Abstract
Novelty processing was studied in patients with lesions centered in either OFC or lateral pFC (LPFC). An auditory novelty oddball ERP paradigm was applied with environmental sounds serving as task irrelevant novel stimuli. Lesions to the LPFC as well as the OFC resulted in a reduction of the frontal Novelty P3 response, supporting a key role of both frontal subdivisions in novelty processing. The posterior P3b to target sounds was unaffected in patients with frontal lobe lesions in either location, indicating intact posterior cortical target detection mechanisms. LPFC patients displayed an enhanced sustained negative slow wave (NSW) to novel sounds not observed in OFC patients, indicating prolonged resource allocation to task-irrelevant stimuli after LPFC damage. Both patient groups displayed an enhanced NSW to targets relative to controls. However, there was no difference in behavior between patients and controls suggesting that the enhanced NSW to targets may index an increased resource allocation to response requirements enabling comparable performance in the frontal lesioned patients. The current findings indicate that the LPFC and OFC have partly shared and partly differential contributions to the cognitive subcomponents of novelty processing.
INTRODUCTION
The pFC constitutes about one third of the human cortex (Stuss & Benson, 1986) and has extensive bidirectional connections to other cortical and subcortical regions (Petrides & Pandya, 2002). This neuroanatomical organization places pFC in a unique position to monitor and control diverse human behaviors with lesions to the frontal lobes resulting in problems with higher-order control of cognition, emotion, and behavior. There is emerging consensus that there is no unitary executive function. Rather, subregions within the frontal lobes are associated with distinct cognitive functions supporting the general concept of cognitive control (Stuss & Alexander, 2000). One major functional anatomical distinction is between lateral pFC (LPFC) and OFC with each region having multiple subareas. Although the LPFC is primarily associated with cognitive executive functions such as controlled attention, working memory, goal selection, planning, sequencing, and set shifting (Royall, 2002), injury to the OFC is associated with altered self-regulatory behavior such as poorly modulated emotional reactions and social interactions and defective decision-making. OFC damage tends to affect the ability to utilize cues in the environment to predict future rewarding or aversive events and the ability to regulate behavioral responses, particularly in the context of changing reinforcement contingencies. Lack of insight into the consequences of the brain injury is typical after OFC damage (Koenigs & Tranel, 2006; Stuss & Levine, 2002).
Although the cognitive executive problems following LPFC lesions are more likely to be detected in neuropsychological evaluations, patients with OFC injury will often display normal results on formal cognitive evaluations despite marked problems with “real-life” decision-making, such as maladaptive personal, social, and occupational functioning (Zald & Andreotti, 2010).
A prominent clinical symptom in patients with frontal lobe injury is a reduced ability to adapt efficiently to changed requirements from their environment (Stuss & Levine, 2002). Coping with change is a prerequisite for survival because failure to detect and respond to salient changes in our surroundings could be fatal. This process of novelty detection is related to the orienting response (Sokolov, 1963), enabling the redirection of attention toward a new stimulus. When a stimulus is perceptually salient, this reorienting of attention is largely reflexive (Corbetta, Patel, & Shulman, 2008), although there is evidence that the automatic bottom–up driven reorienting is also modulated by the top–down attentional set of the subject (Chong et al., 2008; Folk, Leber, & Egeth, 2002) or by the degree of task relevance of the stimulus (Yantis & Egeth, 1999). Subsequent to detecting the occurrence of a novel event, there is a need to rapidly evaluate the significance of this change and to decide whether action is called for.
The ERP method provides a physiological probe well suited to address psychological theories of frontal lobe function (Stuss, Shallice, Alexander, & Picton, 1995). Two frontally distributed ERP components have been proposed to represent a neurophysiological marker of the orienting response; the Novelty P3 and a later negative slow wave (NSW) with a frontal scalp distribution (Rohrbaugh, Syndulko, & Lindsley, 1979; Kok, 1978).
The P3 complex is one of the most widely studied ERP components (for comprehensive reviews, see Polich, 2007; Polich & Criado, 2006; Friedman, Cycowicz, & Gaeta, 2001; Kok, 2001; Soltani & Knight, 2000). The P3b component with a positive polarity and parietal maximum is associated with voluntary target detection (Soltani & Knight, 2000), whereas the earlier and more frontocentrally distributed Novelty P3 is elicited by infrequent, task-irrelevant, but perceptually salient, stimuli (Courchesne, Hillyard, & Galambos, 1975; Squires, Squires, & Hillyard, 1975). The Novelty P3 has been considered to be a neurophysiological marker of the orienting response (Debener, Makeig, Delorme, & Engel, 2005; Debener, Kranczioch, Herrmann, & Engel, 2002; Soltani & Knight, 2000). Although frontal brain structures contribute to generation of the Novelty P3, parietal cortices and the TPJ are associated with the target P3b (Mecklinger & Ullsperger, 1995).
Deviant stimuli can also generate a frontally distributed NSW in the same time window as the posterior P3b (Spencer, Dien, & Donchin, 2001; Näätänen, 1992). The NSW has been discussed in relation to the orienting response and is typically observed in contingent negative variation paradigms where an initial stimulus (S1) signals that the second and imperative stimulus (S2) will follow (Walter, 1964). Rohrbaugh and colleagues (1979) propose that the NSW represents a nonspecific cortical activation reflecting the transient appearance of alerting, orienting, arousal, or activation. The frontal NSW is sensitive to task load, aspects of encoding and retrieval from long-term memory and working memory (Ruchkin, Canoune, Johnson, & Ritter, 1995). Although some debate persists as to what specific cognitive operations are indexed by late slow waves, all current theories of the NSW link this ERP to the level of mental processing (Ruchkin et al., 1995; Ritter & Ruchkin, 1992).
Earlier latency ERP components preceding the P3, such as the N1 and P2 components, are modulated by top–down processes. Knight, Hillyard, Woods, and Neville (1980) showed that, although LPFC damage resulted in an enhanced N1 component, the following P2 was normal, a finding that was interpreted as a demonstration of altered inhibitory control over sensory processing because of prefrontal deficit. Thus, the process of detecting salient novel events is performed using interrelated cognitive operations, where the Novelty P3 represents an important but not exclusive part of the novelty-processing cascade.
Studies of patients with heterogeneous lesion distributions provide mixed results, reporting both attenuation (Solbakk, Reinvang, & Andersson, 2002) and enhancement (Kaipio et al., 1999) of the Novelty P3. Studies of patients with focal brain lesions have, however, provided a strong case for anatomical network specificity. Knight and Scabini (1998) summarized several studies showing that focal lesions to the LPFC result in reduced Novelty P3 amplitudes in visual, auditory, and somatosensory tasks. Superior parietal lesions affect neither the P3b to targets nor the Novelty P3, but lesions to the TPJ attenuate both components. The reduction of the Novelty P3 amplitude following frontal lobe lesions has been confirmed by Daffner and colleagues (2000, 2003). Importantly, these studies demonstrated shorter viewing time to visual novel events in patients with frontal lobe damage, providing key behavioral evidence that patients with frontal lobe injuries exhibit reduced orienting behavior to novel events. Similarly, LPFC lesions eliminate the classic von Restorff memory boost seen in normal subjects for novel events (Kishiyama, Yonelinas, & Knight, 2009).
Whereas the role of the LPFC has been documented, the role of OFC in novelty processing is not well defined. In one study, four OFC patients were reported to have enhanced Novelty P3s, but the stimuli were embedded in an emotionally laden context (Rule, Shimamura, & Knight, 2002). Another study with traumatic brain injury (TBI) patients (Kaipio et al., 1999) also reported enhanced P3s, but the lesions were of mixed etiology compromising strong conclusions on the role of OFC in novelty processing.
In the present study, we examined a large cohort of OFC patients in a cognitive task with no emotional component. An auditory novelty oddball paradigm was administered to one patient group with OFC damage (OFC group) and one with LPFC damage (LPFC group). On the basis of previous studies, we hypothesized that LPFC lesions would result in altered novelty detection reflected in a reduction of the Novelty P3 amplitude (Daffner et al., 2000, 2003; Knight, 1984). Rule et al. (2002) reported an enhanced novelty response in patients with OFC damage. However, as noted, these findings were derived from a design involving affectively laden stimuli and would not necessarily apply to a paradigm where the environmental novels are presented in an emotionally neutral task context. The extant literature did thus not allow for strong predictions about the effects of OFC lesions.
A second objective was to examine the contributions of OFC or LPFC lesions to other aspects of the Novelty processing cascade, indexed by alterations in ERP components both preceding and following the P3 complex. The extant literature suggests an increase in N1 amplitudes after LPFC lesions. As for the P3 complex, previous studies did not give rise to strong predictions about the N1 after OFC lesions. It was expected that later parts of the orienting response would be indexed by slow negative waveforms. Although it is well known that oddball paradigms tend to elicit NSWs following the P3 complex, the literature did not provide a specific hypothesis regarding the effect on this aspect of novelty processing after focal frontal brain injury, and this part of the analysis was exploratory.
METHODS
Participants
Nineteen patients with prefrontal lesions and 15 healthy controls were included in the study. All subjects were right handed. The OFC group consisted of 13 patients, and the LPFC group consisted of 6 patients (see Table 1 and Figures 1 and 2 for patient characteristics). The OFC group consisted of four patients with traumatic brain injury (TBI), one with low-grade glioma (LGG), and the remaining eight who had undergone resection of large meningiomas. The majority (10 of 13) of OFC patients had bilateral damage. All patients in the LPFC group had unilateral lesions because of LGG. All patients with tumors had gone through surgical tumor resection. None of the patients had received radiation therapy, whereas one patient in the LPFC group had received chemotherapy.
Table 1.
