Annals of Tropical Medicine and Parasitology 98509–523. [DOI] [PubMed] [Google Scholar]
- Narita AS.&Taylor HR.(1993)Blindness in the tropics. Medical Journal of Australia 159416–420. [DOI] [PubMed] [Google Scholar]
- Noma M, Nwoke BEB, Nutall I, Tambala PA, Enyong P, Namsenmo A, Remme J, Amazigo UV, Kale OO.&Sékétéli A.(2002)Rapid epidemiological mapping of onchocerciasis (REMO): its application by the African Programme for Onchocerciasis Control (APOC). Annals of Tropical Medicine and Parasitology 96(Suppl. 1)S29–S39. [DOI] [PubMed] [Google Scholar]
- Nwoke BEB.&Dozie INS.(2001)Operational research and its success in onchocerciasis control in Nigeria. Nigerian Journal of Parasitology 223–10. [Google Scholar]
- Nwoke BEB.&Uwazie OU.(1991)Studies on the blackflies Simulium (Diptera: Simuliidae) of Imo state. The distribution of immature stages in Isuikwato-Okigwe area. Nigerian Journal of Parasitology 1229–37. [Google Scholar]
- Ocran MH, Davies JB.&Agoua H.(1982)Water Temperatures in S. damnosum Breeding Rivers of the Onchocerciasis Control Programme Area Document WHO/VBC/82·848Geneva: World Health Organization [Google Scholar]
- Okonkwo P, Akpa A, Ihekwaba A, Nwagbo D, Umeh R, Adibua S, Ezike V.&Ogbuokiri J.(1991)Studies on onchocerciasis in forest–savannah mosaic areas of Nigeria. I. Investigations in Gbaragu, Oji River. Annals of Tropical Medicine and Parasitology 85617–623. [DOI] [PubMed] [Google Scholar]
- Onyenwe E, Ubachukwu PO.&Post RJ.(2007)Simulium sirbanum at a site in SE Nigeria. British Simuliid Group Bulletin 2817–21. [Google Scholar]
- Opara KN.&Fagbemi OB.(2005)Physico–chemical indices of breeding sites of Simulium damnosum in the lower Cross River basin, Nigeria. Journal of Environmental Sciences 17511–517. [PubMed] [Google Scholar]
- Opara KN, Fagbemi OB, Ekwe A.&Okenu DMN.(2005)Status of forest onchocerciasis in the lower Cross river basin, Nigeria: entomologic profile after five years of ivermectin intervention. American Journal of Tropical Medicine and Hygiene 73371–376. [PubMed] [Google Scholar]
- Oyibo WA.&Fagbenro-Beyioko AF.(2003)Effect of repeated community-based ivermectin treatment on the intensity of onchocerciasis in Nigeria. Rural and Remote Health 3211. [PubMed] [Google Scholar]
- Post RJ.(1986)The cytotaxonomy of Simulium sanctipauli and Simulium soubrense (Diptera: Simuliidae). Genetica 69191–207. [Google Scholar]
- Post RJ, Flook PK, Millest AL, Cheke RA, McCall PJ, Wilson MD, Mustapha M, Somiari S, Davies JB, Mank RA, Geenen P, Enyong P, Sima A.&Mas J.(2003)Cytotaxonomy, morphology and molecular systematics of the Bioko form of Simulium yahense (Diptera: Simuliidae). Bulletin of Entomological Research 93145–157. [DOI] [PubMed] [Google Scholar]
- Post RJ, Mustapha M.&Krueger A.(2007)An inventory of the cytospecies and cytotypes of the Simulium damnosum complex (Diptera: Simuliidae). Tropical Medicine and International Health 121342–1353. [DOI] [PubMed] [Google Scholar]
- Quillévéré D.(1975)Étude du complexe Simulium damnosum en Afrique de l’Ouest. I. Techniques d’étude. Identification des cytotypes. Cahiers O.R.S.T.O.M., Série Entomologie Médicale et Parasitologie 1385–98. [Google Scholar]
- Quillévéré D, Gouzy M, Séchan Y.&Pendriez B.(1977)Étude du complexe Simulium damnosum en Afrique de l’Ouest. VI. Analyse de l’eau des gites larvaires en saison des pluies; comparison avec la saison sêche. Cahiers O.R.S.T.O.M., Série Entomologie Médicale et Parasitologie 15195–207. [Google Scholar]
- Roberts DM.(1985)Vertical distribution of flying black flies (Diptera: Simuliidae) in central Nigeria. Tropical Medicine and Parasitology 36102–104. [PubMed] [Google Scholar]
- Roberts DM.&Irving-Bell RJ.(1987)Nigerian blood-fed blackflies (Diptera. Simuliidae) caught in flight: relative activity and host preferences. Tropical Medicine and Parasitology 3823–26. [PubMed] [Google Scholar]
- Roberts DM.&Irving-Bell RJ.(1996)Effect of weather conditions on the flight activity of Nigerian blackflies (Diptera: Simuliidae). Medical and Veterinary Entomology 10137–144. [DOI] [PubMed] [Google Scholar]
