Abstract
The protozoan parasite most frequently associated with diarrhoea worldwide is Giardia intestinalis. In 2005, a study was initiated to identify the genotypes of this parasite infecting children in the Argentinian provinces of Buenos Aires, Mendoza and Chaco, and to explore the associations between the genotype detected in a child, the characteristics of the child’s household and the child’s clinical presentation. Overall, 998 children (504 boys and 494 girls) aged between 2–14 years, with or without symptoms, were enrolled. The G. intestinalis in 94 of the 117 stool samples found positive for the parasite by microscopy were successfully genotyped by PCR. Seventy-seven of the children were found to be infected with genotype B only and 14 with genotype AII only, three children being found to have mixed (AII and B) infections.
Only genotype B was detected in children from rural areas (P<0·05) and most Giardia detected in children from households with a piped water supply were also of this genotype (P<0·05). The other household characteristics investigated (quality of building, history of flooding, type of sanitation, level of overcrowding, and presence/absence of pet dogs) had no significant effect on the genotype distribution. Children infected with genotype AII were significantly younger than those infected with genotype B (P<0·05) and there was a significant positive association between infection with genotype B and abdominal pain (P<0·05). Diarrhoea was not, however, found to be significantly associated with genotype-AII or genotype-B infection.
This is the first published report on the Giardia genotypes circulating in the provinces of Mendoza and Chaco. The results indicate the importance of asymptomatic children in the transmission of Giardia among the young.
The protozoan parasite that causes most cases of diarrhoea worldwide is Giardia intestinalis (G. duodenalis, G. lamblia), this parasite being responsible for an estimated 2·5 million cases of diarrhoea each year among children in developing countries (Mahdy et al., 2009). Giardia affects mainly children and has a wide range of clinical manifestations, from asymptomatic carriage to acute diarrhoea with nutritional malabsorption (Thompson, 2004). The World Health Organization (WHO) recently estimated that about 200 million people in Asia, Africa and Latin America have symptomatic giardiasis (Mahdy et al., 2009).
Human giardiasis is a cosmopolitan (sometimes zoonotic) infection whose prevalence varies from 2% (in some developed countries) to 70% (in some developing countries) (Eligio–García et al., 2008). In Argentina, prevalences of giardial infection have been found to vary from 6% to 36% and to depend largely on the local levels of sanitation and personal hygiene (Minvielle et al., 2004; López Santorno et al., 2006; Basualdo et al., 2007; Milano et al., 2007; Salomón et al., 2007; Gamboa et al., 2011).
Although seven main genotypes of G. intestinalis have been identified (A–G), only genotypes A and B have been associated with human infection (Thompson, 2004). According to Cacciò and Ryan (2008), most human infections involve genotype B, although the relative commonness of genotypes A and B varies geographically. Among Ugandans sharing gorilla habitats (Graczyk et al., 2002), symptomatic patients in Mexico (Ponce–Macotela et al., 2002) and Canada (Van Keulen et al., 2002) and children in Brazilian day-care centres (Volotao et al., 2007), for example, genotype A has been found to be predominant. Among symptomatic and asymptomatic young adults in India (Paintlia et al., 1998), English patients with diarrhoea (Amar et al., 2002), children in Australian day-care centres (Read et al., 2002), symptomatic patients in France (Bertrand et al., 2005) and young children (aged 1 month–9 years) in Peru (Pérez–Cordón et al., 2008), however, B was found to be the most common genotype. The distribution of Giardia genotypes among communities in Argentina has been the subject of just two previous studies: Molina et al. (2007) found genotype B in a rural population in Buenos Aires province, and Minvielle et al. (2008) identified genotypes AII and B in the same province.
The factors behind the variability seen in the morbidity of giardial infection in humans are not clear and there is certainly no consensus on any links between the infecting genotype (A or B) and any clinical manifestation (Homan and Mank, 2001; Read et al., 2002; Cacciò et al., 2005; Haque et al., 2005; Robertson et al., 2009).
The main aims of the present study were to identify the genotypes of G. intestinalis circulating among children living in the Argentinian provinces of Buenos Aires, Mendoza and Chaco, and to explore the associations, if any, between the infecting genotypes, epidemiological characteristics and clinical presentations.
SUBJECTS AND METHODS
Subjects and Study Areas
Between August 2005 and July 2007, 1035 children (533 boys and 502 girls) aged 2–14 years and with or without symptoms were enrolled at health centres or in public schools or soup kitchens. All the subjects were permanent residents in the provinces of Buenos Aires, Mendoza or Chaco, in Argentina. Enrollment was voluntary.
Buenos Aires province, in central–eastern Argentina, has a humid and moderate climate, with a mean annual rainfall of 1100 mm. The subjects from this province lived in Hipólito Vieytes (35°16′S, 57°34′ E), Atalaya (35°01′S, 57°31′E), Barrio Obrero (34°56′S, 57°52′E), El Paligüe (34°58′S, 57°53′E), or 4 de Junio (30°50′S, 58°06′E), which lie 40, 45, 7, 3 and 35 km, respectively, from the provincial capital of La Plata.
