Abstract
This study was designed to study potentiation of fluoxetine's antidepressant effect by curcumin or pindolol. Twenty eight groups of mice (n=8) were used in three sets of experiments. In the first set, 9 groups were subjected to the forced swimming test after being treated intraperitoneally with three vehicles, fluoxetine (5 and 20 mg/kg), curcumin (20 mg/kg), pindolol (32 mg/kg), curcumin+fluoxetine (5 mg/kg) and pindolol+fluoxetine (5 mg/kg). One hour after the test, serum and brain fluoxetine and norfluoxetine levels were measured in mice receiving fluoxetine (5 and 20 mg/kg), curcumin+fluoxetine (5 mg/kg) and pindolol+fluoxetine (5 mg/kg). In the second set, the test was done after pretreatment with p-chlorophenylalanine. In the third set, the locomotor activity was measured. The immobility duration was significantly decreased in fluoxetine (20 mg/kg), curcumin (20 mg/kg), curcumin+fluoxetine (5 mg/kg) and pindolol+fluoxetine (5 mg/kg) groups. These decreases were reversed with p-chlorophenylalanine. Fluoxetine and norfluoxetine levels were significantly higher in fluoxetine (20 mg/kg) group with no differences in fluoxetine (5 mg/kg), curcumin+fluoxetine (5 mg/kg) and pindolol+fluoxetine (5 mg/kg) groups. Moreover, drugs failed to alter the locomotor activity indicating absence of central stimulation. In conclusion, curcumin, more than pindolol enhanced the antidepressant effect of a subeffective dose of fluoxetine in mice without increasing its serum or brain levels excluding any pharmacokinetic interaction. Reversal of this potentiation with p-chlorophenylalanine suggests a pharmacodynamic interaction through involvement of presynaptic 5-HT1A receptors.
Keywords: Curcumin, forced swimming test, fluoxetine, pharmacodynamic, pharmacokinetic, pindolol
Fluoxetine, the prototype of the selective serotonin reuptake inhibitors (SSRIs), has become one of the most widely used antidepressants due to its therapeutic importance and the relative absence of severe adverse reactions. The common adverse reactions of fluoxetine are dry mouth, sweating, headache, diarrhea, sleepiness and insomnia[1,2]. The delay in onset of SSRIs and treatment resistance of sub-populations of depressed patients led to development of new strategies to improve the antidepressant efficacy[3].
There is a renewed public interest in complementary and alternative medicines even in the Western world[4]. Curcumin (diferuloylmethane), derived from Curcuma longa, has been found to enhance the antidepressant effect of fluoxetine. The mechanism of the antidepressant effect of curcumin is not fully understood. It may act through inhibiting the monoamine oxidase enzyme, modulating the release of serotonin (5-HT) and promotion of hippocampal neurogenesis[5] or through an interaction with 5-HT1A/1B and 5-HT2C receptors[6].
Pindolol, a β-adrenoceptor and 5-HT1A/1B receptor antagonist (partial agonist), has been used to compensate for the delay in onset of SSRIs[3]. It completely blocks central β-adrenoreceptors at clinically relevant plasma levels[7] while, at plasma levels in patients after 2.5 mg three times a day, it only partially blocks presynaptic 5-HT1A autoreceptors and does not augment the SSRI-induced 5-HT increase in the guinea pig brain. Therefore, it is unlikely that the favorable effects of combining pindolol with SSRIs are due to 5-HT1A antagonism[8]. Because pindolol augments fluoxetine or paroxetine, but not sertraline, mechanisms other than pharmacodynamics may be involved. There are possible interactions between pindolol and antidepressants that rely on CYP2D6 metabolism, as fluoxetine, causing elevations in their levels[9].
The mouse forced swimming test (FST) is thought to show a state of despair as the animal, realizing no escape route is plausible, becomes immobile in a “state of despair”. The FST is used to evaluate the antidepressant drugs and investigate the underlying mechanisms, due to its easiness, specificity and sensitivity[10]. The FST is straightforward to conduct reliably and it requires minimal specialized equipment[11]. Low doses of fluoxetine (1-5 mg/kg) were ineffective after acute treatment but produced different behavioral patterns in the FST after chronic administration, thus high doses seem to be required in acute testing[12].
