Abstract
BACKGROUND
Rheumatoid arthritis (RA) is associated with cardiovascular disease (CVD) but little is known about its association with another form of vascular disorder, venous thromboembolism (VTE).
METHODS
A retrospective cohort study was conducted using the US insurance claims. RA and non-RA patients were matched on age, sex, and index date. Incidence rates (IR) and rate ratios (RR) of VTE, defined as the composite of deep vein thrombosis (DVT) or pulmonary embolism (PE), were calculated. Cox proportional hazards models compared VTE risks between RA and non-RA patients, adjusting for VTE risk factors such as CVD, surgery, hospitalization, medications, and acute phase reactants (APR).
RESULTS
Over the mean follow-up of 2 years, the IR for VTE among RA patients was 6.1 per 1,000 person-years, 2.4 times higher (95%CI 2.1–2.8) than the rate of non-RA patients. The IRs for both DVT (RR 2.2, 95%CI 1.9–2.6) and PE (RR 2.7, 95%CI 2.2–3.5) were higher in RA compared with non-RA patients. After adjusting for risk factors of VTE, the VTE risk remained elevated in RA (hazard ratio 1.4, 95%CI 1.1–1.7) compared to non-RA patients. The result was similar after further adjustment for elevated APR (hazard ratio 1.5, 95%CI 0.3–6.5). One-third of patients who developed VTE had at least one major VTE risk factors 90 days before and after the VTE event.
CONCLUSION
Our results showed an increased risk of developing VTE for RA patients compared with non-RA patients. The risk was attenuated but remained elevated even after adjusting for various risk factors for VTE.
Keywords: rheumatoid arthritis, venous thromboembolism, pulmonary embolism, deep vein thrombosis
INTRODUCTION
Venous thromboembolism (VTE) which includes deep vein thrombosis (DVT) and pulmonary embolism (PE) is a major health problem and occurs in approximately 1 per 1,000 persons in the U.S.(1) The incidence increases dramatically with age. Other known risk factors for VTE are fracture of lower extremities, joint replacement surgery, major general surgery, major trauma, malignancy, heart or respiratory failure, pregnancy, history of VTE, hormone replacement therapy and oral contraceptive use, but do not traditionally include inflammatory diseases such as rheumatoid arthritis (RA).(2, 3) The link between chronic systemic inflammatory diseases, such as RA, and cardiovascular disease (CVD), including myocardial infarction and stroke, has been well documented.(4–7) Systemic inflammation may also play a important role in the development of VTE as inflammatory cytokines such as interleukin (IL)-6, IL-8, and tumor necrosis factor (TNF) could modulate thrombotic responses by activating coagulation pathways.(8, 9) Markers of systemic inflammation, such as C-reactive protein (CRP), fibrinogen, and factor VIII, are also found at higher levels in patients with VTE, similar to atherothrombosis.(10, 11)
Recent studies report that patients with RA have a 1.5- to 6-fold increased risk of VTE, such as pulmonary embolism (PE) and deep vein thrombosis (DVT) compared to non-RA patients.(12–18) Many of these studies identified their RA cohort based on a hospital discharge diagnosis of RA which could introduce a bias to select patients with severe RA and hence may not be generalizable to typical RA patients seen in the outpatient setting.(13–16) Furthermore, no prior studies have examined the VTE risk in RA adjusting for acute phase reactant levels. The objectives of this study are 1) to examine the rate of incident VTE in a cohort of patients with RA compared with those without RA in the general population, 2) to assess the VTE risk in RA compared to non-RA adjusting for a number of known risk factors for VTE as well as baseline acute phase reactant levels, and 3) to determine the proportion of VTE cases in the presence of major VTE risk factor such as recent hospitalization, surgery and malignancy during the follow-up period.
METHODS
Data Source
We conducted a cohort study using the claims data from a commercial U.S. health plan which insures primarily working adults and their family members, and a small Medicare population for the period January 1, 2001 through June 30, 2008. This database contains longitudinal claims information including medical diagnoses, procedures, hospitalizations, physician visits, and pharmacy dispensing on more than 28 million fully-insured subscribers, with medical and pharmacy coverage, to 14 Blue Cross/Blue Shield health plans across the United States. Results for outpatient laboratory tests, including C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) were available on a subset of beneficiaries. Personal identifiers were removed from the dataset before the analysis to protect subject confidentiality. Patient informed consent was not required. The study protocol was approved by the Institutional Review Board of Brigham and Women’s Hospital.
Study Cohort
Adult patients who had at least two visits seven days apart coded with the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD 9-CM) code, 714.xx, for RA were eligible for the RA cohort. The index date for the RA cohort was the date of the first dispensing of a disease-modifying anti-rheumatic drug (DMARD) after at least 12 months of continuous health plan eligibility; thus, all persons in the RA cohort were required to have had two diagnoses and at least one filled prescription for a DMARD at the start of follow-up. A previous validation study showed that RA patients can be accurately identified using a combination of diagnosis codes and DMARD prescriptions in insurance claims data.(19) Patients with one or more diagnostic codes of DVT or PE, solid tumors, hematologic malignancies, or myelodysplastic syndrome recorded in the 12-month period prior to index dates were excluded. To ensure that we only include incident cases of VTE, subjects with claims for DVT or PE, or dispensings for anticoagulants in the 12-month period prior to index dates were also excluded from both cohorts. (See Appendix 1 for the list of diagnosis codes for VTE and the name of anticoagulants that were excluded).