Lesion Characteristics: Etiology, Time since Injury, Lesion Volume, and Affected Brodmann’s Areas
| Subject | Etiology | Time since Injury (months) |
Lesion Volume (ccm) |
BA Right Hemisphere | BA Left Hemisphere |
|---|---|---|---|---|---|
| OFC group mean | 33 | Total: 50.3 | |||
| RH: 28.9 | |||||
| LH: 21.3 | |||||
| 1 | TBI | 45 | 140 | 6, 8, 9, 10, 11, 32, 45, 46, 47 | 8, 9, 10, 11, 32, 46, 47 |
| 2 | Meningioma | 13 | 69.1 | 10, 11, 32, 46, 47 | 10, 11, 47 |
| 3 | Meningioma | 48 | 79.8 | 10, 11, 47 | 10, 11, 46, 47 |
| 4 | Meningioma | 13 | 39.7 | 10, 11 | 10, 11, 47 |
| 5 | Meningioma | 19 | 5.1 | 11 | |
| 6 | Meningioma | 43 | 134.8 | 9, 10, 11, 32, 46, 47 | 9, 10, 11, 32, 46, 47 |
| 7 | Meningioma | 27 | 7.2 | 11, 47 | 11 |
| 8 | Meningioma | 44 | 2.9 | 10, 11 | |
| 9 | LGG | 7 | 28.6 | 10, 11, 25 | 11, 25 |
| 10 | TBI | 44 | 23.6 | 10, 11 | 11 |
| 11 | TBI | 59 | 33.3 | 10, 11, 47 | 10, 11, 46 |
| 12 | TBI | 15 | 41.1 | 11 | 10, 11, 38, 45, 46, 47 |
| 13 | Meningioma | 52 | 48.7 | 9, 10, 11, 32, 46, 47 | |
| LPFC group mean | 46 | Total: 33.8 | |||
| RH: 55.8 | |||||
| LH: 11.9 | |||||
| 1 | LGG | 30 | 34.4 | 8, 9, 32, 44, 45, 46 | |
| 2 | LGG | 27 | 24.8 | 4, 6, 9, 44 | |
| 3 | LGG | 68 | 60.1 | 4, 6, 8, 9, 32, 44, 45, 46 | |
| 4 | LGG | 112 | 72.8 | 6, 9, 32, 44, 45, 46, 47 | |
| 5 | LGG | 31 | 0.8 | 45 | |
| 6 | LGG | 9 | 10.1 | 6 |
Lesions that comprise<0.5 ccmin any given Brodmann’s area are not reported. BA=Brodmann’s area, RH=right hemisphere, LH=left hemisphere; TBI=traumatic brain injury; LGG = low-grade glioma.
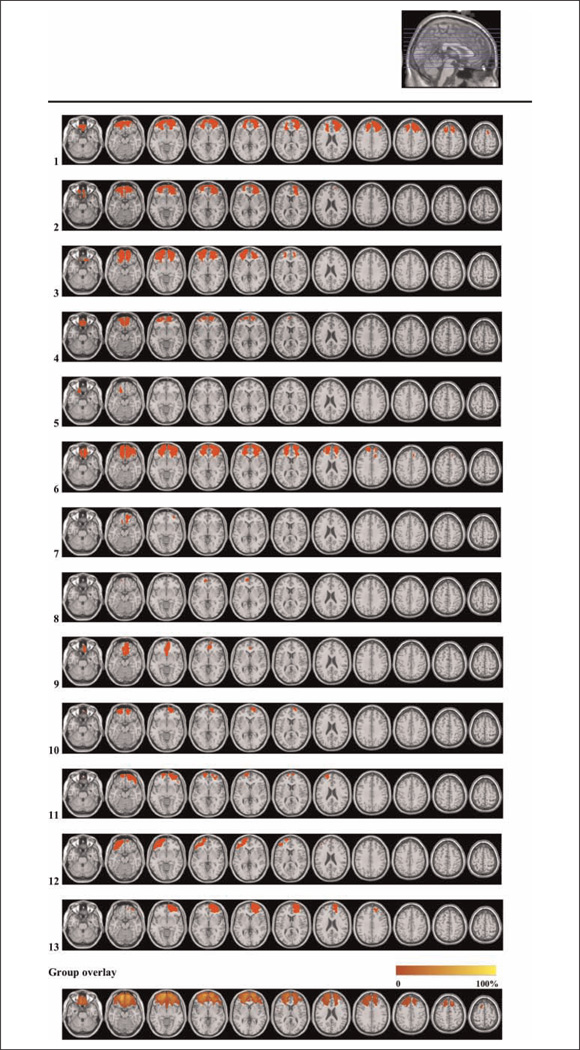
Figure 1.
Lesion reconstructions for the OFC group. Individual patients (1–13) and group overlay (bottom row). Eighty-two percent of the cortical lesion volume was within Brodmann’s areas 10, 11, and 47.
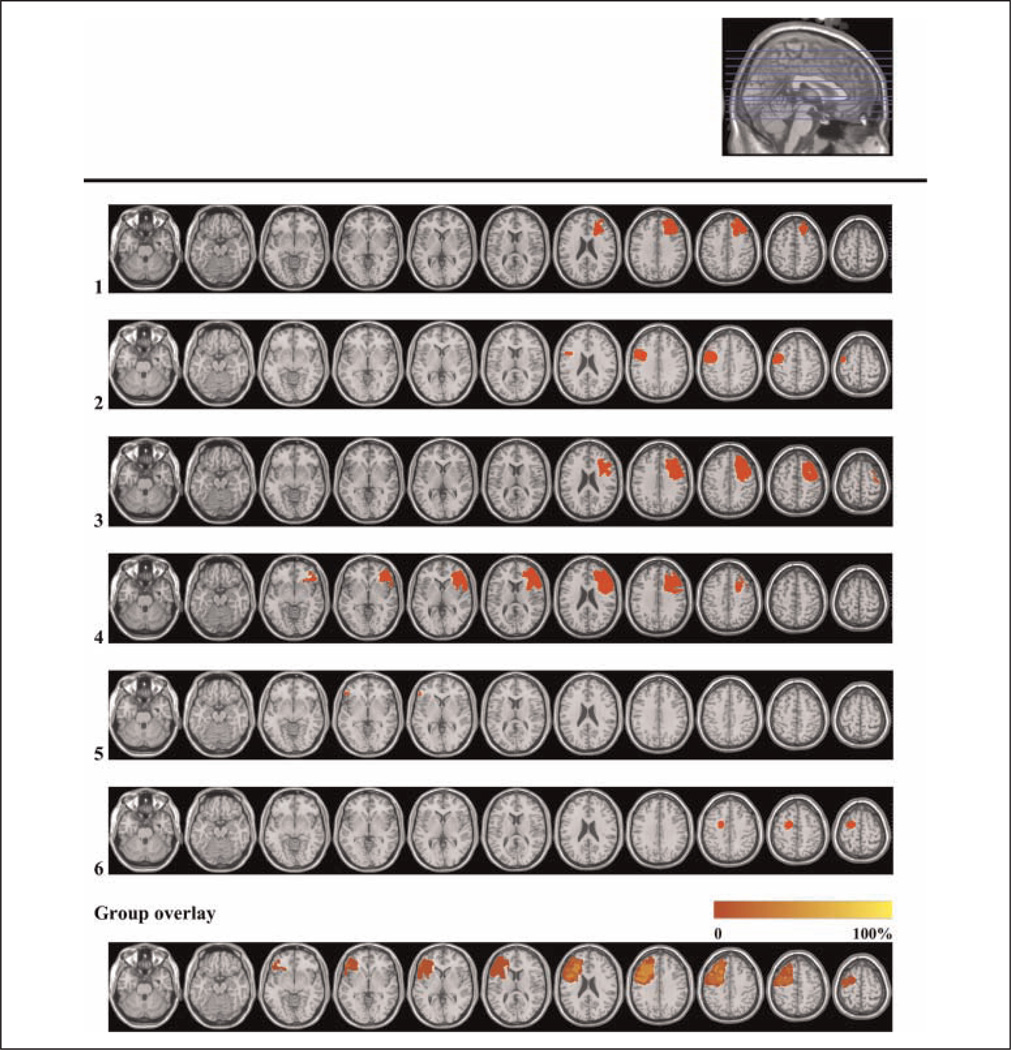
Figure 2.
Lesion reconstructions of the LPFC group. Individual patients (1–6) and group overlay (bottom row). Eighty percent of the cortical lesion volume was within Brodmann’s areas 6, 8, 9, 44, 45, and 46.
Patient inclusion was based on preexisting frontal brain lesions indicated on structural CT and/or MRI scans. Lesion reconstructions were based on structural MRIs obtained after inclusion and have been verified by the neuroradiologist, neurologist, and neurosurgeon in the research group (P. Due-Tønnessen, R. T. Knight, & T. Meling). Testing took place at least 6 months after injury or surgery. Patients were matched with healthy controls by age, sex, and years of education (Table 2). Participants with a history of serious psychiatric disease, drug or alcohol abuse requiring treatment, premorbid head injury, pre-/comorbid neurological disease, IQ below 85, substantial aphasia, visual neglect, or marked sensory impairment were excluded from participation.
Table 2.
Subject Characteristics
| Control | OFC | LPFC | ANOVA | |
|---|---|---|---|---|
| N (% women) | 15 (53) | 13 (54) | 6 (33) | |
| Age in years | 41.6 (12.2) | 45.92 (10.10) | 46.17 (7.25) | ns |
| Education in years | 13.2 (2.1) | 13.0 (2.38) | 14.17 (2.56) | ns |
| Total IQ | 111.93 (9.9) | 107.85 (11.87) | 103.83 (16.92) | ns |
| Performance IQ | 111.6 (9.9) | 109.77 (13.2) | 105 (14.97) | ns |
| Verbal IQ | 109.07 (9.6) | 103.92 (11.71) | 102.33 (17.51) | ns |
Values given are mean (±SD).
The functional outcome of the patients was classified with the Glasgow Outcome Scale Extended (GOS-E; Wilson, Pettigrew, & Teasdale, 1998). GOS-E is a hierarchical scale in which overall rating is based on the lowest outcome indicated. Total, verbal, and performance IQ were estimated on the basis of all four subtests of the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). Two subtests were selected from the WAIS-III and Digit Span and Letter–Number Sequencing (Wechsler, 1997). The following four subtests from the Delis–Kaplan Executive Function System were included: Trail Making Test, Design Fluency, Verbal Fluency, and Color–Word Interference Test (Delis, Kaplan, & Kramer, 2001). Screening of emotional distress was performed using the Symptom Checklist 90 Revised (SCL-90-R; Derogatis, 1994). The presence of obsessive–compulsive symptoms was explored using the Obsessive–Compulsive Inventory Revised (OCI-R; Foa et al., 2002).
Patients and controls gave their informed consent to participation. Controls were paid NOK 500 (approximately USD 80) for participation in the entire research program that included neuropsychological assessment, EEG, as well as structural and functional MRI examination. The study was approved by the Regional Committee for Medical Research Ethics, Region South, and was conducted in agreement with the Helsinki declaration.
Task
Subjects were seated 1 m from a 24-in. computer screen. They were instructed to fixate on a star in the center of the screen during data acquisition. Auditory stimuli were presented binaurally through stereo headphones. The novelty oddball paradigm consisted of 280 (70%) 1000-Hz tones designated standard and 60 (15%) designated target tones of 1500 Hz presented in a pseudorandomized order where a target tone was never followed by another target. The duration of standard and target tones was 50 msec. Sixty (15%) unique environmental sounds (e.g., dog barks, door slams, and laughter) with matched intensity and a presentation time of 400 msec were interspersed in a pseudorandomized order. A novel stimulus never preceded a target tone or another novel. Subjects were instructed to press a button to target stimuli with the index finger of their dominant hand and to ignore all other sounds. They were asked to respond as fast and as accurately as possible. The experiment was presented in two blocks containing 140 standard, 30 target, and 30 novel stimuli each. A training session containing 15 standard tones and five targets, but no novel stimuli, was presented before EEG recording started. Subjects were not informed that novel stimuli would appear during the experimental run. Stimulus presentations and response recordings were controlled using E-prime software, version 2.0 (Psychology Software Tools, Pittsburgh, PA).