Taken together, combination therapy of antidepressants with different mechanisms of action or those having mixed effects on serotonin, norepinephrine and dopamine levels in brain are often required and search for antidepressants with wider and a safer profile continues. Consequently, this study was designed to determine which more effectively enhances the antiimmobility effect of fluoxetine in the FST in mice, curcumin or pindolol, and which interaction (pharmacokinetic or pharmacodynamic) is involved. In order to investigate this, the serum and brain levels of fluoxetine (F) and its active metabolite (norfluoxetine, NF) were measured 1 h after the FST. Moreover, the influence of pretreatment with p-chlorophenylalanine methyl ester (PCPA, an inhibitor of tryptophan hydroxylase and 5-HT synthesis) on the effects of drugs in the FST was examined. To detect if central stimulation contributes to the antiimmobility effect of the drugs, their effect (at the same doses used in the FST) on locomotor activity of mice was measured using the open field test.
MATERIALS AND METHODS
The protocol of the study was approved by the Research Ethics Committee and adhered to the international guidelines for the use of experimental animals. Male albino mice weighed 25-35 g (30.13±4.02) were recruited from the Research Center, acclimated for 6 days before use and housed in plastic cages in an air-conditioned room at 24° in a 12h light–dark cycle (light on at 7.00 am) with food and water available ad libitum. All experiments were carried out during the light cycle with each animal used only once. All drugs and chemicals were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA) unless mentioned otherwise. Fluoxetine hydrochloride and PCPA were dissolved in saline. Curcumin and pindolol were dissolved in 0.4% methylcellulose and a 1% aqueous solution of Tween 80 respectively. Drugs were freshly prepared and protected from light.
Study design and treatment groups:
Twenty eight groups of mice (n=8) were used and three sets of experiments were performed. In the first set, mice were subjected to FST and then 1h later, the serum and brain levels of F and NF were measured by liquid chromatography/mass spectrometry (LC/MS). Propranolol hydrochloride was used as an internal standard (IS) and ultrapure water (Purelab Classic water purification system, Elga, UK) was used. All solvents were of HPLC grade (Merck, Darmstadt, Germany). Ammonium acetate and glacial acetic acid were of analytical grade (BDH, Poole, England). In the second set, the influence of pretreatment with PCPA on the effects of drugs in the FST was tested. In the third set, effect of drugs (at the same doses used in the FST) on locomotor activity of mice was measured using the open field test.
Forced swimming test:
Nine groups of mice were treated as follows: three control groups (vehicle-treated: saline, 0.4% methylcellulose and 1% aqueous solution of Tween 80), two fluoxetine-treated groups (F5 and F20: 5 and 20 mg/kg), curcumin-treated group (C, 20 mg/kg), pindolol-treated group (P, 32 mg/kg), curcumin-fluoxetine5-treated group (CF5: curcumin, 20 mg/kg, then after 20 min fluoxetine 5 mg/kg) and pindolol-fluoxetine5-treated group (PF5: pindolol, 32 mg/kg, then after 20 min fluoxetine 5 mg/kg). Drugs and vehicles were given three times at 24, 5 and 1 h before the FST by intraperitoneal (ip) injection in a volume of 0.1 ml per 10 g body weight. Each mouse was placed inside a vertical glass cylinder (height, 25 cm and diameter, 10 cm) containing 19 cm of water maintained at 23-25°, so mice could not support themselves by touching the bottom with their feet. The movements of the mice were videotaped for recording. All animals were forced to swim for a total of 5 min, and the total duration of immobility was measured. Each mouse was judged to be immobile when it ceased struggling and remained floating motionless in the water, making only those movements necessary to keep its head above water. The doses of the drugs used were selected on the basis of literature data[13,14,15,16].
LC/MS analysis of F and NF in mouse serum and brain:
One hour after the FST, blood samples were collected from mice in the F5, F20, CF5 and PF5 groups. Blood (0.2 ml) was collected by capillary tubes via the retroorbital sinus under light anesthesia by ether. The blood was placed in plain tubes and centrifuged at 3000 g for 5 min for separation of serum which was stored at −80 ° until measurement of the levels of F and NF. Mice were then sacrificed and brains were removed, weighed and stored frozen at −80° until analysis[17]. Concentrations of F and NF were measured using the following procedure. Stock standard solutions (1 mg/ml) of F, NF and IS were prepared by dissolving accurately weighed 10.0 mg of each compound in 5 ml of methanol and diluting to 10 ml with the same solvent. These solutions were stored at -20 ° and working solutions were obtained by diluting stock solutions with ultrapure water.