The subjects from Mendoza province (which lies in central–western Argentina and has a moderate and arid climate, with a mean annual rainfall of 250 mm) were enrolled in Barrio Jardín Aeroparque (32°53′S, 68°41′E), which lies 4 km from the provincial capital of Mendoza (and 1090 km from La Plata).
The subjects from the north–eastern province of Chaco province (which has a warm and humid subtropical climate, with a mean annual rainfall of 1200 mm) were enrolled in the city of Resistencia, which lies at 27°27′S and 58°59′E (and 1070 km from La Plata).
Parasitology and Data Collection
A medical team conducted a copro–parasitological examination of each child and collected relevant clinical–epidemiological data on each child, from the child’s parents, who were asked to complete a standardized questionnaire.
data collection
The questionnaire recorded details of the child’s house — setting (rural or urban), quality [‘precarious’ (built of wood/cardboard/metal sheet) or ‘substantial’ (built of cement and masonry)], sanitation (sewerage, latrine or cesspool), source of water (piped or pump), history of flooding (whether or not the house was flooded more than twice/year), pet dogs (present or absent), level of overcrowding (whether or not there were more than three people/room) — and whether or not the child had any symptoms (presence/absence of diarrhoea in the last week, anorexia, vomiting, asthenia and/or abdominal pain).
faecal samples
Faecal material was collected, by each child’s parents or tutors, on a daily basis for 5 consecutive days, in the same disposable container with 5% formaldehyde. These samples were concentrated using a modification of the Telemann method. Briefly, 10 g of homogenized sample were macerated in 15 ml Telemann solution [made by dissolving 5 g NaCl in 50 ml of 40% (w/v) formaldehyde and 950 ml distilled water]. After filtering the sample through a double layer of gauze, 1 ml of ether was added and then the suspension was centrifuged at 1000×g for 3 min. Three wet smears of each resultant pellet, each smear treated with a drop of Lugol’s solution, were then checked for Giardia and other intestinal parasites under a light microscope, at ×100 and ×400 (Minvielle et al., 2008).
Giardia genotyping
All faecal samples found smear-positive for Giardia cysts were processed further, to concentrate and clean the cysts and extract their DNA, so that the genotype could be identified (Molina et al., 2007). The cysts were first isolated using sucrose-gradient concentration, counted in a Neubauer chamber and preserved at 4°C until the DNA could be isolated.
Prior to the DNA extraction, the cysts were broken open, by three cycles of freezing (at −80°C for 30 min) and thawing (at +80°C for 30 min), and then incubated at 60°C for 24 h with lysis E buffer [100 mm Tris-HCl, 100 mm EDTA, 2% (w/v) SDS, 0·2 m NaCl and 1 mm mercaptoethanol, containing 1 mg proteinase K/ml] before being preserved at −20°C.
The DNA in the suspensions of broken cysts was purified using the QIAamp DNA stool mini kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions and then preserved at −20°C. These DNA samples, once thawed, were used as the templates in a hemi-nested PCR designed to amplify a fragment of the Giardia tpi (triosephosphate isomerase) gene (Amar et al., 2002). In the first round, 3 μl of purified DNA were used in a 30-μl reaction mixture of 1× PCR buffer containing 3 mm MgCl2, 0·25 mm of each deoxyribonucleotide triphosphate (dNTP), 0·25 μm of each primer (TPIAF, TPIAR, TPIBF and TPIBR), 0·1 μg bovine serum albumin (BSA)/μl and 0·1 U Taq DNA polymerase/μl. The thermocycler used was set to give an initial denaturation at 94°C for 4 min, then 30 cycles, each of 30 s at 94°C, 30 s at 52°C and 1 min at 72°C, before a final extension for 10 min at 72°C.
The amplicons from the first round were used as templates for two single PCR (PCRII A and PCRII B). The 30-μl reaction mixture for each of these PCR consisted of 1× PCR buffer containing 1·5 mm MgCl2, 0·25 mm of each dNTP, 1 μm of each primer (TPIAIF and TPIAR in PCRII A, TPIBIF and TPIBR in PCRII B), 0·1 μg BSA/μl and 0·05 U Taq DNA polymerase/μl. For both reactions, the thermocycler was set to give an initial denaturation at 94°C for 4 min, then 33 cycles, each of 30 s at 94°C, 30 s at 54°C and 1 min at 72°C, before a final extension for 10 min at 72°C. In each PCR, samples of DNA from G. intestinalis of genotypes A and B (1 ng/μl; kindly donated by Dr H. van Keulen from Cleveland University, OH) were used as positive controls and double-distilled water was used as the negative control.
The amplicons from PCRII A (5 μl) were incubated with 5 U Rsa1, in a final volume of 30 μl, at 37°C for 3 h, so that they could be investigated by restriction-fragment-length-polymorphism (RFLP) analysis (Amar et al., 2002). The amplicons (from PCRII A and PCRII B) and the PCRII-A digestion products were investigated via electrophoresis in 1·5% and 3% (w/v) agarose gel, respectively, so that genotypes could be identified (Amar et al., 2002).