The non-RA cohort, defined as adults who never had a diagnosis of RA during the study period, were identified and the aforementioned exclusion criteria were applied. The index date for the non-RA cohort was the second physician visit date after at least 12 months of continuous health plan eligibility. The non-RA cohort was then matched to the RA cohort on age, sex and index date (+/− 30 days) with a 4:1 ratio to mirror the health care utilization of the RA cohort. Patients in both cohorts were followed from the index date to the first of any of the following censoring events: development of DVT, PE, loss of health plan eligibility, end of study database, or death.
Outcome Definition
The primary outcome was defined as a hospitalization for the composite endpoint of incident VTE, either PE or DVT, based on discharge diagnosis codes within the study database.(20, 21) The secondary outcome was defined more strictly with a combination of hospital discharge diagnosis codes for VTE and a prescription for anticoagulants within ten days after discharge date (Appendix 1).
Covariates
Variables potentially related to development of VTE were assessed using data from the 12 months before the index date. These variables included demographic factors (age and sex), comorbidities (hypertension, CVD, chronic obstructive pulmonary disease, diabetes, heart failure, chronic kidney disease, liver disease, malignancy, smoking, varicose vein, obesity, and history of various types of surgeries), medications (oral contraceptives and hormone replacement therapy), and health care utilization factors (the number of visits to any physicians and acute care hospitalizations, and the number of different prescription drugs). To quantify patients’ comorbidities, we also calculated the Deyo-adapted Charlson Comorbidity Index based on ICD-9-CM.(22, 23) The Comorbidity Index is a summative score, based on 19 major medical conditions including myocardial infarction, congestive heart failure, peripheral vascular disease, dementia, cerebrovascular disease, pulmonary, renal, hepatic disease, diabetes, ulcer, connective tissue disease, cancer, and HIV infection. A score of 0 representsabsenceof comorbidity and a higher score indicates a greater number of comorbid conditions. Outpatient laboratory data such as levels of acute phase reactants (i.e., ESR or CRP) at baseline were available in a subgroup of the study cohort.
In patients who developed VTE during the follow-up, major clinical risk factors or provoking factors for VTE such as malignancy 90 days before and after the VTE and hospitalization and surgeries 90 days before the date of VTE events were assessed.(24)
Statistical Analyses
We compared the baseline characteristics between RA and non-RA patients. We estimated incidence rate (IR) of VTE events with 95% confidence interval (CI), calculated as the number of subjects who had a hospitalization for either DVT or PE divided by the total person-time, in both RA and non-RA cohorts. The rate ratio (RR) with 95% CI was estimated by dividing the rate of the VTE events among RA patients by that of non-RA.(25) To adjust for potential confounders, separate Cox proportional hazard models were used to compare the rate of VTE events among RA patients with those in non-RA cohort.