Solid phase extraction of serum samples was performed on Chromaband C8 cartridges (1.0 ml, 100 mg, Macherey Nagel, GmbH and Co., Germany). The used HPLC system was an Agilent 1200 series (Agilent Technologies, Waldbronn, Germany) consisted of a solvent delivery module, a quaternary pump, an autosampler and a column compartment. The column effluent was connected to an Agilent 6120 Quadrupole LC-ESI-MS. The control of the HPLC system and data processing were performed using ChemStation (Rev. B.04.02 [96]). The analytes were separated using an AgilentZorbax EclipsePlusC18 column, rapid resolution (4.6×100mm, 3.5 μm, Agilent Technologies, Palo Alto, CA, USA).
The LC system was operated isocratically using a mobile phase consisting of acetonitrile-ammonium acetate buffer, pH 4.7 (40:60, v/v); at a flow rate of 0.5 ml/min. Sample injection volume was 5 μl and each run time was 8.5 min. The condition of the mass spectrometer was optimized by a preliminary direct infusion of standard F, NF and IS solutions. General MS adjustments were set as follows: capillary voltage, 3000 V; nebulizer, 35 psi; drying gas flow, 10 l/min; desolvation temperature, 350°; fragmentor, 70 for F and NF and 125 for IS. The applied MS mode was the positive selected ion monitoring (SIM) mode. The system was adjusted to monitor the ions having m/z values of 260.1, 296.2 and 310.2 corresponding to the molecular ions [M+1]+ of IS, NF and F, respectively.
The solid-phase extraction (SPE) of F and NF from serum samples was achieved by using C8 cartridges. The whole process was controlled with a Visiprep DL vacuum system from Supelco (Bellefonte, PA, USA). SPE cartridges were conditioned by passing 2 ml of methanol then 1 ml of 1% NH4 OH, through the cartridge to establish a slightly basic pH. Thereafter it was loaded with 100 μl of serum and washed sequentially with 1 ml of 1% NH4OH. Retained compounds were eluted with 2 ml of 1% formic acid in methanol followed by 2 ml of CHCl3. An aliquot of 100 μl of the IS (0.5 ng/μl of propranolol hydrochloride in methanol) was added to each extract. The extract was then evaporated to dryness under a stream of nitrogen at 40° and the residue was reconstituted in 100 μl of methanol. Brain tissue samples were weighed, lyophilized and grinded. In a screw capped test tubes, each sample was then mixed with 2 ml of methanol and 2 ml of CHCl3 and an aliquot of 100 μl of the IS was added. After sonication for 10 min, all tubes were subjected to centrifugation at 5000 rpm for 10 min at 4° in a refrigerated centrifuge. The supernatant was filtered and evaporated to dryness under a stream of nitrogen at 40°. The resultant residue was reconstituted in 100 μl of methanol, centrifuged for 5 min and clear supernatant was then injected.
To prepare the standard calibration curves, spiked serum and brain samples of control rats were applied. Serum samples were spiked with appropriate amounts of working solutions of F and NF to obtain serial dilutions spanning the range of 0.1-4 ng/μl and 0.02-2 ng/μl, respectively. Also, brain samples were spiked to obtain serial dilutions spanning the range of 10-600 ng/mg and 0.5-20 ng/mg for F and NF, respectively. These samples were then extracted according to the previously described extraction procedure and analyzed in triplicates using the specified LC/MS method. The calibration curve was constructed by plotting the peak area ratio of F or NF to IS against the concentration of F or NF.
The extraction recoveries were determined to study the effect of extracted serum or brain matrix. For this purpose, the peak area ratios of the extracts were compared with those obtained by direct injection of the same amounts of the compounds. The average recoveries from 10 separated batch assays were 96.1% for F and 94.3% for NF in serum sample and 94.4 and 93.2% for F and NF in brain tissues.