EEG Recording
EEG data were acquired using a 128-channel HydroCel Geodesic Sensor Net and Net Amps 300 amplifier (Electrical Geodesics, Eugene, OR). Impedance was kept below 100 kΩ (Ferree, Luu, Russell, & Tucker, 2001). Recordings were initially referenced to Cz and subsequently re-referenced to an average reference before data analysis. EEG signals were sampled at 250 Hz with a 24-bit analog-to-digital converter and a DC to 125-Hz band pass.
ERP Analysis
ERP analyses and identification of peaks/computation of mean amplitudes from averaged ERP waveforms was carried out using Net Station, Version 4.3.1 software (Electrical Geodesics, Eugene, OR). Continuous EEG data were filtered off-line with 0.3-Hz high pass and 20-Hz low pass filters. Data were epoched, time-locked to stimulus onset in segments from −100 to 900 msec. Artifact detection, artifact correction, and bad channel interpolation were performed using Net Station custom procedures. Channels were marked as bad throughout the entire recording if bad in more than 20% of the segments, and segments were defined as bad if they contained more than 10 bad channels as defined by the computer algorithm or visual inspection. Averaged ERPs were based on correct trials for the three stimulus types (standard, target, and novel).
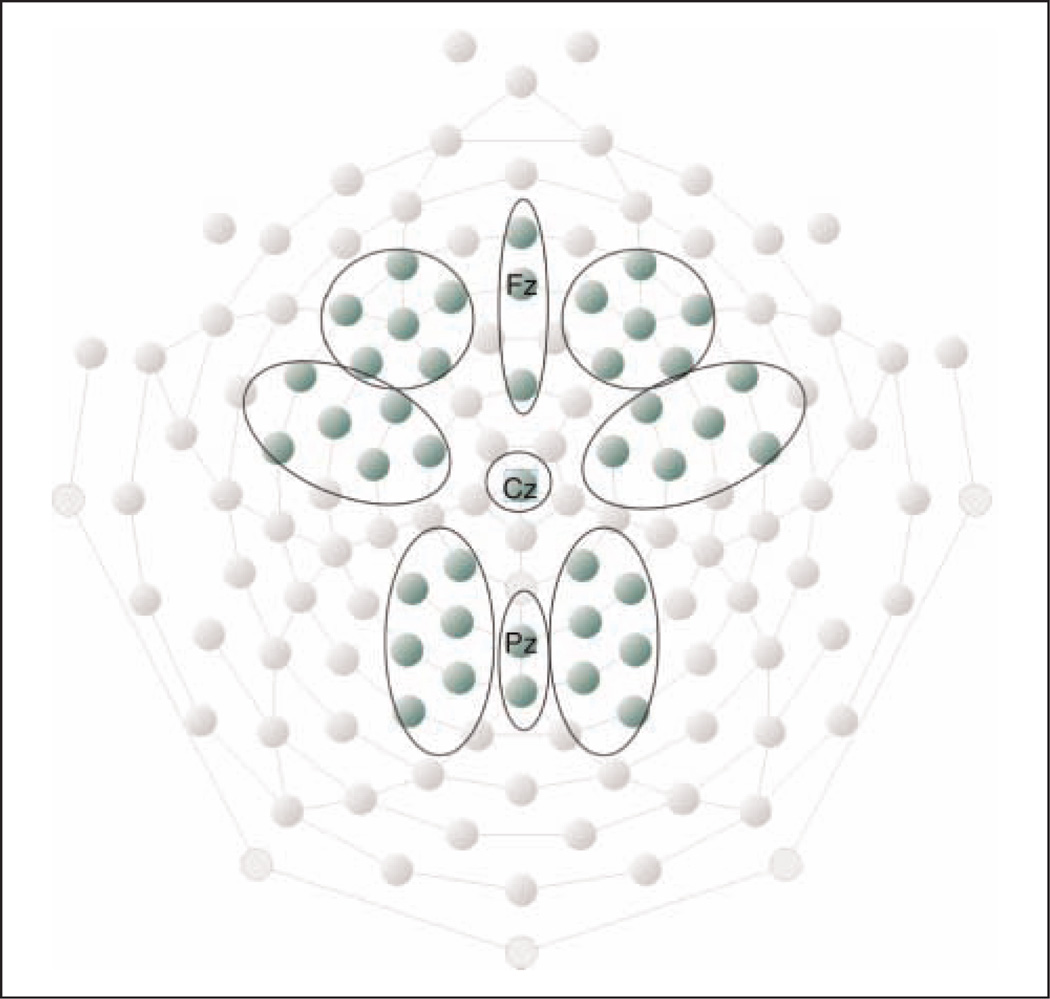
ROI electrode groups were established as shown in Figure 3 with the following anatomical sites: one right, midline, and left frontal group; one right and left frontocentral group with Cz as midline electrode; and one right, left, and midline parietal ROI. Statistical analyses and illustrations were performed on extracted mean values over electrodes in each ROI. All patients in the LPFC group had unilateral lesions, with three patients having right hemisphere lesions. In statistical analyses and illustrations, the electrodes of the group with right hemisphere lesions are exchanged so that left hemisphere electrodes are synonymous with lesioned hemisphere for the whole LPFC group.
Figure 3.
ROI electrode groups. Three electrode groups were established along the anterior–posterior axis (frontal, frontocentral, and parietal) and three groups along the right–left axis (right, center, and left).
The ERP amplitudes and latencies were extracted as follows:
N1: N1 peak was defined as the most negative point 60–120 msec poststimulus. Latency of this point was derived for all three stimulus types and analyzed over frontal and frontocentral electrode groups.
P2: Because of the temporal overlap between the P2 and the P3a component for deviant tones, P2 mean amplitude 100–250 msec poststimulus was analyzed over frontal and frontocentral electrode groups for standard tones only.
N2: The deviance related negativity (N2) is best observed in difference waveforms where the ERP to standard tones is subtracted from ERPs to novel and target sounds. The N2 appeared at a shorter latency to novel stimuli compared with targets. Peak amplitude and latency for novel N2 was derived as the most negative point 125–300 msec after stimulus onset and target N2 as the most negative point 150–300 msec. The N2 was analyzed statistically over the frontocentral midline electrodes Cz and Fcz.
P3b to target: P3b peak amplitude and latency was derived at the most positive amplitude 300–500 msec poststimulus over parietal electrode groups. Mean amplitude in the 300–500 msec time window was also computed.
Novelty P3: Peak amplitude and latency was analyzed at the most positive point 270–400 msec poststimulus with all ROI electrode groups included in the initial overall analysis. Mean amplitude for the same time interval was also calculated. Habituation of the Novelty response was studied by comparing the mean amplitude of the first three novel stimuli over the frontal midline electrodes with the mean amplitudes of novel stimuli numbers 4–6 and 7–9, respectively.
Sustained late negativity: A sustained late NSW following P3 was seen over frontal and frontocentral electrode groups. Visual inspection of group averaged ERPs revealed that the NSW had shorter duration for the target stimuli compared with the novels. Accordingly, the NSW was independently analyzed as the mean amplitude 400–600 msec (early NSW) and 600–800 msec (late NSW) poststimulus over frontal and frontocentral electrode groups for target and novel deviant stimuli.
Statistical Methods
SPSS 17.0 for Windows (SPSS, Inc., Chicago, IL) was used for statistical analyses. ERP data were analyzed using repeated measures ANOVA. There were three levels of stimulus type (standard, target, and novel), three levels of electrode groups along the anterior–posterior axis (frontal, frontocentral, and parietal), and three levels along the left/right (hemisphere) topographical axis (right, midline, and left). There were also three levels of the between-subject factor group (control, OFC, and LPFC group). Greenhouse–Geisser epsilon corrected p values along with uncorrected degrees of freedom are reported for computations involving more than two levels of a repeated measures factor. Analyses that yielded significant interactions between Group, Stimulus Type, Anterior–Posterior, or Hemisphere resulted in planned contrasts between the levels of the variable. In those cases where the patient groups differed from each other, lesion volume was entered as covariate in the ANOVA. Demographic, psychometric, and performance data were analyzed using one-way ANOVA with Group as between-subject factor. Bonferroni corrected p values are reported in post hoc analyses. Effects involving differences between patient groups and controls were of primary interest. The relationship between behavioral responses and ERP measures was explored with Pearson two-tailed correlation coefficients and comparisons of amplitudes over the hemispheres within groups were conducted with paired samples t test. Results are reported with a significance level of ≤.05.
RESULTS
Functional Outcome
Both patient groups had GOS-E scores categorizing them as “Moderately impaired–Upper Level” (OFC: 6.3, SD = 1.1; LPFC: 6.2, SD = 1.0), an outcome level that characterizes patients who are capable of living an independent life despite having disabilities because of the brain injury (Teasdale, Pettigrew, Wilson, Murray, & Jennett, 1998). The patient groups did not differ significantly from each other or the healthy controls in total, verbal, or performance IQ (see Table 2). Performance was within normal range, and there were no significant group effects on any of the Wechsler Abbreviated Scale of Intelligence, WAIS-III, or Delis–Kaplan Executive Function System subtests. However, there was a group effect on the Obsessive–Compulsive subscale of the SCL-90-R (F(2, 31) = 4.62, p < .02) because of the OFC group having higher scores than the controls (OFC: 10, SD = 5.8; controls: 3.5, SD = 4.6, p < .03). There was also a group effect on the Hostility subscale of the SCL-90-R (F(2, 31) = 5.71, p < .01) because of the LPFC group reporting more symptoms of irritability than the controls (LPFC: 3, SD = 2.9; controls: 0.38, SD = 0.7, p < .01). The OFC group reported more obsessive–compulsive symptoms on the OCI-R as well, as there was a significant group effect on the Ordering (F(2, 33) = 5.83, p < .01) and Hoarding (F(2, 32) = 3.58, p < .04) subscales. This effect was due to the OFC group reporting significantly more symptoms than controls on the Ordering subscale (OFC: 3.9, SD = 2.7; controls: 1.1, SD=1.5, p<.01) and near significantly more on the Hoarding subscale (OFC: 4.1, SD = 2.6; controls: 1.9, SD = 2.1, p < .06).
Performance Data
Table 3 displays mean hit rate and RT to targets, as well as false alarms to novels and standards. There were no statistically significant differences between groups. Both patient groups and healthy controls had a high hit rate to targets (>99%) and showed few commission errors to nontargets (<2%). RTs to targets did not differ significantly between groups.
Table 3.