Ethics
Stool samples were collected only after parents or tutors gave their informed consent.
The study protocol, which was approved by the Ethical Review Committees of La Plata National University, Roemmers Foundation and the Argentinian Ministry of Education, met the requirements of the Declaration of Helsinki (1964), the Nuremberg Code (1947) and the Argentinian National Act 25·326 (on the protection of personal information).
Data Analysis
Pearson’s χ2 and Fisher’s exact tests were used to explore the relationships between each of the categorical variables investigated in the present study and the relative frequency of each Giardia genotype. Version 14.0 of the SPSS software package (SPSS Inc, Chicago, IL) was used to store, edit and analyse the data. A P-value of <0·05 was considered indicative of a statistically significant difference or association.
RESULTS
Although stool samples were collected from 1035 children, the samples and data from only 998 children (504 boys and 494 girls) were investigated, the other 37 children being excluded because their stool samples had not been collected correctly (19) or because their questionnaires were incomplete (18).
The microscopical examination of the stool samples from the 998 children revealed infections with Blastocystis hominis (in 39·1%), Giardia intestinalis (11·7%), Entamoeba coli (13·8%), Endolimax nana (10·1%), Enterobius vermicularis (5·3%), Ascaris lumbricoides (2·2%), Hymenolepis nana (1·2%), Chilomastix mesnili (1·1%) and Trichuris trichiura (0·5%).
Although Giardia cysts were found in the faeces of 117 children, the Giardia in only 94 (80·3%) of the 117 cyst-positive samples were successfully genotyped. Seventy-seven children were found to be infected with Giardia only of genotype B, and 14 with Giardia only of genotype AII, while three children (from the same school) were each found positive for both genotype AII and genotype B.
Faeces that apparently contained Giardia cysts of genotype B only, genotype AII only and both genotype AII and genotype B contained similar mean (s.d.) numbers of giardial cysts: 153 (184), 123 (146) and 147 (119) cysts/μl, respectively (P>0·05).
Of the 52 boys with genotyped giardial infection, 39 (75%) appeared to be infected with genotype B only, 11 (21%) with AII only, and two (4%) with both genotypes AII and B. Although PCR was more often successful with the cyst-positive faecal samples from boys than with similar samples from girls, the difference was not statistically significant (P>0·05).
The mean (s.d.) age of the 94 children with genotyped giardial infection was 6·0 (3·4) years, 49 (52·1%) being aged between 2 and 5 years (preschool) and the other 45 (47·9%) being schoolchildren aged 6–14 years. A child found infected with genotype B only was, on average, significantly older than a child found infected with genotype AII only (Fig. 1; P<0·05).
Figure 1.
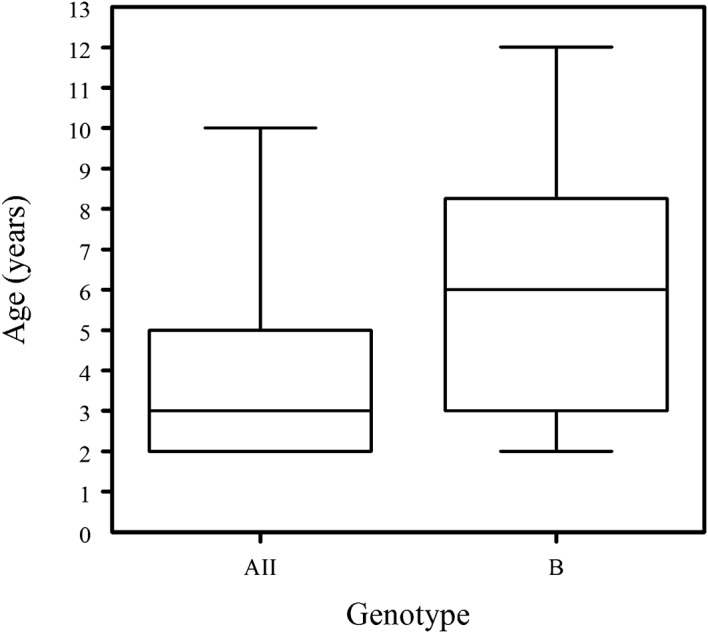
A ‘box-and-whisker’ plot of the ages of the Argentinian children found infected with Giardia intestinalis of genotype AII only or B only. In each plot, the horizontal bar indicates the median, the box the range between the 25th and 75th percentiles, and the ‘whiskers’ the range between the fifth and 95th percentiles.
Both Giardia genotypes detected in the present study (B and AII) were found in each of the three provinces under study (Fig. 2). Of the 94 children with genotyped giardial infection, 72 (76·5%) lived in urban areas [55 of them infected with genotype B, 14 with AII and three with mixed (AII+B) infections] and 22 (23·5%), all infected with genotype B, lived in rural areas. Household setting (rural v. urban) had a statistically significant effect on the recorded genotype distribution, with a giardial infection in a child from a rural household more likely to be identified as genotype B than such an infection in an urban child (P<0·05).