The applied LC/MS method was fully validated according to ICH guidelines. The calibration curves of F showed linearity in the range of 0.1-4 ng/μl for spiked serum samples and from 10 to 600 ng/mg for brain tissue. The calibration curves of NF were linear in the range of 0.02-2 ng/μl for spiked serum samples and from 0.5 to 20 ng/mg for brain tissue. In all cases, the determination coefficient (r2) was more than 0.999 indicating the linearity of the analytical curve. The interday and intraday precision of the method was estimated by using serum samples spiked with three different levels of concentrations and subjected to the proposed extraction process. The RSD value for the measured concentrations was less than 10%. Specificity of the method was established by verifying the purity of the F and NF peak and also by applying the SIM mode of the MS system.
Pretreatment with PCPA:
In order to investigate the possible contribution of the serotoninergic system to the effects of drugs alone or in combinations, ten groups of mice (n=8) were used. Nine groups were pretreated with PCPA (100 mg/kg) while the tenth group received saline, once a day, for 4 consecutive days. Twenty min after the last PCPA injection, the nine groups received drugs and vehicles at the same doses used in the first set by ip injection in a volume of 0.1 ml per 10 g body weight and then were tested in the FST 30 min later[18].
Locomotor activity in the open field test:
In order to exclude central stimulation as a possible cause of the antiimmobility effect of the drugs in the FST, their effect, at the same doses, on locomotor activity of mice was measured using the open field test. Nine groups of mice (n=8) were treated in the same schedule as in the first set. Drugs or vehicles were given three times at 24, 5 and 1 h before the test. A black circular platform 1 m in diameter without walls was divided into six symmetrical sectors and elevated 50 cm above the floor. The laboratory room was dark and only the center of the open field was illuminated, with a 75 W bulb placed 75 cm above the platform (a pilot study was done to determine the proper illumination level). Mice were placed gently in the center of the platform. Mice tried to explore the area and during their movement, locomotion (exploratory activity) was expressed in terms of total number of ambulations (sector line crossings) during a 5-min test for each mouse[16,19].
Statistical analysis:
The data were expressed as means±SEM and analyzed using GraphPad_Prism 5 statistical software. Comparisons for two groups were made using Student's T-test. One-way analysis of variance (ANOVA) was used for experiments in which more than two groups were compared with Tukey post hoc testing. P<0.05 was considered to be statistically significant.
RESULTS
FST without and after pretreatment with PCPA:
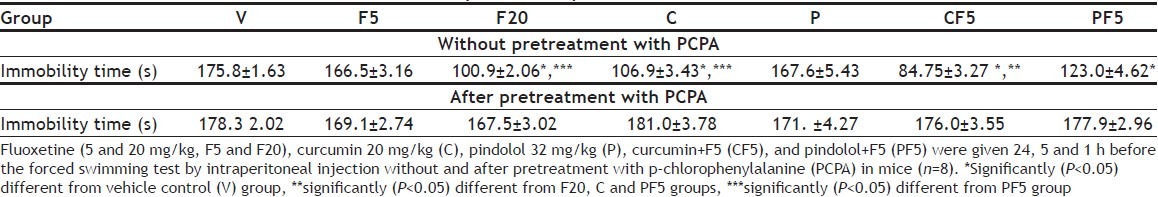
The differences among the three control vehicle-treated groups were non-significant, thus all were pooled in one group. All except fluoxetine-5 and pindolol groups showed significant decreases in the immobility duration in the FST as compared to the vehicle control group (p<0.05). The combination therapies (CF5 and PF5) further decreased the immobility duration with significant differences from fluoxetine-20 and curcumin groups (p<0.05). Pretreatment with curcumin (20 mg/kg) enhanced the subeffective dose of fluoxetine (5 mg/kg) more significantly than that with pindolol (32 mg/kg) (p <0.05). In the second set of experiments, pretreatment with PCPA alone did not alter the immobility duration while it prevented the antiimmobility effects of fluoxetine-20 and curcumin and reversed the augmentation of the subeffective dose of fluoxetine (F5) with curcumin or pindolol in the FST (Table 1).
TABLE 1.