(26) The relationship between RA and the proportion of patients free of VTE was plotted on a Kaplan-Meier curve. Additionally, we conducted subgroup analyses for the primary outcome in patients in whom we had an acute phase reactant measurement at baseline. Among these subjects, the acute phase reactant levels were used as a covariate in Cox proportional hazard models comparing the risk of a hospitalization for VTE in RA with non-RA. All analyses were done using SAS 9.2 Statistical Software (SAS Institute Inc., Cary, NC).
RESULTS
Cohort Selection
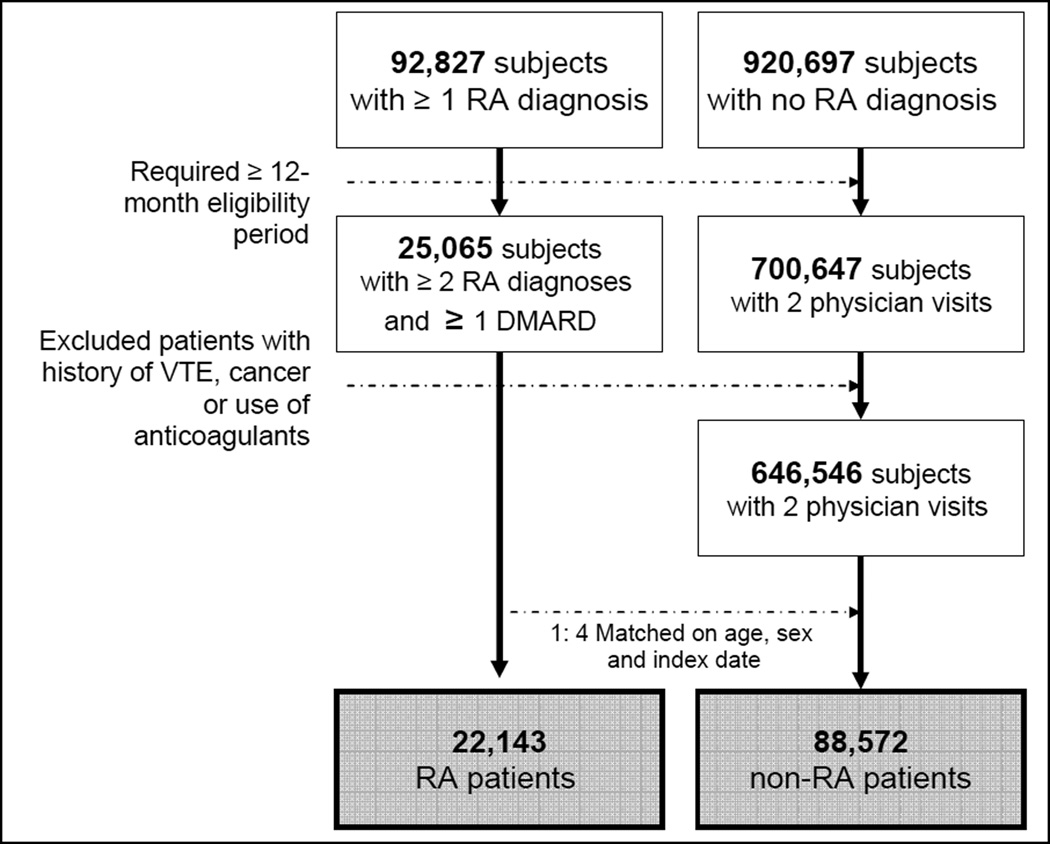
There were initially 92,827 subjects with at least one RA diagnosis after a 12-month-enrollment period and 920,697 subjects with no RA diagnosis at any time during the entire study period. Of the potential 44,978 RA patients with at least two diagnoses of RA at least seven days apart, 25,065 patients had at least one prescription for DMARDs. 700,647 patients with no RA had at least two physician visits after a 12-month-enrollment period. There were 6,138 patients with a history of VTE in the 12-month baseline period. After applying the exclusion criteria, we selected four non-RA patients matched to each RA patient on age, sex, and index date (+/− 30 days). Our final study cohort includes 22,143 RA patients and 88,572 non-RA patients. Figure 1 displays our cohort selection process. The mean (SD) follow-up time was 2.0 (1.6) years for RA and 2.0 (1.5) years for non-RA patients.
Figure 1. Selection of the study cohort.
The final study cohort included 22,143 RA patients and 88,572 non-RA patients matched on age, sex and index date.
RA: rheumatoid arthritis, DMARD: disease-modifying antirheumatic drug, VTE: venous thromboembolism
Patient Characteristics
Baseline characteristics of the age- and sex-matched cohorts were compared (Table 1). The mean age was 52 years and 75% were women in both cohorts. Substantial differences across almost all other baseline characteristics were observed between the cohorts, with the prevalence of comorbid conditions that may be related to VTE risks more common in RA patients than non-RA subjects. A recorded diagnosis of smoking, hypertension, CVD, chronic obstructive pulmonary disease, and intra-abdominal surgery, hormone replacement therapy and oral contraceptive use, and health care utilization including physician visits, hospitalization, and number of prescription drugs were more commonly noted in patients with RA. The mean (SD) length of hospitalization among those who had at least one hospitalization in the baseline 365-day period was 7.2 (12.0) days for the RA cohort and 5.9 (11.1) days for the non-RA cohort. 15% of RA patients and 1% of non-RA patients had their baseline acute phase reactants measured (see Appendix 2). Thirty-seven percent of RA and 18% of non-RA patients with a baseline acute phase reactant level measurement available had elevated levels. The mean (SD) CRP level at baseline was 15.2 (28.1) milligram per liter for RA and 3.4 (6.1) milligrams per liter for non-RA patients. The mean (SD) ESR at baseline was 19.3 (21.9) millimeters per hour for RA and 10.2 (14.0) millimeters per hour for non-RA patients.
Table 1.