Figure 2.
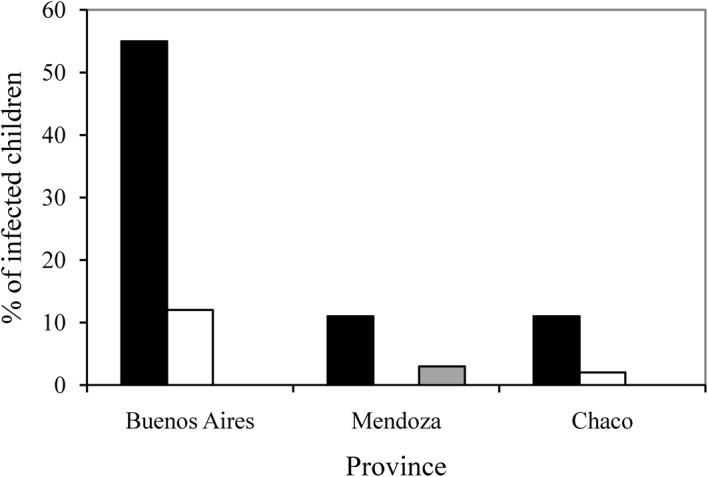
The numbers of children from Buenos Aires, Mendoza and Chaco provinces found infected with Giardia intestinalis of genotype B only (▪), genotype AII only (□) or both genotypes B and AII ( ).
).
The Table shows the relationships between the recorded distribution of Giardia genotypes and the variables investigated via the epidemiological questionnaire. Only one such relationship — that between the type of water-supply system and infecting genotype — appeared statistically significant (P<0·05); the Giardia-infected study children from households with a piped water supply were less likely to be infected with genotype B than their counterparts from households that depended on wells for their water supply.
The recorded epidemiological variables and their links with the Giardia genotypes detected in children in the Argentinian provinces of Buenos Aires, Mendoza and Chaco, in 2005–2007.
| No. and (%) of the children found infected with genotype(s): | |||
| Variable | AII only | B only | Both AII and B |
| build quality of house | |||
| Precarious | 7 (50) | 26 (34) | 0 (0) |
| Substantial | 7 (50) | 51 (66) | 3 (100) |
| sanitation | |||
| Latrine | 7 (50) | 19 (25) | 0 (0) |
| Cesspool | 6 (43) | 42 (55) | 0 (0) |
| Sewerage | 1 (7) | 16 (20) | 3 (100) |
| water supply | |||
| Piped | 12 (86) | 24 (31) | 3 (100) |
| Well | 2 (14) | 53 (69) | 0 (0) |
| House flooded at least twice/year | 8 (57) | 33 (43) | 0 (0) |
| Household owns pet dog | 9 (64) | 60 (78) | 3 (100) |
| Household overcrowded | 5 (36) | 17 (22) | 0 (0) |
Fifty (53·2%) of the 94 children with genotyped giardial infection (42 apparently infected with genotype B only and eight apparently infected with genotype AII only) appeared to be suffering from symptomatic giardiasis (Fig. 3). Twenty (47·6%) of the symptomatic children found infected with genotype B and seven (87·5%) of the symptomatic children found infected with genotype AII were aged 2–5 years. All three children found to have mixed (AII+B) infection were asymptomatic. Genotype-B infection was significantly associated with the presence of abdominal pain (P<0·05).
Figure 3.
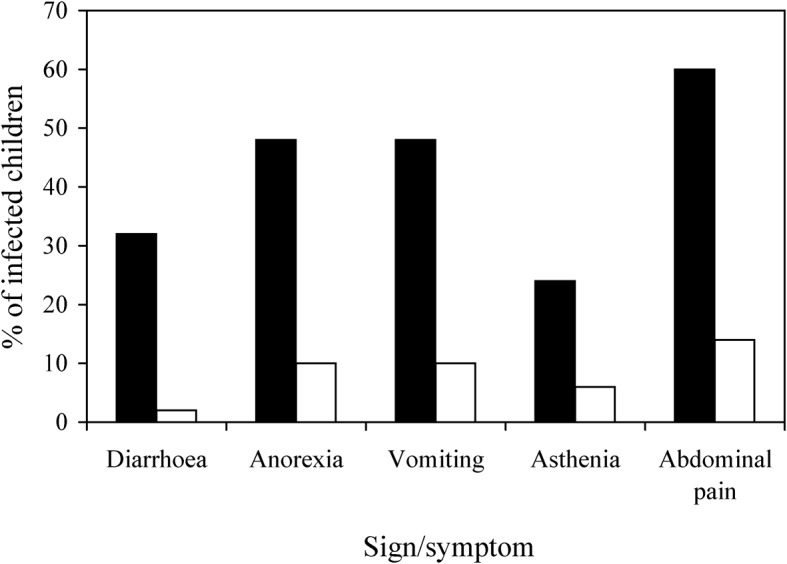
The percentages of the children found infected with Giardia intestinalis of genotype B only (▪) or genotype AII only (□) who were suffering from diarrhoea, anorexia, vomiting, asthenia and/or abdominal pain when they were enrolled.