THE EFFECTS ON IMMOBILITY TIME (SECONDS)
LC/MS analysis of F and NF in mouse serum and brain:
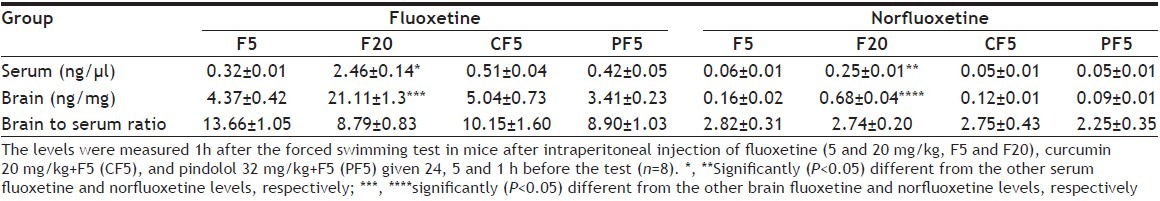
The serum levels of F and NF, measured 1 h after the FST in fluoxetine-5, fluoxetine-20, curcumin+fluoxetine-5 and pindolol+fluoxetine-5 groups, were significantly higher in fluoxetine-20 group (p<0.05) compared with the other groups. The brain levels showed similar changes. In addition, pretreatment with curcumin or pindolol in CF5 and PF5, respectively did not significantly affect the serum or brain concentrations of F and NF with non-significant difference between the two combination groups. NF showed lower levels than F while both F and NF showed much higher concentrations in brain than in serum. Thus, the fluoxetine brain to serum ratio was 8.79 to 13.66 and that of NF was 2.25-2.82 (Table 2).
TABLE 2.
THE SERUM AND BRAIN LEVELS OF FLUOXETINE AND NORFLUOXETINE
Locomotor activity in the open field test:
In the third set of experiments, the drugs given alone or in combinations at 24, 5 and 1 h before the open field test, at the doses used in the FST, failed to change the exploratory activity compared to the vehicle control group (Table 3).
TABLE 3.
THE EFFECTS ON LOCOMOTOR ACTIVITY (NUMBER OF TOTAL COUNTS)

DISCUSSION
The present study showed that the immobility duration in the FST was significantly decreased in fluoxetine-20, curcumin, curcumin+fluoxetine-5 and pindolol+fluoxetine-5 groups mostly in curcumin+fluoxetine-5 group, while it was not altered in pindolol group as compared to the vehicle control group. Pretreatment with curcumin enhanced the subeffective dose of fluoxetine more significantly than that with pindolol. Pretreatment with PCPA alone did not alter the immobility duration while it prevented the antiimmobility effects of fluoxetine-20 and curcumin and reversed the augmentation of the subeffective dose of fluoxetine with curcumin or pindolol in the FST. The serum and brain levels of fluoxetine and norfluoxetine were significantly higher in fluoxetine-20 group compared to the other groups. In addition, pretreatment with curcumin or pindolol in curcumin+fluoxetine-5 and pindolol+fluoxetine-5 groups respectively did not significantly affect the serum or brain concentrations of fluoxetine and norfluoxetine. All drugs failed to affect the locomotor activity.
In previous studies[13,20,21], ip injection of fluoxetine (10-40 mg/kg) reduced significantly, in a dose-dependent fashion, the duration of immobility in FST in mice, while a dose of 5 mg/kg was ineffective. Moreover, curcumin (10-80 mg/kg, ip) dose-dependently inhibited the immobility period, and in the higher doses it increased brain levels of 5-HT and dopamine and inhibited the monoamine oxidase enzymes in mice. When a subthreshold dose of fluoxetine (5 mg/kg, ip) was combined with curcumin (20 mg/kg, ip), the antidepressant activity was potentiated as compared to the effect per se of curcumin or fluoxetine[15]. The antidepressant activity of curcumin may involve the central neurotransmitter systems increasing release of 5-HT, noradrenaline and dopamine in addition to inhibiting the monoamine oxidase enzyme[22]. In chronic studies[5,23], curcumin has been shown to enhance neurogenesis, notably in the frontal cortex and hippocampal regions of the brain and increase brain derived neurotrophic factor (BDNF) protein levels in the amygdale.