Behavioral Results from the Novelty Oddball Task
| Control | OFC | LPFC | ANOVA | |
|---|---|---|---|---|
| Hit rate target (%) | 99.7 (0.9) | 99.6 (1.0) | 99.2 (2.0) | ns |
| False alarms (%) | ns | |||
| Standard | 0.6 (0.3) | 0.6 (0.3) | 0.7 (0.4) | ns |
| Novel | 1.2 (2.3) | 1.1 (1.4) | 1.1 (1.3) | ns |
| RT target (msec) | 381.9 (66.2) | 422.5 (73.5) | 421.9 (46.7) | ns |
Results are reported as mean values (±SD).
ERP Data
Overview
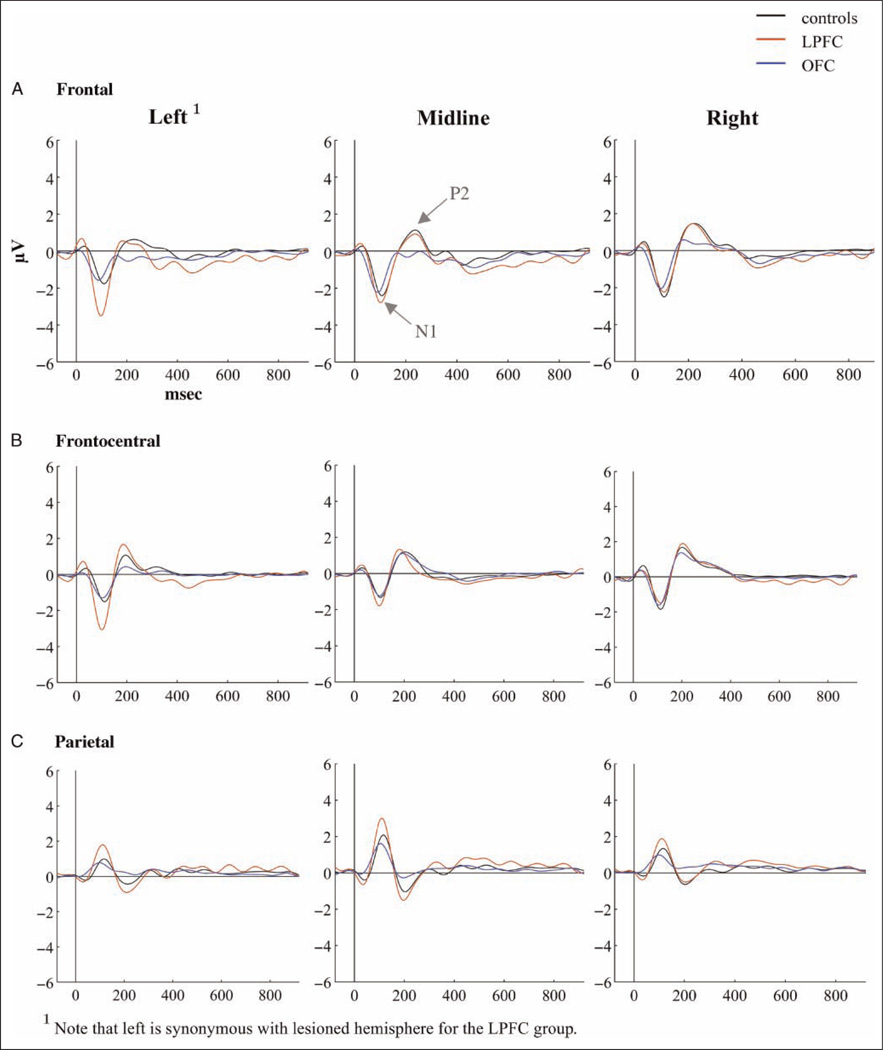
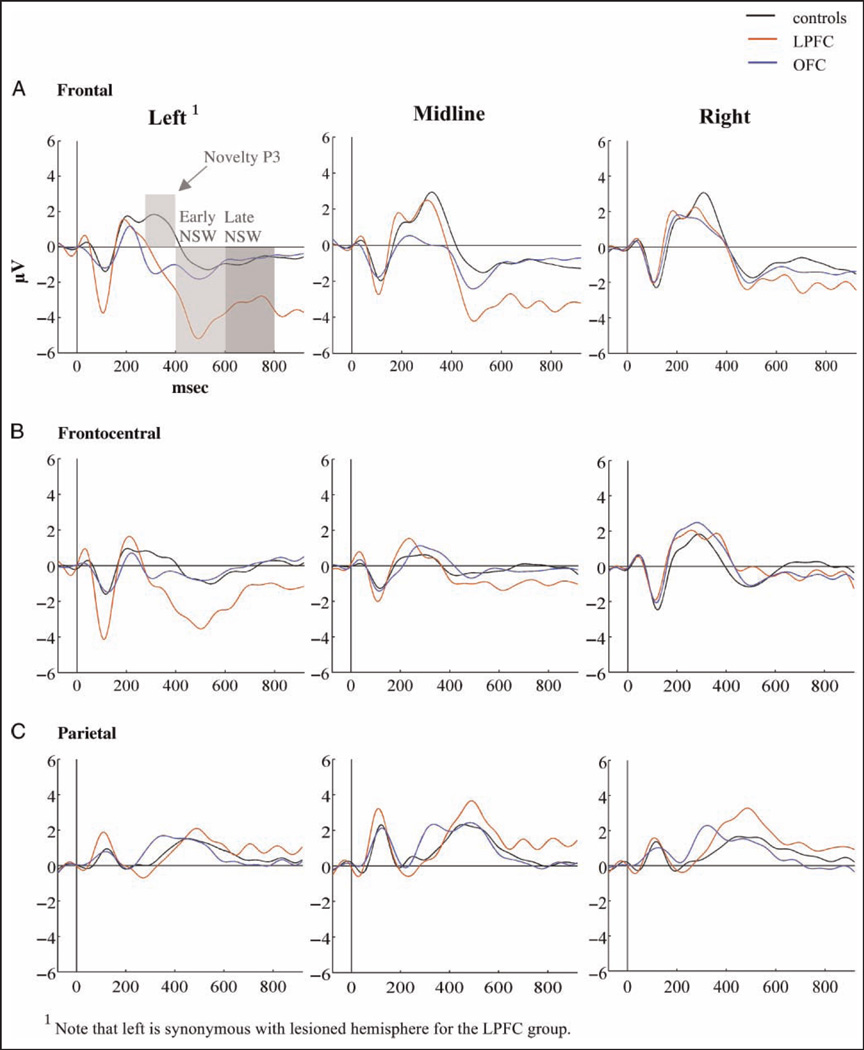
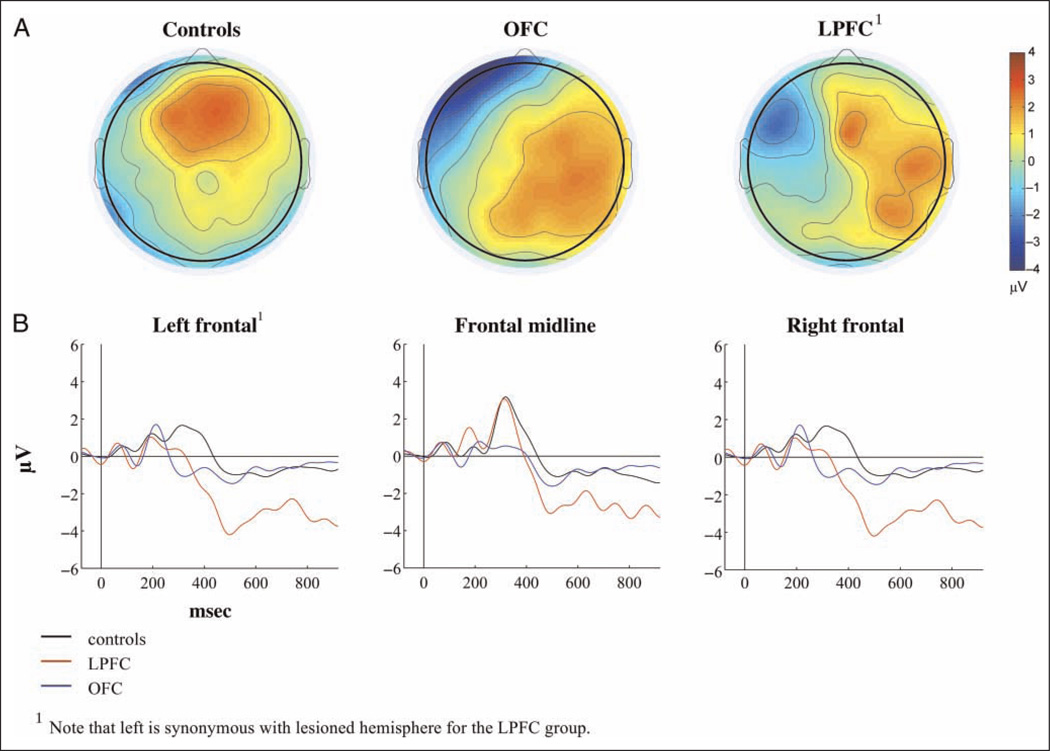
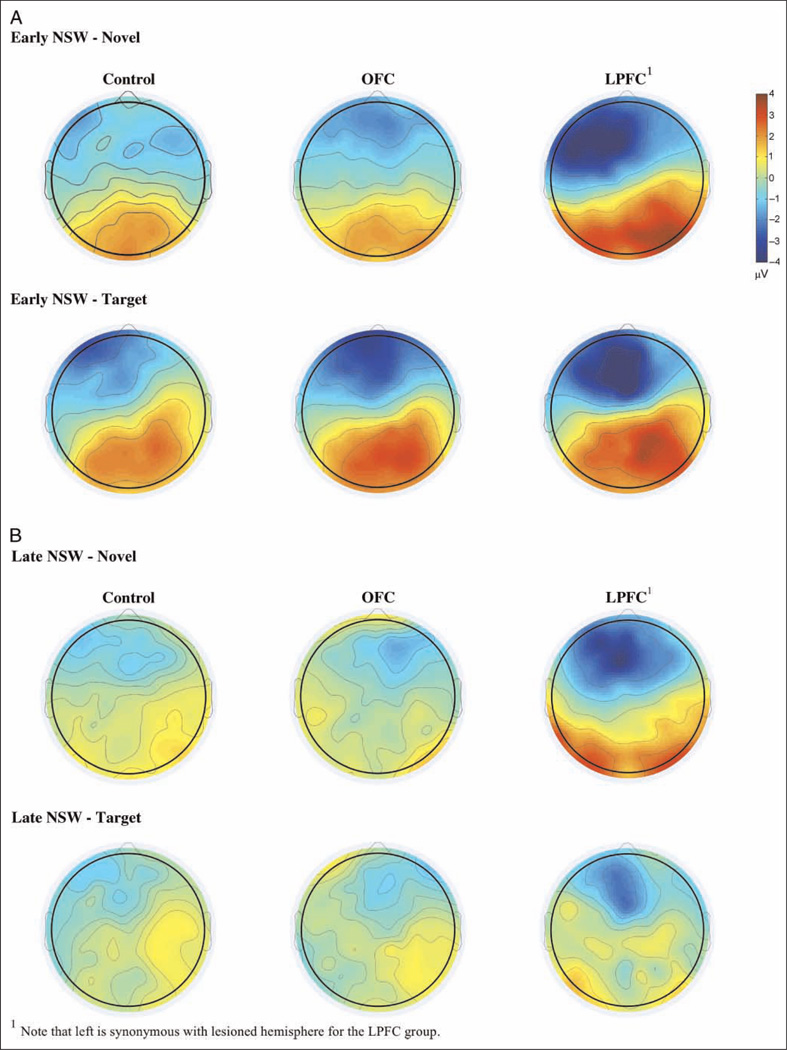
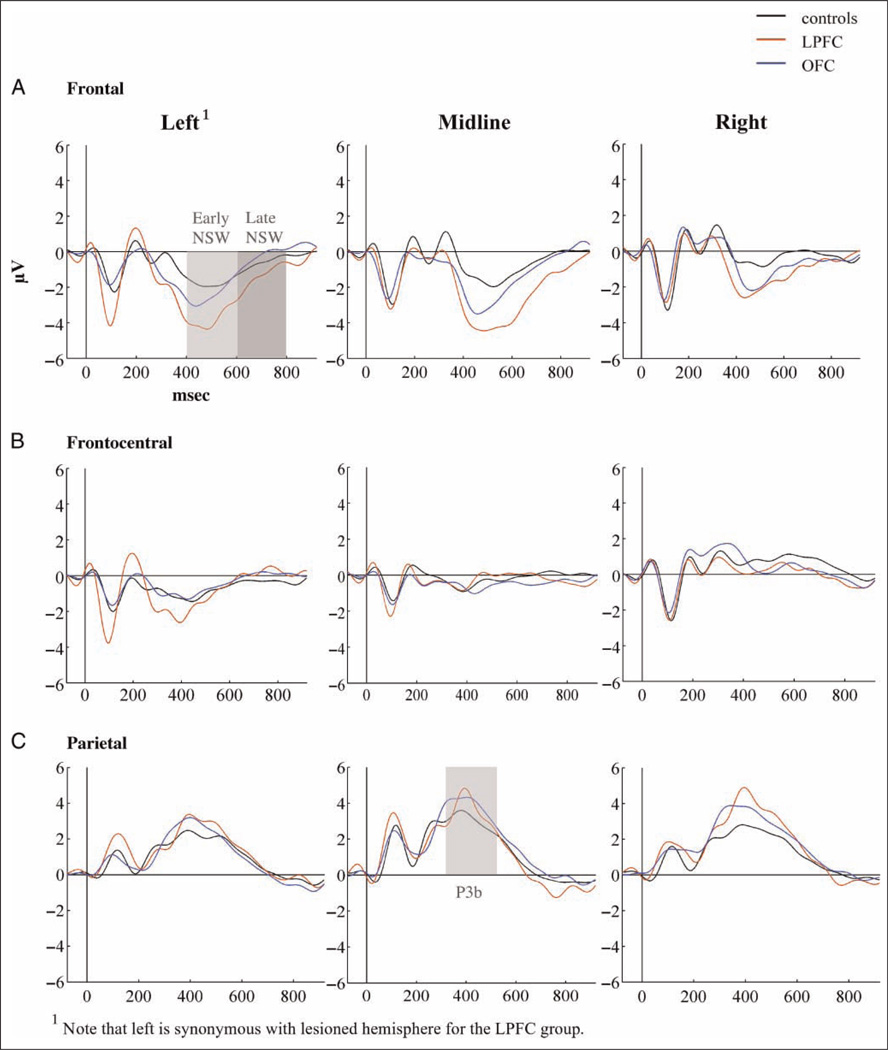
Grand average ERPs for each of the three groups to standard, target, and novel stimuli are presented in Figures 4–6. Scalp topographies for the Novelty P3 response along with ERP difference waves for novel minus standard tones over frontal electrode sites are depicted in Figure 7, and scalp topographies for the NSW to deviant sounds are illustrated in Figure 8. Visual inspection suggested that the task elicited the expected frontally distributed Novelty P3 to novel sounds (Figures 6A and 7) and that both patient groups displayed an amplitude reduction of the novelty response. The parietally maximal P3b to target stimuli was present in all groups (Figure 5C). Both types of deviant sounds elicited a frontal/frontocentral sustained NSW that was more pronounced with a longer duration for novel stimuli compared with targets. The NSW was particularly pronounced for the LPFC group (Figures 5A, 6A, and 8).
Figure 4.
ERPs to standard tones over (A) frontal, (B) frontocentral, and (C) parietal electrode groups.
Figure 6.
ERPs to novel sounds over (A) frontal, (B) frontocentral, and (C) parietal electrode groups.
Figure 7.
Novelty P3. (A) Scalp topography of the Novelty response (270–400 msec) for each group. (B) ERP difference waves (Novels minus standards) over frontal electrode groups.
Figure 8.
Scalp topographies for (A) early (400–600 msec) and (B) late (600–800 msec) NSW for each group.
Figure 5.
ERPs to target tones over (A) frontal, (B) frontocentral, and (C) parietal electrode groups.
Early Latency ERP Components—N1, P2 and N2
N1
Regardless of stimulus type, the LPFC group had enhanced N1 amplitudes over the lesioned hemisphere compared with both the OFC group ( p < .01) and controls ( p < .01). This effect was evident in a significant interaction between Hemisphere and Group (F(4, 62) = 9.61, p < .001). The OFC group and controls did not differ significantly from each other. Both patient groups displayed a shorter left/lesioned frontal N1 latency to targets than the control group (controls: 99.3 msec, SD = 7.9 msec; OFC: 88.5 msec, SD = 17 msec; LPFC: 90.78 msec, SD = 5.5 msec). This was seen in a significant effect of Group over this electrode location for target stimuli (F(2, 31) = 5.48, p < .01). The difference between OFC patients and controls was significant ( p < .05) and approached significance for the LPFC group compared with the controls ( p< .06). The two patient groups did not differ significantly from each other.
The LPFC patients had all undergone craniotomy, which could potentially influence amplitude levels over the lesioned hemisphere through current shunting caused by skull defects or to changes of current flow patterns due to the surgical resection cavities filled with cerebrospinal fluid. To partly address this issue, an analysis of signal-to-noise ratio over lesioned versus nonlesioned electrode sites was performed by computing mean standard deviations of the amplitudes in the baseline period. There was not a significantly larger variation in amplitudes over the lesioned compared with the nonlesioned hemisphere in the LPFC group ( p < .3), indicating that the signal-to-noise ratio was comparable across hemispheres.
P2
Over the frontal electrodes, there was a main effect of Group (F(2, 31) = 5.07, p < .01) as the OFC group had significantly smaller mean P2 amplitude to standards than the control group ( p < .001). The LPFC group did not differ significantly from either of the two other groups. See Figure 4.