DISCUSSION
Given its frequently high prevalence among children, giardiasis is an important infection from an epidemiological viewpoint. It is therefore surprising that, although there have been several previous studies on the prevalence of giardial infection in Argentina (López Santorno et al., 2006; Milano et al., 2007; Salomón et al., 2007; Gamboa et al., 2011), there were no attempts before 2007 to identify the G. intestinalis genotypes circulating in this country. A few years ago, genotype B was detected in two cities in Buenos Aires province (Molina et al., 2007; Minvielle et al., 2008). One aim of the present study was to identify the genotypes circulating, among children, in other Argentinean provinces, and this article represents the first report of G. intestinalis genotypes in the provinces of Mendoza and Chaco. Genotype B appeared to be the predominant form of G. intestinalis infecting children in all three provinces under study. This genotype has also been found to predominate in recent studies on schoolchildren in the Philippines (Yason and Rivera, 2007), Thailand (Ratanapo et al., 2008) and India (Ajjampur et al., 2009).
In the present study, as in an earlier study in Nicaragua (Lebbad et al., 2008) and the previous studies in Argentina (Molina et al., 2007; Minvielle et al., 2008), genotype AII appeared rarer in humans than genotype B. Elsewhere in Latin America, however, in Cuba (Pelayo et al., 2008) and Peru (Pérez–Cordón et al., 2008), AII has been found more common than genotype B, while the predominant G. intestinalis genotype infecting humans in Brazil appears to vary according to the region (Souza et al., 2007; Volotao et al., 2007). The predominance of one G. intestinalis genotype over another in a particular area has been attributed to biological as well as geographical factors and, in certain endemic areas, all the giardial infections in humans appear to involve just one genotype (Volotao et al., 2007). Despite the geographical differences, the predominant genotype in the present study was the same in each study province, perhaps indicating that historical immigration and other mass movements of humans (Ponce–Macotela et al., 2002) have reduced the geographical variation in genotype distribution across Argentina. Given that most immigrants who settled in Argentina during the 19th and 20th Centuries were European and most giardial infections found in Europeans are now of genotype B, it is perhaps not surprising that genotype B is now so common in Argentina. More than 60% of the 803 isolates of G. intestinalis from Europeans recently investigated by Sprong et al. (2009) and 57% of the 108 isolates from Spaniards studied by Sahagún et al. (2008) were of genotype B. It is, however, unclear how immigration from areas where genotype B predominates could explain the apparent absence of genotype AII from the rural children investigated in the present study. Pelayo et al. (2008) found genotypes A and B to be equally common among children from a rural Cuban community and Gelanew et al. (2007) found genotype A to predominate in rural communities in Ethiopia.
In the present study, three children were each found infected with two genotypes (i.e. AII and B). The fact that the three attended the same school may indicate that they were exposed to the same source of infection. Mixed infections reflect the complex circulation of the parasites in the environment, and potential exposure to sources of multiple infection, such as sewage (Hopkins et al., 1999; Lalle et al., 2005; Gelanew et al., 2007).
In the present study, the relative frequencies of the B and AII genotypes (among children with giardial infection) appeared to be unaffected by most environmental and socio–cultural conditions, such as the quality of the housing and sanitation, level of overcrowding, frequency of flooding and the presence/absence of household dogs. Kohli et al. (2008) found no statistically significant links between the relative frequencies of infecting genotypes in Brazilian children and any of the epidemiological variables that they investigated. Only the source of household water appeared to affect the relative frequencies of the B and AII genotypes significantly in the present study, perhaps indicating that there is more transmission of genotype B from well water than of genotype AII.
Person-to-person transmission of genotype AII may explain why, in the present study, compared with their older counterparts, the preschool children (with their relatively low standards of personal hygiene and high levels of mouth–hand contact) who were Giardia-positive were significantly more likely to be carrying this genotype. Pet–human transmission of any genotype may also be important in Argentina and merits further investigation. Although no significant link was observed, in the present study, between household ownership of a pet dog and the relative frequencies of the B and AII genotypes, it remains possible that other types of pet or livestock are involved in transmission.
In this study, the cyst loads in faecal material were similar whatever the infecting genotype. Although Hussein et al. (2009) found that people infected with genotype B excreted greater numbers of giardial cysts than those infected with genotype AII, their subjects were all patients who were seeking medical treatment for abdominal problems (not, as in the present study, a mixture of children with and without intestinal symptoms).