Pindolol alone did not modify the immobility time[6] while prior administration of pindolol (32 mg/kg, ip) enhanced a subactive dose of paroxetine, an SSRI, in the mouse FST[24]. The mechanism of action by which pindolol improves the antidepressant action of SSRIs is not fully understood. Pindolol may prevent the activation of 5-HT1A autoreceptors in the raphe nuclei, by the increased 5-HT in synaptic cleft by the SSRIs, hence preventing the inhibition of 5-HT release, as well as increasing hippocampal and cortical 5-HT content. However, the partial agonist properties of pindolol at 5-HT1A receptors and β-adrenoceptors suggest that other explanations for its action are also possible[25]. Pindolol may increase brain availability of tryptophan, the precursor amino acid of 5-HT, through its sympathomimetic effects especially its stimulatory influences of the sympathetic system on the blood brain barrier transporter that carries tryptophan into the brain compartment[26]. Using positron emission tomography, pindolol was more potent at blocking presynaptic 5-HT1A than postsynaptic receptors[27] and its dose used to augment antidepressants in clinical trials (2.5 mg tid) was found to be suboptimal because it did not achieve a significant occupancy of 5-HT1A autoreceptor in depressed patients. Therefore, testing pindolol's efficacy will necessitate higher doses (5.0 mg tid)[28]. Moreover, although pindolol alone has sufficient intrinsic activity to produce a desensitization of the 5-HT1A receptor, when given in combination with fluoxetine it is able to prevent the desensitization induced by not only fluoxetine but also itself. This may suggest that the clinical augmentation of antidepressant action by pindolol, when co-administered with a SSRI, is through antagonism of the 5-HT1A receptor[29]. Thus, the clinical efficacy of pindolol in augmenting the antidepressant response to SSRIs, such as fluoxetine, is generally attributed to a blockade of the feedback inhibition of serotonergic neuronal activity mediated by somatodendritic 5-HT1A autoreceptors and unrelated to a restoration of serotonergic neuronal activity[30,31].
Failure of curcumin or pindolol to increase the serum and brain levels of fluoxetine or norfluoxetine, measured at 1 h after the FST, in spite of potentiating its antidepressant effect, excludes pharmacokinetic interactions. In a previous study[19], amantadine did not significantly change the level of imipramine and its metabolite, desipramine, in the rat plasma and brain, 1 h after the FST. Hence, any contribution of a pharmacokinetic interaction to the potentiation of imipramine effect by amantadine, observed in vivo in the FST, was excluded.
In order to confirm the involvement of the presynaptic 5-HT1A receptors in the antidepressant-like effects of curcumin and pindolol, a second set of experiments was conducted after pretreatment with PCPA (100 mg/kg/day for 4 consecutive days). The results presented here show that pretreatment with PCPA alone did not alter the immobility time while it prevented the antiimmobility effects of fluoxetine-20 and curcumin and reversed the augmentation of a subeffective dose of fluoxetine (F5) with curcumin or pindolol in the FST. Ability of PCPA, given in this schedule, to deplete the endogenous store of 5-HT successfully without affecting noradrenaline and dopamine levels was confirmed by previous studies[18,32]. Wang et al.[6] mentioned that pretreatment with PCPA blocked the antiimmobility effect of fluoxetine and curcumin in the FST. Moreover, pretreatment with pindolol, p-MPPI (5-HT1A receptor antagonist) or isamoltane (5-HT1B receptor antagonist) blocked that of curcumin suggesting that it is related to the serotonergic system and may be mediated by, at least in part, an interaction with 5-HT1A/1B and 5-HT2C receptors. In addition, pretreatment with PCPA prevented the antidepressant-like effect of agmatine in the FST. Considering that PCPA acts presynaptically, it is likely that presynaptic 5-HT1A receptors are involved in the antidepressant-like effect of agmatine, although the involvement of postsynaptic 5-HT1A receptors cannot be ruled out[14].