N2
There were no significant main effects or interactions involving Group on amplitude (Fs=0.21–2.0, ps = .08–.94) or latency (Fs = 0.27–1.67, ps = .21–.76) of the N2 difference waves (target minus standard and novel minus standard computations).
ERPs to Target and Novel Deviants
Parietal P3b to targets
There was no significant main effect involving Group for the parietal P3b latency (F(2, 31) = .17, p < .85; controls: 386.1 msec, SD = 57.3; OFC: 394.9 msec, SD = 54.1; LPFC: 398.9 msec, SD = 28). The analysis revealed no significant main effect of Group on the parietal P3b peak (F(2, 31) = 1.17, p < .32) or mean (F(2, 31) = 1.34, p < .28) amplitude, as well as no significant interactions between Group and Hemisphere neither for the peak (F(4, 62) = 1.46, p < .23) nor the mean (F(4, 62) = 1.42, p < .24) amplitude analysis.
Novelty P3
There was no significant main Group latency effect for the frontal Novelty P3 (F(2, 31) = .36, p < .7; controls: 314.7 msec, SD = 17.6; OFC: 328.1 msec, SD = 34.5; LPFC: 309.5 msec, SD = 28.2). Amplitude analysis was performed on mean values. Both patient groups displayed attenuated Novelty P3 amplitudes compared with healthy controls. The overall analysis showed both significant Anterior–Posterior × Group (F(4, 62) = 3.53, p < .05) and Hemisphere × Group (F(4, 62) = 3.65, p < .05) interactions. Follow-up analyses were, thus, performed on the right, midline, and left/lesioned electrode groups separately. Compared with controls, the OFC group had attenuated mean amplitudes over frontal left ( p < .001) and frontal midline ( p < .05) electrode groups. The LPFC group differed from the control group by displaying smaller Novelty P3 amplitudes over the lesioned hemisphere only, a difference that was significant both over frontal ( p < .01) and frontocentral ( p < .01), but not midline, electrode sites. The patient groups did not differ significantly from each other (see Figures 6 and 7).
Novelty P3 was compared between controls and the patients with OFC lesions due to tumor (n = 9) and TBI (n = 4), respectively, and both etiologies resulted in Novelty P3 reductions. The patients with tumor resections had significantly reduced Novelty P3 amplitudes over both left (F(1, 23) = 16.82, p < .001) and midline (F(1, 23) = 10.32, p < .005) frontal electrode groups, whereas the smaller TBI group had reduced Novelty P3s only over the left hemisphere (F(1, 18) = 5.35, p < .05).
Amplitudes to the first nine novel stimuli were examined over the frontal midline electrodes by comparing the first three novel stimuli to the next three (Novel Stimuli 4–6) and again to Novel Stimuli 7–9. The raw data of the control group showed the expected decline in amplitudes over the three stimulus groups (Novel Stimuli 1–3: 3.7 µV, SD = 3.6 µV; Novel Stimuli 4–6: 3.0 µV, SD = 2.7 µV; Novel Stimuli 7–9: 1.1 µV, SD = 5.3 µV), whereas the OFC group had negative polarity ERP amplitudes to novel stimuli from all three groups with no evidence of habituation (Novel Stimuli 1–3: −0.5 µV, SD = 4.1 µV; Novel Stimuli 4–6: −2.1 µV, SD = 4.5 µV; Novel Stimuli 7–9: −0.6 µV, SD = 4.6 µV). The LPFC group had a small Novelty P3 of positive polarity over the mean of the first three stimuli only (Novel Stimuli 1–3: 1.9 µV, SD = 4 µV; Novel Stimuli 4–6: −0.9 µV, SD = 2.6 µV; Novel Stimuli 7–9: −0.4 µV, SD = 2.6 µV). The change in amplitudes over stimulus groups did not turn out as significant for any of the subject groups. There was, however, a main effect of Group (F(2, 29) = 5.12, p < .01), indicating that the Novelty P3 diminishment was evident already from the first novel stimuli presented (mean amplitudes of Novel Stimuli 1–9 controls: 2.6 µV, SD = 2.6 µV; OFC: −0.9 µV, SD = 3.3; LPFC: .23 µV, SD = 2.1). Post hoc analysis showed that the OFC group differed significantly from controls (p < .01).