Baseline characteristics of the study cohort in 12 months prior to the index date
| Rheumatoid arthritis (N=22,143) |
Non-rheumatoid arthritis (N=88,572) |
|
|---|---|---|
| N (%) or mean ± SD | ||
| Follow-up period, years | 2 (1.5) | 2 (1.5) |
| Demographic | ||
| Age*, years | 52.2 ± 12 | 52.2 ± 12 |
| Female* | 16,513 (75) | 66,052 (75) |
| Comorbidities | ||
| Comorbidity Index | 1.2 ± 0.8 | 0.2 ± 0.6 |
| Diabetes | 1,132 (5) | 3,827 (4) |
| Obesity | 531 (2) | 1,809 (2) |
| Smoking | 1,446 (7) | 3,614 (4) |
| Varicose vein | 180 (1) | 494 (1) |
| Chronic kidney disease | 333 (2) | 651 (1) |
| Liver disease | 604 (3) | 1,370 (2) |
| Hypertension | 6,747 (30) | 20,880 (24) |
| Cardiovascular disease | 1,496 (7) | 4,222 (5) |
| Stroke | 658 (3) | 1,841 (2) |
| COPD | 2,754 (12) | 6,467 (7) |
| Heart failure | 410 (2) | 915 (1) |
| Pregnancy | 253 (1) | 1,531 (2) |
| Hormone replacement therapy | 3,304 (15) | 10,234 (12) |
| Oral contraceptives | 1,805 (8) | 6,416 (7) |
| Oral steroids | 11,730 (53) | 5,948 (7) |
| Surgery, musculoskeletal | 1,095 (5) | 1,389 (7) |
| Surgery, cardiovascular | 501 (2) | 1,120 (1) |
| Surgery, intra-abdominal | 1,465 (7) | 3,481 (4) |
| Health care utilization | ||
| No. of total physician visits | 10.4 ± 7.6 | 3.8 ± 4.4 |
| No. of hospitalizations | 0.2 ± 0.7 | 0.1 ± 0.5 |
| No. of prescription drug | 10.3 ± 6.4 | 4.4 ± 4.6 |
| Laboratory data | ||
| APR levels available | 3,313 (15) | 840 (1) |
| Elevated APR levelsa | 1,210 (37) | 149 (18) |
matched
SD: standard deviation, COPD: chronic obstructive pulmonary disease, APR: acute phase reactant
the proportion was calculated among the subjects with APR levels available
Risk of Venous Thromboembolism
During the study follow-up, 713 (0.6%) of the study population were hospitalized for VTE. Of these patients with VTE, 42.8% received a dispensing for an anticoagulant within 10 days after hospital discharge and 47.1% received a dispensing for an anticoagulant within 30 days after hospital discharge. Among those who developed VTE events during the follow-up time, the mean (SD) length of hospitalization was 13.8 (19.1) days for the RA cohort and 12.9 (18.9) days for the non-RA cohort. As shown in Table 2, the IR of the primary outcome, hospitalization for VTE among RA patients was 6.1 per 1,000 person-years and 2.4 times higher than that of non-RA patients (2.5 per 1,000 person-years). The age and sex-adjusted RR of RA was 2.2 (95 % CI, 1.9–2.6) for experiencing DVT and 2.7 (95% CI, 2.2–3.5) for PE, compared to non-RA. Similar risks were observed for the secondary outcomes, defined by both diagnosis codes and dispensings of anticoagulants.
Table 2.
Incidence rates and rate ratios (RR) of VTE (per 1,000 person-years), age, sex, and index date matched
| Rheumatoid arthritis (N=22,143) |
Non-rheumatoid arthritis (N=88,572) |
RR (95% CI) |
|||||
|---|---|---|---|---|---|---|---|
| Cases (n) | Person years |
Rates (95% CI) |
Cases (n) | Person- years |
Rates (95% CI) |
||
| Primary definition | |||||||
| VTE (DVT or PE) | 265 | 43,278 | 6.1 (5.4–6.9) | 448 | 176,866 | 2.5(2.3–2.8) | 2.4(2.1–2.8) |
| DVT | 197 | 43,371 | 4.5 (4.0–5.2) | 364 | 177,018 | 2.1 (1.9–2.3) | 2.2 (1.9–2.6) |
| PE | 111 | 43,490 | 2.6 (2.1–3.1) | 164 | 177,276 | 0.9 (0.8–1.1) | 2.7 (2.2–3.5) |
| Secondary definition | |||||||
| VTE (DVT or PE) | 115 | 43,462 | 2.7 (2.2–3.2) | 190 | 177,209 | 1.1 (0.9–1.2) | 2.5 (2.0–3.1) |
| DVT | 80 | 43,514 | 1.8 (1.5–2.3) | 156 | 177,284 | 0.9 (0.8–1.0) | 2.1 (1.6–2.7) |