- Traoré S, Wilson MD, Sima A, Barro T, Diallo A, Aké A, Coulibaly S, Cheke RA, Meyer R, Mas J, McCall PJ, Post RJ, Zouré H, Noma M, Yameogo L, Sékétéli AV.&Amazigo UV.(2009)The elimination of onchocerciasis vectors from the island of Bioko as a result of larviciding by the WHO African Programme for Onchocerciasis Control. Acta Tropica 111211–218. [DOI] [PubMed] [Google Scholar]
- Traore-Lamizana M, Somiari S, Mafuyai HB, Vajime CG.&Post RJ.(2001)Sex chromosome variation and cytotaxonomy of the onchocerciasis vector Simulium squamosum in Cameroon and Nigeria. Medical and Veterinary Entomology 15219–223. [DOI] [PubMed] [Google Scholar]
- Ubachukwu PO.(2004)Human onchocerciasis: epidemiological status of Uzo-Uwani local government area of Enugu state, Nigeria. Nigerian Journal of Parasitology 2593–99. [Google Scholar]
- Ubachukwu PO.&Anya AO.(2001)Studies on the diurnal biting activity pattern of Simulium damnosum complex (Dipteran: Simuliidae) in Uzo-Uwani local government area of Enugu state, Nigeria. Nigerian Journal of Parasitology 22163–168. [Google Scholar]
- Usip LPE, Udonsi JK, Ibanga ES.&Opara KN.(2003)A survey of breeding sites and variation of Simulium damnosum in Ini L.G.A. of Akwa Ibom, Nigeria. Nigerian Journal of Parasitology 24149–154. [Google Scholar]
- Vajime CG.(1986)Cytotaxonomy. II. Standard chromosomes of Simulium (Edwardsellum) damnosum Theobald (Diptera; Simuliidae). Nigerian Journal of Entomology 752–67. [Google Scholar]
- Vajime CG.(1989)Cytotaxonomy of Sirba form populations of the Simulium damnosum complex in West Africa: amendments to sex chromosomes and sibling status. Tropical Medicine and Parasitology 40464–467. [PubMed] [Google Scholar]
- Vajime CG.&Dunbar RW.(1975)Chromosomal identification of eight species of the subgenus Edwardsellum near and including Simulium (Edwardsellum) damnosum Theobald (Diptera: Simuliidae). Tropenmedizin und Parasitologie 26111–138. [PubMed] [Google Scholar]
- Vajime CG.&Gregory WG.(1990)Species complex of vectors and epidemiology. Acta Leidensia 59235–252. [PubMed] [Google Scholar]
- Vajime CG.&Quillévéré D.(1978)The distribution of the Simulium damnosum complex in West Africa with particular reference to the Onchocerciasis Control Programme area. Tropenmedizin und Parasitologie 29473–481. [PubMed] [Google Scholar]
- Walsh JF, Davies JB.&Garms R.(1981)Further studies on the reinvasion of the onchocerciasis control programme by Simulium damnosum s.l.: the effects of an extension of control activities into southern Ivory Coast during 1979. Tropenmedizin und Parasitologie 32269–273. [PubMed] [Google Scholar]
- Wilson MD, Post RJ.&Gomulski LM.(1993)Multivariate morphotaxonomy in the identification of adult female Simulium damnosum Theobald complex (Diptera: Simuliidae) in the Onchocerciasis Control Programme area of West Africa. Annals of Tropical Medicine and Parasitology 8765–82. [DOI] [PubMed] [Google Scholar]
- Wilson MD, Mafuyai HB.&Post RJ.(1994)Morphological identification of sibling species of the Simulium damnosum (Diptera: Simuliidae) complex from Nigeria, Cameroun and Bioko. Proceedings of the Section Experimental and Applied Entomology of the Netherlands Entomological Society 5181–185. [Google Scholar]
- World Health Organization(1995)Onchocerciasis and its Control. Report of a WHO Expert Committee on Onchocerciasis Technical Report Series No. 852Geneva: WHO; [PubMed] [Google Scholar]
- World Health Organization(2002)Success in Africa: the Onchocerciasis Control Programme in West Africa 1974–2002 Geneva: WHO [Google Scholar]
- World Health Organization(2004)Year 2004 Progress Report of WHO/APOC Ouagadougou: African Programme for Onchocerciasis Control [Google Scholar]
- Yaméogo L, Resh VH.&Molyneux DH.(2004)Control of river blindness in West Africa: case history of biodiversity in a disease control programme. EcoHealth 1172–183. [Google Scholar]