Early (400–600 msec) NSW
There was a main effect of Group (F(2, 31) = 9.16, p < .001) because of larger amplitudes in the LPFC group than in controls (p < .01). There was an additional Stimulus Type × Hemisphere × Group interaction (F(8, 124) = 3.08, p < .01; see Figures 6 and 8). For the target sounds, there was a magnitude difference between the LPFC group and controls (p < .05) over left/lesioned electrode sites, and both OFC and LPFC groups had larger amplitudes than controls over frontal midline electrodes (p < .05 and p < .01, respectively). For the novel sounds, there was an interaction between Group and Hemisphere for both frontal (F(4, 62) = 7.30, p < .001) and frontocentral electrodes (F(4, 62) = 6.53, p < .001). Over the lesioned hemisphere, the LPFC group had larger frontal and frontocentral NSW to novel sounds than both OFC patients and controls (p < .001). The effect was still present when lesion volume was entered as covariate in the model (F(2/30) = 6.66, p < .005). The LPFC group also differed from the other groups over frontal midline sites (p < .001). There were no significant differences between the OFC group and the controls. Irrespective of group, the early NSW was most pronounced over the left (lesioned for the LPFC group) hemisphere for both targets (F(2, 62) = 15.75, p < .0005) and novels (F(2, 62) = 8.32, p < .001). There were no significant effects involving standard tones.
Late (600–800 msec) NSW
A main effect of Group (F(2, 31) = 5.15, p < .01) reflected that the LPFC group had a larger mean amplitude than the other groups (see Figure 6). There were additional Stimulus Type × Group (F(4, 62) = 2.62, p < .05) and Anterior–Posterior × Hemisphere × Group (F(4, 62) = 2.59, p < .05) interactions. Follow-up analyses revealed no significant Group differences for the target stimuli. For the novel sounds, however, there was a main effect of Group (F(2, 31) = 8.37, p < .001) but also an interaction between Hemisphere and Group (F(4, 62) = 2.52, p < .05). Over the lesioned hemisphere, the LPFC group had a significantly larger NSW than both controls (p < .01) and the OFC group (p < .01). This effect was still present when lesion volume was entered as covariate (F(2, 30) = 5.73, p < .001). This was also the case for the midline electrodes (p < .01). Over the nonlesioned hemisphere, the LPFC group differed significantly from controls only (p < .05). There were no significant differences between controls and the OFC group. As for the early NSW, there were no significant effects involving standard tones.
Relationship between RT and ERP Measures
In the healthy controls, but not the patient groups, there was a significant negative correlation between the amplitude of the early NSW to target stimuli and RT to successful target trials over the left hemisphere (r = −.53, p < .04). There were no other significant correlations between RT to target stimuli and ERP measures.
DISCUSSION
Neurophysiological markers of novelty processing were studied in patients with lesions to the LPFC or the OFC in an auditory oddball task containing unexpected and task-irrelevant novel environmental sounds. The patient groups were classified as moderately impaired by the GOS-E. Despite this, their IQ and neuropsychological test results were normal, indicating that the patients experience functional deficits that were not readily detected by traditional neuropsychological measures. Of note, the OFC group reported more obsessive–compulsive symptoms and the LPFC group reported more irritability than controls. Obsessive–compulsive symptoms have been described in patients with OFC damage (Coetzer, 2004; Woessner & Caplan, 1995). Both patient groups displayed few commission errors to task-irrelevant stimuli, and their RTs and hit rates to target sounds were comparable to healthy controls, in accord with earlier studies showing typical behavioral performance in patients with focal frontal lesions on simple oddball tasks (Knight & Scabini, 1998; Knight, 1984, 1997; Yamaguchi & Knight, 1991). Despite normal scores on neuropsychological tests and normal performance on the experimental task, there were robust effects of frontal lesions on ERP measures of novelty processing.
Dissociation of Target and Novelty Processing after Frontal Lobe Damage
All three groups displayed a parietal maximum P3b to target stimuli supporting normal target detection in this simple task, a finding previously demonstrated in patients with LPFC lesions (Knight & Scabini, 1998). The present study indicates that the same conclusion can be extended to the role of OFC lesions on parietal-dependent target processing.
LPFC damage resulted in reduced amplitudes of the Novelty P3 response as has also been reported. Novelty P3 attenuation was evident for patients with OFC lesions as well, indicating that both OFC and LPFC participate in novelty processing. The reduction of the Novelty P3 was predominantly found at frontal and frontocentral electrode sites over the lesioned hemisphere in the LPFC group. The relative sparing of midline novelty P3 activity in the LPFC group might be because of reorganization of frontal function in the spared cortex of these patients (Voytek, Davis, et al., 2010).
In the OFC group, the Novelty P3 reduction was seen over frontal electrodes only, but the effect was present both over midline and left hemisphere electrodes. A habituation analysis of the Novelty response to the first nine Novel stimuli showed that the OFC patients failed to generate a Novelty P3 over frontal midline electrodes for any of the novel stimuli, providing additional support for the role of OFC in novelty processing. Of interest, the LPFC group showed habituation of the Novelty P3 response.
The lateralized reduction of the Novelty response in OFC patients was unexpected. The majority of OFC patients had bilateral lesions. In fact, the OFC group had a larger mean lesion volume over the right hemisphere (28.9 ccm) compared with the left (21.3 ccm), indicating that the laterality effect was not merely a product of the amount of damaged cortex. One possibility is that the Novel sounds used were meaningful environmental sounds and could have given rise to semantic processing (Mecklinger, Opitz, & Friederici, 1997). It has been demonstrated that novel environmental sounds activate left frontal brain regions in a verbal encoding task (Opitz, Mecklinger, & Friederici, 2000). Although speculative, it is possible that the effect of OFC lesions on novelty processing was larger over the left hemisphere because of altered processing of acoustic meaning.
Differential Frontal Lesion Effects on the NSW
The NSW to targets was enhanced for both patient groups with a maximum around the time of manual response delivery (mean RT = 422 msec in both patient groups). A left and midline frontal maximum was observed, corresponding to the lesioned hemisphere for the LPFC patients. Novel stimuli elicited a larger frontal negativity with a longer duration than the NSW to target stimuli for the LPFC group compared with both the OFC group and healthy controls. The enhanced NSW to novels in the LPFC group was predominantly present over the lesioned hemisphere.
Studies of healthy subjects (Schroger & Wolff, 1998), neurological populations (Potter, Bassett, Jory, & Barrett, 2001), and children (Maatta et al., 2005) suggest a link between auditory novelty-related NSW and controlled allocation of attentional resources. It has been proposed that the orienting response consists of two stages; first, the reaction that something novel has appeared, and second, an evaluation of stimulus characteristics and response requirements (Germana, 1968). Kok (1978) proposes that the Novelty P3 and the NSW reflect these two decision stages of the orienting response. A seeming paradox has been noted in Knight’s (1984) conclusion that lateral frontal lesions result in a deficit, both in inhibitory control and in novelty detection, implying that patients with frontal lobe lesions are both more distractible and less susceptible to deviant events (Kok, 1999). Kok (1999) resolves this paradox by assuming that these two phenomena reflect deficits in separable attentional mechanisms. Reduced novelty detection could reflect a deficit in automatic or involuntary aspects of attention, whereas increased distractibility could result from a deficit in active focusing of selective attention. This implies that it should be possible to observe differential effects on ERP indicators of novelty processing. The OFC group in the present study presented with reduced Novelty P3 only, whereas the LPFC group displayed both a reduced Novelty P3 and an enhanced novelty-related NSW indicating that the Novelty P3 and the NSW can be differentially affected by brain injuries.
A second possibility is that the Novelty P3 is not as automatic or reflexive as traditionally believed. The Novelty P3 amplitude is modulated by familiarity and semantic context (Friedman, Cycowicz, & Dziobek, 2003), implying that the Novelty P3 also is affected by the process of bringing the event to consciousness for evaluation of salience and appropriate action. If a Novelty P3 reduction indexes changes in the cognitive evaluation of novel events in addition to an involuntary orienting response, one might expect to observe signs of subsequent prolonged processing. This notion would be in line with the proposal that late NSW is associated with working memory and level of mental processing (Ruchkin et al., 1995). The prolonged enhancement of the NSW to meaningful novel sounds could, thus, index a tendency for sustained stimulus processing in the LPFC group even after a decision has been made to not respond.
A related question is the functional association between the NSW to target and novel stimuli. These stimuli are both deviant events of low frequency and both elicit slow waves in an oddball paradigm (Ritter & Ruchkin, 1992). The novel stimuli, unlike the target sounds, are acoustically complex and meaningful. They are also unique on every presentation, whereas the targets are identical but task relevant. The amplitude of the frontal NSW can be affected by a range of factors, such as degree of novelty, stimulus probability, response probability, and task relevance (Kok, 1978). The NSW elicited by target stimuli could be because of factors related both to physical deviance, task relevance, and motor response requirements. The novel sounds might elicit a slow negativity largely because of perceptual novelty and inherent meaningfulness. The NSW to target and novel deviants could thus be reflecting both distinct and overlapping cognitive processes that we could not disentangle in the current study.
Increased NSW amplitudes were associated with longer RTs. This has been demonstrated in earlier studies, as well, and has been interpreted as indicating that slow waves are related to task demand (Roth, Ford, & Kopell, 1978). Thus, an enhanced NSW in patients with frontal lobe injury might be associated with an abnormal allocation of “mental effort” to the deviant stimuli to cope efficiently with the task (Voytek, Davis, et al., 2010).
Distinct Lesion Effects on Early ERP Potentials: N1 and P2
Enhancement of the N1 in patients with frontal lobe injury (Knight et al., 1980) and in older subjects (Kok, 2000) has previously been interpreted as indexing altered inhibitory control because of prefrontal deficit. However, in this study, there was a possibility that the ERP effects in the LPFC group could be influenced by surgical skull defects over the lesioned hemisphere because of current shunting caused by craniotomy defects in the skull or to changes of current flow patterns due to the resection cavities being filled with cerebrospinal fluid (Voytek, Secundo, et al., 2010). The spontaneous EEG of the pre-stimulus baseline period did not show significantly larger amplitude variation over the lesioned compared with the nonlesioned hemisphere in the LPFC group. Thus, the amplitude enhancements are not likely because of increased noise or activity not related to task requirements. It is not possible to entirely rule out a contribution of craniotomy effects because the increased N1 amplitude in the LPFC group generalized across stimulus types. Although factors not related to the task such as current shunting may have contributed to the N1, the effect might also reflect changed inhibitory top–down control over early perceptual processes.