| PE | 61 | 43,551 | 1.4 (1.1–1.8) | 84 | 177,380 | 0.5 (0.4–0.6) | 3.0 (2.1–4.1) |
VTE: venous thromboembolism, DVT: deep vein thrombosis, PE: pulmonary embolism, CI: confidence interval
Primary definition: based on a discharge diagnosis
Secondary definition: based on a discharge diagnosis and a dispensing of anticoagulants within 10 days after the discharge date
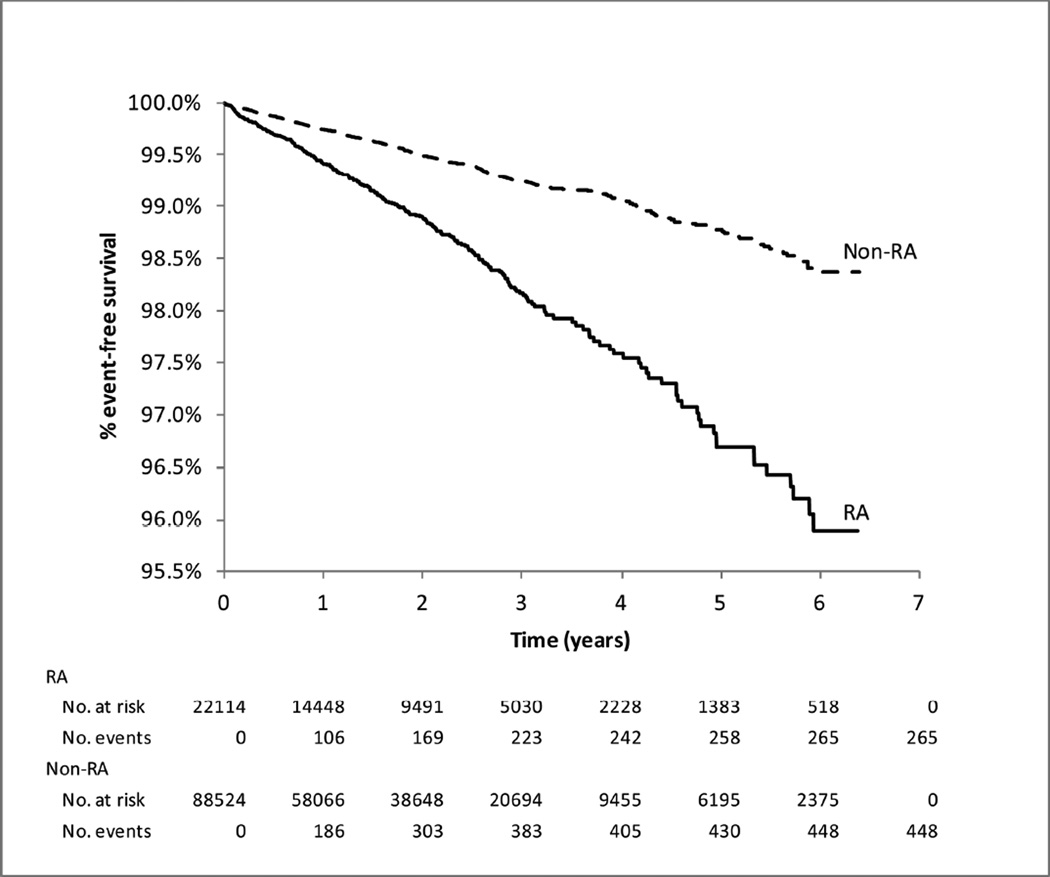
Age and sex-adjusted hazard ratio (HR) of VTE for RA compared with non-RA was 2.5 (95% CI, 2.2–2.9). After adjusting for all the potential confounders of VTE including demographic factors, comorbidities, medications, and health care utilization characteristics (listed in Table 1), the VTE risk remained elevated in RA (HR 1.4, 95% CI, 1.1–1.7) compared to non-RA patients. The fully adjusted HR was 1.2 (95% CI, 0.9–1.5) for DVT and 1.9 (95% CI, 1.3–2.7) for PE. The HRs were very similar for the secondary definitions of VTE (Table 3). Figure 2 shows the Kaplan-Meier curves for hospitalization for VTE in RA and non-RA patients. 84.2% of VTE events in RA and 85.5% of VTE events in non-RA occurred in the first three years of follow-up.
Table 3.
Fully adjusted hazard ratios (HRs)* and 95% confidence intervals (CIs) for VTE in rheumatoid arthritis compared to non-rheumatoid arthritis
| Rheumatoid arthritis | |
|---|---|
| Primary definition | |
| VTE (DVT or PE) | 1.4 (1.1–1.7) |
| DVT | 1.2 (0.9–1.5) |
| PE | 1.9 (1.3–2.7) |
| Secondary definition | |
| VTE (DVT or PE) | 1.7 (1.2–2.3) |
| DVT | 1.2 (0.8–1.7) |
| PE | 2.5 (1.6–4.2) |
Adjusted for age, sex, Comorbidity Index, comorbid conditions, medications and health care utilization patterns listed in Table 1
Figure 2. Kaplan–Meier survival curves for hospitalization for VTE in patients with and without rheumatoid arthritis (RA).
Number of subjects at risk and events were stratified by time since the index date.
Among 713 patients who developed incident VTE, 57% of RA and 60% of non-RA patients had a hospitalization, surgery or a new diagnosis of malignancy, which are considered major VTE risk factors or provoking factors 90 days before the VTE event and a diagnosis of malignancy 90 days after the time of the VTE event. (Table 4) About one-third of those with incident VTE had at least one antecedent major risk factor in the 90 days prior to VTE events.
Table 4.
Presence (%) of major risk factors for VTE among patients with incident VTE (n=713) during the follow-up period
| Rheumatoid arthritis (n=265) |
Non-rheumatoid arthritis (n=448) |
|
|---|---|---|
| Percentages | ||
| 90 days before the outcome | ||
| Hospitalization | 29 | 32 |
| Any surgery | 38 | 42 |
| Malignancy | 30 | 19 |
| 90 days after the outcome | ||
| Malignancy | 11 | 19 |
| 90 days before and after the outcome | ||
| Provoked VTE* | 57 | 60 |
Provoked VTE was defined as occurring in the presence of known malignancy 90 days before and after the VTE, acute hospitalization and surgery 90 days before the VTE.(24)
Subgroup Analyses on Laboratory Data
In a subgroup of patients (N=4, 153) with the baseline acute phase reactant levels available, a total of 22 (0.5%) hospitalizations for VTE occurred. The multivariable HR for VTE associated with RA was 1.7 (95% CI, 0.5–5.9) adjusted for age, sex, and elevated acute phase reactants. After adjusting for age, sex, Comorbidity index and elevated acute phase reactants, the multivariable HR was 1.3 (95% CI, 0.4–4.4). The fully adjusted model that further adjusted for elevated acute phase reactants showed the HR of 1.5 (95% CI, 0.3–6.5) associated with RA.