In the present study, the children found infected with genotype AII were significantly younger than those found infected with genotype B (Fig. 1). This observation matches that made earlier, in Argentina, by Minvielle (2005). When Kohli et al. (2008) carried out a diachronic analysis of 47 children infected with Giardia, they concluded that all the children had been (re)infected with genotype B, irrespective of the initial infecting genotype. Genotype-B infection in older children might be a consequence of various factors: the genotype is endemic in schools, it causes more persistent infections than genotype AII, and/or it is more resistant to antigiardiasic drugs.
Infection with Giardia may often be asymptomatic. In this study, the frequency of symptomatic illness (presumably symptomatic giardiasis) tended to decrease with age, probably as the result of acquired immunity (Hughes and Kelly, 2006). Pezzani (2007) found that, in rural Argentina, most intestinal parasites cause more symptoms in young children than in older children and adults. Although, in the present study, infection with genotype AII appeared to be no more or less likely to be symptomatic than infection with genotype B, genotype-B infection did appear to be significantly linked to abdominal pain. Other studies have reported a significant association between genotype A and the presence of symptoms (Read et al., 2002; Aydin et al., 2004; Haque et al., 2005; Sahagún et al., 2008) and other genotype-linked differences in clinical symptoms (Homan and Mank, 2001; Gelanew et al., 2007).
Diarrhoea was reported for <20% of the Giardia-infected children investigated in the present study and was not significantly associated with the infecting genotype in any age-group. These results are very similar to those of Read et al. (2002), who studied Dutch children aged <5 years, and those of Haque et al. (2005), who genotyped the Giardia cysts excreted by patients in Bangladesh, only 29% of whom had diarrhoea.
To conclude, this is the first report on the Giardia genotypes in the provinces of Mendoza and Chaco. Genotype B was predominant in the children of these provinces and in Buenos Aires province. There was a significant association between genotype B and both residence in a rural area and the use of well water. Other environmental and socio–cultural conditions had no apparent effect on the infecting genotype of Giardia. Children infected with genotype AII were significantly younger than those infected with genotype B but symptomatic giardiasis was detected at similar frequency among children infected with each genotype. There was a statistically significant association between genotype-B infection and abdominal pain. Diarrhoea was found in <20% of the children infected with Giardia and was not significantly associated with the infecting genotype in any age-group. Future research to expand our knowledge on the relationships between Giardia genotype and symptoms in children should include studies on larger groups of children with symptomatic and asymptomatic infection and on children from other regions of Argentina.
Acknowledgments
The authors would like to thank the communities involved in this study, the staff of the health centres, schools and soup kitchens where enrollment occurred, and E. Ledesma (who provided stool samples from Chaco province). This work was supported financially by La Plata National University, Roemmers Foundation, and the Secretary of University Policies, Ministry of Education, Argentina.
REFERENCES
- Ajjampur SR, Sanzaran P, Kannan A, Sathyakumar K, Sarkar R, Gladstone B.&Kang G.(2009)Giardia duodenalis assemblages associated with diarrhea in children in South India identified by PCR–RFLP. American Journal Tropical Medicine and Hygiene 8016–19. [PMC free article] [PubMed] [Google Scholar]
- Amar CF, Dear PH, Pedraza-Díaz S, Looker N, Linnane E.&McLauchlin J.(2002)Sensitive PCR-restriction fragment length polymorphism assay for detection and genotyping of Giardia duodenalis in human feces. Journal of Clinical Microbiology 40446–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydin AF, Besirbellioglu BA, Avci IY, Tanyuksel M, Araz E.&Pahsa A.(2004)Classification of Giardia duodenalis parasites in Turkey into Groups A and B using restriction fragment length polymorphism. Diagnostic Microbiology and Infectious Diseases 50147–151. [DOI] [PubMed] [Google Scholar]
- Basualdo JA, Cordoba MA, de Luca MM, Ciarmela ML, Pezzani BC, Grenovero MS.&Minvielle MC.(2007)Intestinal parasitoses and environmental factors in a rural population of Argentina, 2002–2003. Revista do Instituto de Medicina Tropical de São Paulo 49251–255. [DOI] [PubMed] [Google Scholar]