There are several reasons why current shunting cannot explain the results observed for the P2, Novelty P3, and NSW components. First, there is not a general amplitude enhancement across all ERP components or stimulus types for the P2, Novelty P3, and NSW. Second, the N1 enhancement was not accompanied by a comparably negative shift or enhancement in the following P2 component to standard tones. Third, the NSW was condition specific. Finally, a regression analysis showed that the N1 amplitude did not predict NSW amplitude.
Although N1 was unaffected in the OFC group, these patients had a reduced P2 to standard tones. The functional significance of the P2 is poorly understood, but it has been shown to be related to aspects of auditory discrimination and stimulus classification as well as attentional processing (Tong, Melara, & Rao, 2009; Crowley & Colrain, 2004; Näätänen, 1992). The N1 and P2 have been shown to be differentially affected by frontal brain injury as patients with lateral frontal damage displayed enhanced N1 and normal P2 when stimuli were presented to the ear contralateral to lesion site compared with ipsilateral stimulation (Knight et al., 1980). The results of the current study are in line with these findings, as the LPFC group displayed an increased N1 amplitude and a normal P2 over the lesioned hemisphere. The OFC group had a normal N1 but reduction of the P2 to standard stimuli. One hypothesis is that the OFC patients might be presenting signs of dampened perceptual classification, although not to the degree where it caused a breakdown in target discrimination in this fairly simple auditory oddball task.
Novelty P3 and OFC Lesions
Two earlier studies have described enhancement of P3 amplitudes after frontal lobe injury. The Rule et al. (2002) study is the only work that has explored the effects of OFC lesions. A parietal (Pz) Novelty P3 distribution seen in controls was enhanced in OFC patients. The parietal distribution of the Novelty P3 stands in contrast to many studies showing a more frontal–central novelty distribution. A passive novelty task was used by Rule et al. The patients watched a silent movie during stimulus presentation, and auditory and somatosensory stimuli were interspersed among each other in an unpredictable fashion at long ISI, causing the somatosensory and the auditory stimuli to be emotionally laden because they automatically pulled attention away from the movie. Of note, the study included only four OFC patients, of which one had additional lesion to the temporal lobe. The extent of OFC damage was comparable between our study and the study by Rule and colleagues.
The second study reporting enhancement of the P3 is Kaipio et al. (1999), wherein 11 patients with closed head injuries were included and exposed to a passive design as they were instructed to ignore standard and deviant (600 and 660 Hz, respectively) tones as well as complex Novel sounds presented during a visuomotor tracking task. Enhancement of a later portion of the Novelty P3 (350–450 msec) was shown over Cz. No lesion effects were seen over frontal electrode sites. The findings were taken to indicate enhanced processing of novel sounds. Six patients were described to have predominantly frontal damage, one no parenchymal lesion, one retained fluid in the sphenoid sinus, one subcortical diffuse axonal injury, and two temporal lobe lesions. Information on exact lesion site or size was not provided. Although this study might elucidate general effects of acquired brain injury, it is not well suited to provide information about the distinct relationship between subregions of the frontal lobes and novelty processing.
Taken together, differences in lesion location, study design, Novelty P3 scalp distribution, and sample size across studies render direct comparisons with earlier studies difficult. However, the differing results might indicate that an enhancement of the posterior P3 is associated with passive paradigms and emotionally laden stimuli. Contrasting the effect of predominantly cognitive and emotionally charged tasks on the P3 complex in patients with OFC lesions is needed to address this issue. A strength of the current study is the size of the OFC group and the active nature of the task. The findings in this study are in line with an animal study where neurons that responded to novel but not to familiar visual stimuli and habituated rapidly were demonstrated in the anterior OFC of the rhesus macaque monkey (Rolls, Browning, Inoue, & Hernandi, 2005).
Variation in lesion etiology between the two patient groups and within the OFC group might contribute to the findings in this study. All LPFC patients had undergone resections of unilateral LGG. The majority (8 of 13) of patients with OFC lesions had undergone resection of large meningiomas, four suffered TBI, and one had an LGG. The studies performed by Daffner et al. (2000, 2003) included only patients with cerebrovascular insults, whereas in Knight’s initial ERP study of frontal novelty processing (Knight, 1984), 7 of 14 patients had tumor resections, 5 had cerebrovascular, 1 had trauma, and 1 had abscess resection. Stuss and Alexander (2007) note that their studies of the neuropsychological effects of focal frontal lesions have demonstrated that lesion location is more important than etiology. Of note, despite the differing etiologies, the Daffner et al. and Knight studies yielded parallel results on Novelty P3 reductions after LPFC damage. The subgroup analysis of the OFC group in this study demonstrated that the Novelty P3 reduction was evident both in patients with tumor resections and in the TBI patients, indicating that the findings were not restricted to a specific etiology.
Although studies of the effect of LPFC lesions to the ERP complex are almost exclusively performed on patients with unilateral lesions, both Rule et al.’s work (Rule et al., 2002) and our study included OFC patients with predominantly bilateral damage. Whether the Novelty P3 reduction observed in the patients with OFC damage would have been seen in a sample with unilateral OFC damage awaits further study.
Conclusion
This study showed that despite normal task execution and neuropsychological profiles, patients with LPFC and OFC lesions present distinct neurophysiological evidence of alterations in novelty processing. Patients with LPFC and OFC lesions exhibited a normal parietal P3b response to target stimuli, indicating unaffected target detection. Conversely, both patient groups displayed attenuation of the Novelty P3 component, indicating an altered orienting response to unexpected and task irrelevant novel events. Previous work has demonstrated this for patients with LPFC lesions, and here, we extend this finding to OFC damage patients. Both patient groups displayed enhanced NSW to target deviants, possibly related to increased processing to successfully performing the task. Only the LPFC group showed an additional enhanced NSW to novel sounds, an effect that might index prolonged processing of task-irrelevant sounds in this group. Taken together, the results suggest that OFC and LPFC lesions have a partly shared and partly differential effect on the cascade of cognitive subcomponents involved in novelty processing. Normal novelty processing is the result of a cascade of sensory/perceptual and cognitive processes, with subregions of the frontal lobes providing critical input throughout the process of deviance detection and evaluation of stimulus significance.
Acknowledgments
We would like to thank Haakon Engen and Clay Campbell Clayworth for support in establishing routines for lesion reconstructions and Torgeir Moberget for valuable assistance in various stages of the work. This research is supported by the Southeastern Norway Regional Health Authority (grants SUN-001-SS and 2008047), the Research Council of Norway (grant 1865)04/V50), and the National Institute of Neurological Disorders (NS21135 and PO 40813). This work forms part of a doctoral thesis to be submitted to the Department of Psychology, University of Oslo.
REFERENCES
- Chong H, Riis JL, McGinnis SM, Williams DM, Holcomb PJ, Daffner KR. To ignore or explore: Top-down modulation of novelty processing. Journal of Cognitive Neuroscience. 2008;20:120–134. doi: 10.1162/jocn.2008.20003. [DOI] [PubMed] [Google Scholar]
- Coetzer BR. Obsessive–compulsive disorder following brain injury: A review. International Journal of Psychiatry in Medicine. 2004;34:363–377. doi: 10.2190/XENN-NNWT-7N2K-R26A. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: From environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E, Hillyard SA, Galambos R. Stimulus novelty, task relevance and the visual evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1975;39:131–143. doi: 10.1016/0013-4694(75)90003-6. [DOI] [PubMed] [Google Scholar]
- Crowley KE, Colrain IM. A review of the evidence for P2 being an independent component process: Age, sleep and modality. Clinical Neurophysiology. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Mesulam MM, Scinto LF, Acar D, Calvo V, Faust R, et al. The central role of the prefrontal cortex in directing attention to novel events. Brain. 2000;123:927–939. doi: 10.1093/brain/123.5.927. [DOI] [PubMed] [Google Scholar]
- Daffner KR, Scinto LF, Weitzman AM, Faust R, Rentz DM, Budson AE, et al. Frontal and parietal components of a cerebral network mediating voluntary attention to novel events. Journal of Cognitive Neuroscience. 2003;15:294–313. doi: 10.1162/089892903321208213. [DOI] [PubMed] [Google Scholar]
- Debener S, Kranczioch C, Herrmann CS, Engel AK. Auditory novelty oddball allows reliable distinction of top-down and bottom-up processes of attention. International Journal of Psychophysiology. 2002;46:77–84. doi: 10.1016/s0167-8760(02)00072-7. [DOI] [PubMed] [Google Scholar]
- Debener S, Makeig S, Delorme A, Engel AK. What is novel in the novelty oddball paradigm? Functional significance of the novelty P3 event-related potential as revealed by independent component analysis. Brain Research, Cognitive Brain Research. 2005;22:309–321. doi: 10.1016/j.cogbrainres.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Delis D, Kaplan E, Kramer J. Delis-Kaplan executive function system. San Antonio, TX: The Psychological Corporation; 2001. [Google Scholar]
- Derogatis LR. Symptom Checklist-90-R: Administrative scoring and procedures manual. 3rd ed. Minneapolis, MN: National Computer Systems, Inc.; 1994. [Google Scholar]
- Ferree TC, Luu P, Russell GS, Tucker DM. Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology. 2001;112:536–544. doi: 10.1016/s1388-2457(00)00533-2. [DOI] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, et al. The Obsessive-compulsive inventory: Development and validation of a short version. Psychological Assessment. 2002;14:485–496. [PubMed] [Google Scholar]