DISCUSSION
The link between RA and VTE is not yet clearly understood, it has been thought that hypercoagulability is induced by active systemic inflammation and production of cytokines such as TNF–alpha and IL-1. These inflammatory cytokines can lead to endothelial dysfunction, down-regulation of protein C and inhibition of fibrinolysis.(8, 9, 27) It is also possible that patients with RA have more risk factors of VTE such as acute hospitalization, surgical procedures, physical inactivity, and other cardiovascular comorbidities compared to non-RA. This study found that the occurrence of hospitalization for VTE in patients with both RA and non-RA is uncommon, but the risk of incident VTE is increased by 40% in patients with RA compared to non-RA in a fully adjusted analysis accounting for more than 20 different potential confounders. The result remain consistent even after adjusting for elevated acute phase reactants, albeit not statistically significant due the small number of events in this subgroup.
It is well-known that VTE is associated with a number of risk factors such as lower extremity fracture, joint replacement surgery, major general surgery, major trauma, spinal cord injury, congestive heart failure, hormone replacement therapy and oral contraceptive use, and older age.(2) It is also known that nearly all patients who develop VTE have at least one recognized clinical factor associated with VTE, and the risk of VTE increases as the number of VTE risk factors increases.(2, 28, 29) In the present study, 57% of RA and 60% of non-RA patients who developed VTE during the follow-up time had at least one major VTE risk factor, such as a recent hospitalization, surgery, or a diagnosis of malignancy 90 days before or a diagnosis of malignancy 90 days after the date of VTE events.
Several strengths of this study are worth noting. First, we examined a large cohort of RA and non-RA patients in a population that is representative of the U.S. commercially-insured population. Second, although we mainly relied on diagnosis codes for RA and VTE which could potentially lead to exposure and outcome misclassification, both the ICD diagnosis codes for RA and VTE have been validated and used in a number of studies. (19, 20) Moreover, event rates observed in this study are consistent with prior studies: the IR of VTE in non-RA patients, 1 to 2 per 1,000 person-years, is consistent with prior studies,(1, 17,18, 30, 31) and the IR in RA patients was 6 per 1,000 person-years, similar to a previously reported IR which used medical records to define the outcome.(12) Third, we conducted a subgroup analysis of patients with baseline CRP or ESR levels measured and noted a consistently elevated risk of VTE, albeit not statistically significant due to a smaller size of the subgroup, between the RA and non-RA cohorts.
Finally, unlike most published studies that examined the risk of VTE in RA patients based on hospital diagnosis of RA,(13–16) our study cohort was mainly selected based on outpatient RA diagnoses (94%) to be representative of RA patients seen in an ambulatory setting. Our results are also consistent with findings from a recently published cohort study using data from the U.K outpatient medical records.(17) Acute medical hospitalization is a known risk factor for VTE regardless of RA diagnosis.(32) Furthermore, very active RA requiring acute hospitalization could be a potential confounder for the association between RA and VTE. A Swedish study based on patients admitted to hospital for an autoimmune disease reported that PE is a serious problem in this population and the standardized incidence ratio for RA was 6.0 (95% CI 5.6–6.4) in the first year after hospital admission.(16) Another Swedish population-based study also showed a higher rate of VTE in the first year following hospitalization in both RA patients (13.1 per 1,000 person-years) and the general population (2.4 per 1,000 person-years).(18) In a study from the U.S., the adjusted RR of VTE in hospitalized RA patients who did not have an orthopaedic surgery was 2.0 (95% CI 2.0–2.0) compared to non-RA patients.(13) Similarly, a Danish population-based study showed that the adjusted RR for VTE was 1.2 (95% CI 1.0–1.3) among patients with a history of hospitalization for RA.(15)
There are, however, limitations to our study. First, this cohort study is likely subject to residual confounding by body mass index, immobility, severity of heart failure or chronic obstructive pulmonary disease, hereditary hypercoagulable conditions, and other unmeasured risk factors including RA disease severity as well as surveillance bias.(2, 33) A previous U.S. population-based study of 464 RA and 464 non-RA patients, however, reported that there was no significant association between VTE risk and RA disease activity such as presence of rheumatoid factor, presence of joint erosions or destructive changes, or rheumatoid nodules.(12) We assessed a prior diagnosis of VTE, use of anticoagulants and a number of variables potentially related to a future VTE event using the data from the 12 months prior to the index date, but this time period might not be long enough to capture all the information on preexisting diagnosis of VTE and/or potential confounders. We also used multivariable Cox models that were simultaneously adjusted for more than 20 risk factors of VTE, the Comorbidity Index and health care utilization patterns to minimize the effect of such confounders.
Second, we relied on prescription dispensing records in the database to determine patients’ drug exposures including anticoagulants. This may not be the most accurate way to verify individuals’ daily drug exposures, but it is considered one of the best ways to ascertain drug exposure status in non-experimental settings.(34) Lastly, this study was not designed to address a potential role of DMARDs including TNF-α inhibitors in the risk of incident VTE among RA patients. There is limited data suggesting an increased risk of VTE associated with use of DMARDs.(12, 35, 36) The exact effects of DMARDs, either traditional or biologic DMARDs, on the risk of VTE in RA should be further studied, given the large degree of potential for confounding by indication.