- Bertrand I, Albertini L.&Schwartzbrod J.(2005)Comparison of two target genes for detection and genotyping of Giardia lamblia in human feces by PCR and PCR–restriction fragment length polymorphism. Journal of Microbiology 435940–5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacciò SM.&Ryan U.(2008)Molecular epidemiology of giardiasis. Molecular and Biochemical Parasitology 16075–80. [DOI] [PubMed] [Google Scholar]
- Cacciò S, Thompson RCA, McLauchlin J.&Smith HV.(2005)Unravelling Cryptosporidium and Giardia epidemiology. Trends in Parasitology 21430–437. [DOI] [PubMed] [Google Scholar]
- Eligio-García L, Cortes-Campos A, Cota-Guajardo S, Gaxiola S.&Jiménez-Cardoso E.(2008)Frequency of Giardia intestinalis assemblages isolated from dogs and humans in a community from Culiacan, Sinaloa, Mexico using beta-giardin restriction gene. Veterinary Parasitology 156205–209. [DOI] [PubMed] [Google Scholar]
- Gamboa M, Navone G, Orden A, Torres M, Castro L.&Oyhenart E.(2011)Socio–environmental conditions, intestinal parasitic infections and nutritional status in children from a suburban neighborhood of La Plata, Argentina Acta Tropica in press [DOI] [PubMed] [Google Scholar]
- Gelanew T, Lalle M, Hailu A, Pozio E.&Cacciò S(2007)Molecular characterization of human isolates of Giardia duodenalis from Ethiopia. Acta Tropica 10292–99. [DOI] [PubMed] [Google Scholar]
- Graczyk TK, Bosco-Nizeyi J, Ssebide B, Thompson RCA, Read C.&Cranfield MR.(2002)Anthropozoonotic Giardia duodenalis genotype (assemblage) A infections in habitats of free-ranging human-habituated gorillas, Uganda. Journal of Parasitology 88905–909. [DOI] [PubMed] [Google Scholar]
- Haque R, Roy S, Kabir M, Stroup SE, Mondal D.&Houpt E. R(2005)Giardia assemblage A infection and diarrhea in Bangladesh. Journal of Infectious Diseases 1922171–2173. [DOI] [PubMed] [Google Scholar]
- Homan WL.&Mank TG.(2001)Human giardiasis: genotype linked differences in clinical symptomatology. International Journal for Parasitology 31822–826. [DOI] [PubMed] [Google Scholar]
- Hopkins RM, Constantine CC, Groth DA, Wetherall JD, Reynoldson JA.&Thompson RCA.(1999)PCR-based DNA fingerprinting of Giardia duodenalis isolates using the intergenic rDNA spacer. Parasitology 118531–539. [DOI] [PubMed] [Google Scholar]
- Hughes S.&Kelly P.(2006)Interactions of malnutrition and immune impairment, with specific reference to immunity against parasites. Parasite Immunology 28577–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussein AI, Yamaguchi T, Nakamoto K, Iseki M.&Tokoro M.(2009)Multiple-subgenotype infections of Giardia intestinalis detected in Palestinian clinical cases using a subcloning approach. Parasitology International 58258–262. [DOI] [PubMed] [Google Scholar]
- Kohli A, Bushen OY, Pinkerton RC, Houpt E, Newman RD, Sears CL, Lima AM.&Guerrant RL.(2008)Giardia duodenalis assemblage, clinical presentation and markers of intestinal inflammation in Brazilian children. Transactions of the Royal Society of Tropical Medicine and Hygiene 102718–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalle M, Pozio E, Capelli G, Bruschi F, Crotti D.&Cacciò M.(2005)Genetic heterogeneity at the beta-giardin locus among human and animal isolates of Giardia duodenalis and identification of potentially zoonotic subgenotypes. International Journal for Parasitology 35207–213. [DOI] [PubMed] [Google Scholar]
- Lebbad M, Ankarklev J, Tellez A, Leiva B, Andersson JO.&Svard S.(2008)Dominance of Giardia assemblage B in León, Nicaragua. Acta Tropica 10644–53. [DOI] [PubMed] [Google Scholar]
- López Santorno MS, Garraza M, Gamboa MI, Zonta ML.&Navone GT.(2006)Enteroparasitosis en la población infantil de Fuerte Esperanza, Chaco, Argentina. Acta Bioquimica Clinica Latinoamericana 3, (Suppl. 3)235 [Google Scholar]
- Mahdy AK, Surin J, Wan KL, Mohd-Adnan A, Al-Mekhlafi MS.&Lim YAL.(2009)Giardia intestinalis genotypes: risk factors and correlation with clinical symptoms. Acta Tropica 11267–70. [DOI] [PubMed] [Google Scholar]
- Milano A, Oscherov E, Palladino A.&Bar A.(2007)Enteroparasitosis infantil en un área urbana del nordeste argentino. Medicina (Buenos Aires) 67238–242. [PubMed] [Google Scholar]
- Minvielle MC.(2005)Detección de genotipos A (I y II) y B por PCR–RFLP de Giardia lamblia en heces humanas y animales de La Plata y su área de influencia MSc thesisUniversidad Nacional de San Martín; San Martín, Argentina [Google Scholar]