- Folk CL, Leber AB, Egeth HE. Made you blink! Contingent attentional capture produces a spatial blink. Perception & Psychophysics. 2002;64:741–753. doi: 10.3758/bf03194741. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Dziobek I. Cross-form conceptual relations between sounds and words: Effects on the novelty P3. Brain Research, Cognitive Brain Research. 2003;18:58–64. doi: 10.1016/j.cogbrainres.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Friedman D, Cycowicz YM, Gaeta H. The novelty P3: An event-related brain potential (ERP) sign of the brain’s evaluation of novelty. Neuroscience and Biobehavioral Reviews. 2001;25:355–373. doi: 10.1016/s0149-7634(01)00019-7. [DOI] [PubMed] [Google Scholar]
- Germana J. Response characteristics and the orienting reflex. Journal of Experimental Psychology. 1968;78:610–616. doi: 10.1037/h0026626. [DOI] [PubMed] [Google Scholar]
- Kaipio ML, Alho K, Winkler I, Escera C, Surma-aho O, Näätänen R. Event-related brain potentials reveal covert distractibility in closed head injuries. NeuroReport. 1999;10:2125–2129. doi: 10.1097/00001756-199907130-00024. [DOI] [PubMed] [Google Scholar]
- Kishiyama MM, Yonelinas AP, Knight RT. Novelty enhancements in memory are dependent on lateral prefrontal cortex. Journal of Neuroscience. 2009;29:8114–8118. doi: 10.1523/JNEUROSCI.5507-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight R. Decreased response to novel stimuli after prefrontal lesions in man. Electroencephalography and Clinical Neurophysiology. 1984;59:9–20. doi: 10.1016/0168-5597(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Knight R. Distributed cortical network for visual stimulus detection. Journal of Cognitive Neuroscience. 1997;9:75–91. doi: 10.1162/jocn.1997.9.1.75. [DOI] [PubMed] [Google Scholar]
- Knight RT, Hillyard SA, Woods DL, Neville HJ. The effects of frontal and temporal-parietal lesions on the auditory evoked potential in man. Electroencephalography and Clinical Neurophysiology. 1980;50:112–124. doi: 10.1016/0013-4694(80)90328-4. [DOI] [PubMed] [Google Scholar]
- Knight RT, Scabini D. Anatomic bases of event-related potentials and their relationship to novelty detection in humans. Journal of Clinical Neurophysiology. 1998;15:3–13. doi: 10.1097/00004691-199801000-00003. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Pseudopsychopathy: A perspective from cognitive neuroscience. In: Zald DH, Rauch SL, editors. The orbitofrontal cortex. New York: Oxford University Press; 2006. pp. 597–619. [Google Scholar]
- Kok A. The effect of warning stimulus novelty on the P300 and components of the contingent negative variation. Biological Psychology. 1978;6:219–233. doi: 10.1016/0301-0511(78)90024-8. [DOI] [PubMed] [Google Scholar]
- Kok A. Varieties of inhibition: Manifestations in cognition, event-related potentials and aging. Acta Psychologica (Amsterdam) 1999;101:129–158. doi: 10.1016/s0001-6918(99)00003-7. [DOI] [PubMed] [Google Scholar]
- Kok A. Age-related changes in involuntary and voluntary attention as reflected in components of the event-related potential (ERP) Biological Psychology. 2000;54:107–143. doi: 10.1016/s0301-0511(00)00054-5. [DOI] [PubMed] [Google Scholar]
- Kok A. On the utility of P3 amplitude as a measure of processing capacity. Psychophysiology. 2001;38:557–577. doi: 10.1017/s0048577201990559. [DOI] [PubMed] [Google Scholar]
- Maatta S, Saavalainen P, Kononen M, Paakkonen A, Muraja-Murro A, Partanen J. Processing of highly novel auditory events in children and adults: An event-related potential study. NeuroReport. 2005;16:1443–1446. doi: 10.1097/01.wnr.0000177014.36979.3f. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Opitz B, Friederici AD. Semantic aspects of novelty detection in humans. Neuroscience Letters. 1997;235:65–68. doi: 10.1016/s0304-3940(97)00712-x. [DOI] [PubMed] [Google Scholar]
- Mecklinger A, Ullsperger P. The P300 to novel and target events: A spatiotemporal dipole model analysis. NeuroReport. 1995;7:241–245. [PubMed] [Google Scholar]
- Näätänen R. Attention and brain function. Hillsdale, NJ: Lawrence Erlbaum Associates; 1992. [Google Scholar]
- Opitz B, Mecklinger A, Friederici AD. Functional asymmetry of human prefrontal cortex: Encoding and retrieval of verbally and nonverbally coded information. Learning and Memory. 2000;7:85–96. doi: 10.1101/lm.7.2.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Association pathways of the prefrontal cortex and functional observations. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York: Oxford University Press; 2002. pp. 31–50. [Google Scholar]
- Polich J. Updating P300: An integrative theory of P3a and P3b. Clinical Neurophysiology. 2007;118:2128–2148. doi: 10.1016/j.clinph.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polich J, Criado JR. Neuropsychology and neuropharmacology of P3a and P3b. International Journal of Psychophysiology. 2006;60:172–185. doi: 10.1016/j.ijpsycho.2005.12.012. [DOI] [PubMed] [Google Scholar]
- Potter DD, Bassett MR, Jory SH, Barrett K. Changes in event-related potentials in a three-stimulus auditory oddball task after mild head injury. Neuropsychologia. 2001;39:1464–1472. doi: 10.1016/s0028-3932(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Ritter W, Ruchkin DS. A review of event-related potential components discovered in the context of studying P3. Annals of the New York Academy of Sciences. 1992;658:1–32. doi: 10.1111/j.1749-6632.1992.tb22837.x. [DOI] [PubMed] [Google Scholar]
- Rohrbaugh JW, Syndulko K, Lindsley DB. Cortical slow negative waves following non-paired stimuli: Effects of modality, intensity and rate of stimulation. Electroencephalography and Clinical Neurophysiology. 1979;46:416–427. doi: 10.1016/0013-4694(79)90143-3. [DOI] [PubMed] [Google Scholar]
- Rolls ET, Browning AS, Inoue K, Hernandi I. Novel visual stimuli activate a population of neurons in the primate orbitofrontal cortex. Neurobiology of Learning and Memory. 2005;84:111–123. doi: 10.1016/j.nlm.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Roth WT, Ford JM, Kopell BS. Long-latency evoked potentials and reaction time. Psychophysiology. 1978;15:17–23. doi: 10.1111/j.1469-8986.1978.tb01328.x. [DOI] [PubMed] [Google Scholar]
- Royall DR. Executive control function: A review of its promise and challenges for clinical research. A report from the Committee on Research of the American Neuropsychiatric Association. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14:377–405. doi: 10.1176/jnp.14.4.377. [DOI] [PubMed] [Google Scholar]
- Ruchkin DS, Canoune HL, Johnson R, Jr, Ritter W. Working memory and preparation elicit different patterns of slow wave event-related brain potentials. Psychophysiology. 1995;32:399–410. doi: 10.1111/j.1469-8986.1995.tb01223.x. [DOI] [PubMed] [Google Scholar]
- Rule RR, Shimamura AP, Knight RT. Orbitofrontal cortex and dynamic filtering of emotional stimuli. Cognitive, Affective & Behavioral Neuroscience. 2002;2:264–270. doi: 10.3758/cabn.2.3.264. [DOI] [PubMed] [Google Scholar]
- Schroger E, Wolff C. Attentional orienting and reorienting is indicated by human event-related brain potentials. NeuroReport. 1998;9:3355–3358. doi: 10.1097/00001756-199810260-00003. [DOI] [PubMed] [Google Scholar]
- Sokolov EN. Higher nervous functions; the orienting reflex. Annual Review of Physiology. 1963;25:545–580. doi: 10.1146/annurev.ph.25.030163.002553. [DOI] [PubMed] [Google Scholar]
- Solbakk AK, Reinvang I, Andersson S. Assessment of P3a and P3b after moderate to severe brain injury. Clinical Electroencephalography. 2002;33:102–110. doi: 10.1177/155005940203300306. [DOI] [PubMed] [Google Scholar]
- Soltani M, Knight RT. Neural origins of the P300. Critical Reviews in Neurobiology. 2000;14:199–224. [PubMed] [Google Scholar]
- Spencer KM, Dien J, Donchin E. Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology. 2001;38:343–358. [PubMed] [Google Scholar]
- Squires NK, Squires KC, Hillyard SA. Two varieties of long-latency positive waves evoked by unpredictable auditory stimuli in man. Electroencephalography and Clinical Neurophysiology. 1975;38:387–401. doi: 10.1016/0013-4694(75)90263-1. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Executive functions and the frontal lobes: A conceptual view. Psychological Research. 2000;63:289–298. doi: 10.1007/s004269900007. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP. Is there a dysexecutive syndrome? Philosophical Transactions of the Royal Society of London, Series B, Biological Sciences. 2007;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss DT, Benson DF. The frontal lobes. New York: Raven Press; 1986. [Google Scholar]
- Stuss DT, Levine B. Adult clinical neuropsychology: Lessons from studies of the frontal lobes. Annual Review of Psychology. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Annals of the New York Academy of Sciences. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Teasdale GM, Pettigrew LE, Wilson JT, Murray G, Jennett B. Analyzing outcome of treatment of severe head injury: A review and update on advancing the use of the Glasgow Outcome Scale. Journal of Neurotrauma. 1998;15:587–597. doi: 10.1089/neu.1998.15.587. [DOI] [PubMed] [Google Scholar]
- Tong Y, Melara RD, Rao A. P2 enhancement from auditory discrimination training is associated with improved reaction times. Brain Research. 2009;1297:80–88. doi: 10.1016/j.brainres.2009.07.089. [DOI] [PubMed] [Google Scholar]
- Voytek B, Davis M, Yago E, Barcelo F, Vogel EK, Knight RT. Dynamic neuroplasticity after human prefrontal cortex damage. Neuron. 2010;68:401–408. doi: 10.1016/j.neuron.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voytek B, Secundo L, Bidet-Caulet A, Scabini D, Stiver SI, Gean AD, et al. Hemicraniectomy: A new model for human electrophysiology with high spatio-temporal resolution. Journal of Cognitive Neuroscience. 2010;22:2491–2502. doi: 10.1162/jocn.2009.21384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter WG. Slow potential waves in the human brain associated with expectancy, attention and decision. Archiv für Psychiatrie und Nervenkrankheiten. 1964;206:309–322. [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Third Edition. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Wilson JT, Pettigrew LE, Teasdale GM. Structured interviews for the Glasgow Outcome Scale and the extended Glasgow Outcome Scale: Guidelines for their use. Journal of Neurotrauma. 1998;15:573–585. doi: 10.1089/neu.1998.15.573. [DOI] [PubMed] [Google Scholar]
- Woessner R, Caplan B. Affective disorders following mild to moderate brain injury: Interpretive hazards of the SCL-90-R. Journal of Head Trauma and Rehabilitation. 1995;10:78–89. [Google Scholar]
- Yamaguchi S, Knight RT. Anterior and posterior association cortex contributions to the somatosensory P300. Journal of Neuroscience. 1991;11:2039–2054. doi: 10.1523/JNEUROSCI.11-07-02039.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantis S, Egeth HE. On the distinction between visual salience and stimulus-driven attentional capture. Journal of Experimental Psychology: Human Perception and Performance. 1999;25:661–676. doi: 10.1037//0096-1523.25.3.661. [DOI] [PubMed] [Google Scholar]
- Zald DH, Andreotti C. Neuropsychological assessment of the orbital and ventromedial prefrontal cortex. Neuropsychologia. 2010;48:3377–3391. doi: 10.1016/j.neuropsychologia.2010.08.012. [DOI] [PubMed] [Google Scholar]