Third, because our primary outcomes were based on a hospital discharge diagnosis of VTE, not an admission diagnosis, some of the VTE cases might have occurred during the hospitalization for a different reason. In this study, mild cases of VTE treated in the outpatient setting were not included.
In conclusion, our results showed an increased risk of incident VTE, both DVT and PE, for RA patients compared with non-RA patients. The risk was attenuated but remained elevated after adjusting for known risk factors for VTE such as CVD, surgery, hospitalization, and medications and elevated acute phase reactants levels. Future research is needed whether treatment with DMARDs could modify the risk of VTE in patients with RA.
Supplementary Material
Significance and Innovation.
Incident VTE occurred in 6 per 1,000 RA patient-years, 2.4 times higher than the rate of age, sex, index date-matched patients without RA.
There was a 40% increased risk of developing VTE in RA patients compared with non-RA patients after adjusting for baseline VTE risk factors.
One-third of patients who developed VTE had at least one major VTE risk factors such as acute hospitalization, surgery and malignancy diagnosis 90 days before and after the VTE event.
Acknowledgments
Kim is supported by the NIH grant K23 AR059677. Kim has full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Schneeweiss is principal investigator of the Brigham and Women's Hospital DEcIDE Center on Comparative Effectiveness Research, funded by the Agency for Healthcare Research and Quality, and of the Harvard-Brigham Drug Safety and Risk Management Research Contract, funded by the US Food and Drug Administration.
Solomon is supported by the NIH grants K24 AR055989, P60 AR047782, R21 DE018750, and R01 AR056215.
Kim received research support from Takeda Pharmaceuticals North America and Pfizer and tuition support for the Pharmacoepidemiology Program at the Harvard School of Public Health funded by Pfizer and Asisa.
Schneeweiss received consulting fees from WHISCON, LLC and Booz & Company and research grants from Pfizer, Novartis and Boehringer Ingelheim.
Solomon received research support from Abbott Immunology, Amgen and Lilly. He serves in unpaid roles on two analgesic trials sponsored by Pfizer.
Footnotes
Competing interests
Liu has nothing to disclose for financial support or conflict of interest
REFERENCES
- 1.White RH. The epidemiology of venous thromboembolism. Circulation. 2003;107:I4–I8. doi: 10.1161/01.CIR.0000078468.11849.66. [DOI] [PubMed] [Google Scholar]
- 2.Anderson FAJ, Spencer FA. Risk factors for venous thromboembolism. Circulation. 2003;107:I9–I16. doi: 10.1161/01.CIR.0000078469.07362.E6. [DOI] [PubMed] [Google Scholar]
- 3.Goldhaber SZ, Bounameaux H. Pulmonary embolism and deep vein thrombosis. Lancet. 2012;379:1835–1846. doi: 10.1016/S0140-6736(11)61904-1. [DOI] [PubMed] [Google Scholar]
- 4.Solomon DH, Kremer J, Curtis JR, et al. Explaining the cardiovascular risk associated with rheumatoid arthritis: traditional risk factors versus markers of rheumatoid arthritis severity. Ann Rheum Dis. 2010;69:1920–1925. doi: 10.1136/ard.2009.122226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Solomon DH, Karlson EW, Rimm EB, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 6.Del Rincón I, Williams K, Stern MP, et al. Association between carotid atherosclerosis and markers of inflammation in rheumatoid arthritis patients and healthy subjects. Arthritis Rheum. 2003;48:1833–1840. doi: 10.1002/art.11078. [DOI] [PubMed] [Google Scholar]
- 7.van Halm VP, Peters MJ, Voskuyl AE, et al. Rheumatoid arthritis versus diabetes as a risk factor for cardiovascular disease: a cross-sectional study, the CARRE Investigation. Ann Rheum Dis. 2009;68:1395–1400. doi: 10.1136/ard.2008.094151. [DOI] [PubMed] [Google Scholar]
- 8.Fox EA, Kahn SR. The relationship between inflammation and venous thrombosis. A systematic review of clinical studies. Thromb Haemost. 2005;94:362–365. doi: 10.1160/TH05-04-0266. [DOI] [PubMed] [Google Scholar]
- 9.van der Poll T, Büller HR, ten Cate H, et al. Activation of coagulation after administration of tumor necrosis factor to normal subjects. N Engl J Med. 1990;322:1622–1627. doi: 10.1056/NEJM199006073222302. [DOI] [PubMed] [Google Scholar]
- 10.Folsom AR, Lutsey PL, Astor BC, et al. C-reactive protein and venous thromboembolism. A prospective investigation in the ARIC cohort. Thromb Haemost. 2009;102:615–619. doi: 10.1160/TH09-04-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luxembourg B, Schmitt J, Humpich M, et al. Cardiovascular risk factors in idiopathic compared to risk-associated venous thromboembolism: A focus on fibrinogen, factor VIII, and high-sensitivity C-reactive protein (hs-CRP) Thromb Haemost. 2009;102:668–675. doi: 10.1160/TH-09-02-0104. [DOI] [PubMed] [Google Scholar]