- Minvielle MC, Pezzani BC, Córdoba MA, De Luca MM, Apezteguía MC.&Basualdo JA.(2004)Epidemiological survey of Giardia spp. and Blastocystis hominis in an Argentinian rural community. Korean Journal of Parasitology 42121–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minvielle M, Molina N, Polverino D.&Basualdo J.(2008)First genotyping of Giardia lamblia from human and animal feces in Argentina, South America. Memórias do Instituto Oswaldo Cruz 10398–103. [DOI] [PubMed] [Google Scholar]
- Molina N, Polverino D, Minvielle M.&Basualdo J.(2007)PCR amplification of triosephosphate isomerase gene of Giardia lamblia in formalin-fixed feces. Revista Latinoamericana de Microbiologia 496–11. [PubMed] [Google Scholar]
- Paintlia AS, Descoteaux S, Spencer B, Chakraborti A, Mahajan RC, Ganguly NK.&Samuelson J.(1998) Giardia lamblia groups A and B among young adults in India. Clinical Infectious Diseases 26190–191. [DOI] [PubMed] [Google Scholar]
- Pelayo L, Nuñez FA, Rojas L, Furuseth Hansen E, Gjerde B, Wilke H, Mulder B.&Robertson L.(2008)Giardia infections in Cuban children: the genotypes circulating in a rural population. Annals of Tropical Medicine and Parasitology 102585–595. [DOI] [PubMed] [Google Scholar]
- Pérez-Cordón G, Cordova Paz Soldán O, Vargas Vásquez F, Velasco Soto J, Sempere Bordes L, Sánchez Moreno M.&Rosales M.(2008)Prevalence of enteroparasites and genotyping of Giardia lamblia in Peruvian children. Parasitology Research 103459–465. [DOI] [PubMed] [Google Scholar]
- Pezzani BC.(2007)Estrategias de control de las parasitosis intestinales en una comunidad rural PhD thesis, Facultad de Ciencias Naturales y MuseoUniversidad Nacional de La Plata; La Plata, Argentina [Google Scholar]
- Ponce-Macotela M, Martínez-Gordillo MN, Bermúdez-Cruz RM, Salazar-Schettino PM, Ortega-Pierres G.&Ey PL.(2002)Unusual prevalence of the Giardia intestinalis A-II subtype amongst isolates from humans and domestic animals in Mexico. International Journal for Parasitology 321201–1202. [DOI] [PubMed] [Google Scholar]
- Ratanapo S, Mungthin M, Soontrapa S, Faithed C, Siripattanapipong S, Rangsin R, Naaglor T, Piyaraj P, Taamasri P.&Leelayoova S.(2008)Multiple modes of transmission of giardiasis in primary schoolchildren of a rural community, Thailand. American Journal of Tropical Medicine and Hygiene 78611–615. [PubMed] [Google Scholar]
- Read C, Walters J, Robertson ID.&Thompson RCA.(2002)Correlation between genotype of Giardia duodenalis and diarrhoea. International Journal for Parasitology 32229–231. [DOI] [PubMed] [Google Scholar]
- Robertson LJ, Hanevik K, Escobedo AA, Mørch K.&Langeland N.(2009)Giardiasis — why do the symptoms sometimes never stop? Trends in Parasitology 2678–82. [DOI] [PubMed] [Google Scholar]
- Sahagún J, Clavel A, Goñi P, Seral C, Llorente MT, Castillo FJ, Capilla S, Arias A.&Gómez-Lus R.(2008)Correlation between the presence of symptoms and the Giardia duodenalis genotype. European Journal of Clinical Microbiology and Infectious Diseases 2781–83. [DOI] [PubMed] [Google Scholar]
- Salomón C, Tonelli R, Borremans C, Bertello D, De Jong L, Jofré C, Enriquez V, Carrizo L.&Costamagna S.(2007)Prevalencia de parásitos intestinales en niños de la ciudad de Mendoza, Argentina. Parasitologia Latinoamericana 6249–53. [Google Scholar]
- Souza SLP, Gennari SM, Richtzenhain LJ, Pena HFJ, Funada MR, Cortez A, Gregori F.&Soares RM.(2007)Molecular identification of Giardia duodenalis isolates from humans, dogs, cats and cattle from the state of Sao Paulo, Brazil, by sequence analysis of fragments of glutamate dehydrogenase (gdh) coding gene. Veterinary Parasitology 149258–264. [DOI] [PubMed] [Google Scholar]
- Sprong H, Cacciò SM.&van der Giessen JW.(2009)Identification of zoonotic genotypes of Giardia duodenalis. PLoS Neglected Tropical Diseases 3e558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson RCA.(2004)The zoonotic significance and molecular epidemiology of Giardia and giardiasis. Veterinary Parasitology 12615–35. [DOI] [PubMed] [Google Scholar]
- Van Keulen H, Macechko PT, Wade S, Schaaf S, Wallis PM.&Erlandsen SL.(2002)Presence of human Giardia in domestic, farm and wild animals, and environmental samples suggest a zoonotic potential for giardiasis. Veterinary Parasitology 10897–107. [DOI] [PubMed] [Google Scholar]
- Volotao AC, Costa-Macedo LM, Haddad FSM, Brandão A, Peralta JM.&Fernandes O.(2007)Genotyping of Giardia duodenalis from human and animal samples from Brazil using beta-giardin gene: a phylogenetic analysis. Acta Tropica 10210–19. [DOI] [PubMed] [Google Scholar]
- Yason JA.&Rivera WL.(2007)Genotyping of Giardia duodenalis isolates among residents of slum area in Manila, Philippines. Parasitology Research 101681–687. [DOI] [PubMed] [Google Scholar]