False positive results can be obtained in the FST by various dopamine or noradrenaline stimulants if used at doses increasing the locomotor activity[33]. In order to exclude this in the present study, the locomotor activity of mice was measured using the open field test, at the same doses used in the FST given at 24, 5 and 1 h before the test. In this third set of experiments, it was found that none of the drugs given alone or in combination changed the exploratory activity in the open field test in mice. In a previous study[16], curcumin did not increase the locomotor activity in mice or rats as compared to vehicle control, showing that the antidepressant like effect was not due to central nervous stimulation. In addition, the specificity of synergistic interactions between amantadine (an uncompetitive NMDA receptor antagonist) and fluoxetine or imipramine is supported by the control open field test in rats which demonstrated no significant increase, or even a decrease in general locomotion after administration of the compounds alone or in combination[19,34].
Based on results of the present study and previous literature, curcumin may be more ideal to be used for potentiating fluoxetine because it is relatively nontoxic and has few side effects[35] in contrast to the broad-spectrum pharmacodynamics and the potentially fatal consequences of overdose of pindolol[9]. Moreover, curcumin has been proved to be safe and devoid of adverse effects even in high doses of up to 8 g/day taken for several weeks in human studies[36]. Pindolol seemed to hasten the response to SSRIs in depressive patients with no evidence of improved efficacy beyond the first two weeks of treatment[37] and with a limited effect in treatment-resistant patients[38]. The use of higher doses of pindolol, recommended by certain studies[28,38], in future augmentation trials may lead to more adverse effects. However, the use of curcumin in clinics for the treatment of major depression is limited due to its poor gastrointestinal absorption. In order to overcome this problem, ip piperine (a bioavailability enhancing agent) was co-administered with ip curcumin resulting in potentiation of its activities[15]. BCM-95 (biocurcumax), an oral curcumin formulation with a 7-fold enhanced bioavailability, was found to have an antidepressant-like action in mice and rats; however, it did not increase the antidepressant action of fluoxetine and imipramine[16,39].
In conclusion, co-administration of curcumin, more effectively than pindolol, enhanced the antidepressant effect of a subeffective dose of fluoxetine in the FST in mice. Failure of curcumin or pindolol to increase the serum and brain levels of fluoxetine or norfluoxetine, in spite of potentiating its antidepressant effect, excludes any pharmacokinetic interactions. Reversal of this potentiation by pretreatment with PCPA (an inhibitor of 5-HT synthesis acting presynaptically) suggests a pharmacodynamic interaction through the presynaptic 5-HT1A receptors. Failure of the drugs, at the doses used in the FST, to change the exploratory activity in the open field test, excludes any possible central stimulation. Thus, curcumin is recommended as a safe and effective additive therapy to fluoxetine in treatment of resistant cases of depression. Further research, digging more into the underlying mechanisms, is recommended.
ACKNOWLEDGEMENTS
This project was funded by the Deanship of Scientific Research, (DSR), King Abdulaziz University (KAU), Jeddah, under grant number (169/828/1432). The authors acknowledge with thanks DSR technical and financial support. The authors also thank Mr. Tarek Elawady (Pharmaceutical Chemistry Dept., KAU) for technical support in LC/MS analysis.
Footnotes
Murad, et al.: Potentiation of Fluoxetine by Curcumin
REFERENCES
- 1.Diniz JB, Shavitt RG, Fossaluza V, Koran L, Pereira CA, Miguel EC. A double-blind, randomized, controlled trial of fluoxetine plus quetiapine or clomipramine versus fluoxetine plus placebo for obsessive-compulsive disorder. J Clin Psychopharmacol. 2011;31:763–8. doi: 10.1097/JCP.0b013e3182367aee. [DOI] [PubMed] [Google Scholar]