- 12.Bacani AK, Gabriel SE, Crowson CS, et al. Noncardiac vascular disease in rheumatoid arthritis: increase in venous thromboembolic events? Arthritis Rheum. 2012;64:53–61. doi: 10.1002/art.33322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matta F, Singala R, Yaekoub A, et al. Risk of venous thromboembolism with rheumatoid arthritis. Thromb Haemost. 2009;101:134–138. [PubMed] [Google Scholar]
- 14.Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: record-linkage study. BMC Med. 2011;9:1. doi: 10.1186/1741-7015-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannesdottir SA, Schmidt M, Horváth-Puhó E, et al. Autoimmune skin and connective tissue diseases and risk of venous thromboembolism: a population-based case-control study. J Thromb Haemost. 2012;10:815–821. doi: 10.1111/j.1538-7836.2012.04666.x. [DOI] [PubMed] [Google Scholar]
- 16.Zöller B, Li X, Sundquist J, et al. Risk of pulmonary embolism in patients with autoimmune disorders: a nationwide follow-up study from Sweden. Lancet. 2012;379:244–249. doi: 10.1016/S0140-6736(11)61306-8. [DOI] [PubMed] [Google Scholar]
- 17.Choi HK, Rho YH, Zhu Y, et al. The risk of pulmonary embolism and deep vein thrombosis in rheumatoid arthritis: a UK population-based outpatient cohort study. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201669. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 18.Holmqvist M, Neovius M, Eriksson J, et al. Risk of venous thromboembolism in patients with rheumatoid arthritis and association with disease duration and hospitalization. JAMA. 2012;308:1350–1356. doi: 10.1001/2012.jama.11741. [DOI] [PubMed] [Google Scholar]
- 19.Kim SY, Servi A, Polinski JM, et al. Validation of rheumatoid arthritis diagnoses in health care utilization data. Arthritis Res Ther. 2011;13:R32. doi: 10.1186/ar3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White RH, Garcia M, Sadeghi B, et al. Evaluation of the predictive value of ICD-9-CM coded administrative data for venous thromboembolism in the United States. Thromb Res. 2010;126:61–67. doi: 10.1016/j.thromres.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 21.Tagalakis V, Kahn SR. Determining the test characteristics of claims-based diagnostic codes for the diagnosis of venous thromboembolism in a medical service claims database. Pharmacoepidemiol Drug Saf. 2011;20:304–307. doi: 10.1002/pds.2061. [DOI] [PubMed] [Google Scholar]
- 22.Deyo R, Cherkin D, Ciol M. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 23.Schneeweiss S, Seeger J, Maclure M, et al. Performance of comorbidity scores to control for confounding in epidemiologic studies using claims data. Am J Epidemiol. 2001;154:854–864. doi: 10.1093/aje/154.9.854. [DOI] [PubMed] [Google Scholar]
- 24.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005;162:975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 25.Rothman K, Greenland S, Lash T. Modern Epidemiology. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 26.Cox D. Regression models and life-tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 27.Zöller B, Li X, Sundquist J, et al. Autoimmune diseases and venous thromboembolism: a review of the literature. Am J Cardiovasc Dis. 2012;2:171–183. [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson FJ, Wheeler H. Physician practices in the management of venous thromboembolism: a community-wide survey. J Vasc Surg. 1992;16:707–714. doi: 10.1067/mva.1992.41080. [DOI] [PubMed] [Google Scholar]
- 29.Nicolaides AN, Irving D. Clinical factors and the risk of deep venous thrombosis. In: Nicolaides AN, editor. Thromboembolism: Etiology, advances in prevention, and management. Baltimore, MD: University Park Press; 1975. [Google Scholar]
- 30.Anderson FAJ, Wheeler HB, Goldberg RJ, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–938. [PubMed] [Google Scholar]
- 31.Silverstein M, Heit J, Mohr D, et al. Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 32.Heit JA, O'Fallon WM, Petterson TM, et al. Relative impact of risk factors for deep vein thrombosis and pulmonary embolism: a population-based study. Arch Intern Med. 2002;162:1245–1248. doi: 10.1001/archinte.162.11.1245. [DOI] [PubMed] [Google Scholar]
- 33.Tosetto A, Iorio A, Marcucci M, et al. Predicting disease recurrence in patients with previous unprovoked venous thromboembolism: a proposed prediction score (DASH) J Thromb Haemost. 2021;10:1019–1025. doi: 10.1111/j.1538-7836.2012.04735.x. [DOI] [PubMed] [Google Scholar]
- 34.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58:323–337. doi: 10.1016/j.jclinepi.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Korswagen L, Bartelds G, Krieckaert C, et al. Venous and arterial thromboembolic events in adalimumab-treated patients with antiadalimumab antibodies: a case series and cohort study. Arthritis Rheum. 2011;63:877–883. doi: 10.1002/art.30209. [DOI] [PubMed] [Google Scholar]
- 36.Makol A, Grover M, Guggenheim C, et al. Etanercept and venous thromboembolism: a case series. J Med Case Rep. 2010;4:12. doi: 10.1186/1752-1947-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.