- 2.Homberg JR, Olivier JD, Blom T, Arentsen T, van Brunschot C, Pieter S, et al. Fluoxetine exerts age-dependent effects on behavior and amygdala neuroplasticity in the rat. PLoS One. 2011;6:e16646. doi: 10.1371/journal.pone.0016646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hughes ZA, Starr KR, Scott CM, Newson MJ, Sharp T, Watson JM, et al. Simultaneous blockade of 5-HT1A/B receptors and 5-HT transporters results in acute increases in extracellular 5-HT in both rats and guinea pigs: in vivo characterization of the novel 5-HT1A/B receptor antagonist/5-HT transport inhibitor SB-649915-B. Psychopharmacology (Berl) 2007;192:121–33. doi: 10.1007/s00213-006-0691-x. [DOI] [PubMed] [Google Scholar]
- 4.Firenzuoli F, Gori L. Herbal medicine today: clinical and research issues. Evid Based Complement Alternat Med. 2007;4(Suppl 1):37–40. doi: 10.1093/ecam/nem096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni S, Dhir A, Akula KK. Potentials of curcumin as an antidepressant. ScientificWorldJournal. 2009;9:1233–41. doi: 10.1100/tsw.2009.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang R, Xu Y, Wu HL, Li YB, Li YH, Guo JB, et al. The antidepressant effects of curcumin in the forced swimming test involve 5-HT1 and 5-HT2 receptors. Eur J Pharmacol. 2008;578:43–50. doi: 10.1016/j.ejphar.2007.08.045. [DOI] [PubMed] [Google Scholar]
- 7.Cremers TI, Wiersma LJ, Bosker FJ, den Boer JA, Westerink BH, Wikström HV. Is the beneficial antidepressant effect of coadministration of pindolol really due to somatodendritic autoreceptor antagonism? Biol Psychiatry. 2001;50:13–21. doi: 10.1016/s0006-3223(00)01093-3. [DOI] [PubMed] [Google Scholar]
- 8.Béïque JC, Blier P, de Montigny C, Debonnel G. Potentiation by (-) pindolol of the activation of postsynaptic 5-HT1A receptors induced by venlafaxine. Neuropsychopharmacology. 2000;23:294–306. doi: 10.1016/S0893-133X(00)00112-3. [DOI] [PubMed] [Google Scholar]
- 9.Olver JS, Cryan JF, Burrows GD, Norman TR. Pindolol augmentation of antidepressants: A review and rationale. Aust N Z J Psychiatry. 2000;34:71–9. doi: 10.1046/j.1440-1614.2000.00681.x. [DOI] [PubMed] [Google Scholar]
- 10.Porsolt RD, Le Pichon M, Jalfre M. Depression: A new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–2. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 11.Can A, Dao DT, Arad M, Terrillion CE, Piantadosi SC, Gould TD. The mouse forced swim test. J Vis Exp. 2012;59:e3638. doi: 10.3791/3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Detke MJ, Johnson J, Lucki I. Acute and chronic antidepressant drug treatment in the rat forced swimming test model of depression. Exp Clin Psychopharmacol. 1997;5:107–12. doi: 10.1037//1064-1297.5.2.107. [DOI] [PubMed] [Google Scholar]
- 13.Holladay JW, Dewey MJ, Yoo SD. Pharmacokinetics and antidepressant activity of fluoxetine in transgenic mice with elevated serum alpha-1-Acid glycoprotein levels. Drug Metab Dispos. 1998;26:20–4. [PubMed] [Google Scholar]
- 14.Dias Elpo Zomkowski A, Oscar Rosa A, Lin J, Santos AR, Calixto JB, Lúcia Severo Rodrigues A. Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test. Brain Res. 2004;1023:253–63. doi: 10.1016/j.brainres.2004.07.041. [DOI] [PubMed] [Google Scholar]
- 15.Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology (Berl) 2008;201:435–42. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 16.Sanmukhani J, Anovadiya A, Tripathi CB. Evaluation of antidepressant like activity of curcumin and its combination with fluoxetine and imipramine: An acute and chronic study. Acta Pol Pharm. 2011;68:769–75. [PubMed] [Google Scholar]
- 17.Uhr M, Steckler T, Yassouridis A, Holsboer F. Penetration of amitriptyline, but not of fluoxetine, into brain is enhanced in mice with blood-brain barrier deficiency due to mdr1a P-glycoprotein gene disruption. Neuropsychopharmacology. 2000;22:380–7. doi: 10.1016/S0893-133X(99)00095-0. [DOI] [PubMed] [Google Scholar]
- 18.Eckeli AL, Dach F, Rodrigues AL. Acute treatments with GMP produce antidepressant-like effects in mice. Neuroreport. 2000;11:1839–43. doi: 10.1097/00001756-200006260-00008. [DOI] [PubMed] [Google Scholar]
- 19.Rogóz Z, Skuza G, Kuśmider M, Wójcikowski J, Kot M, Daniel WA. Synergistic effect of imipramine and amantadine in the forced swimming test in rats. Behavioral and pharmacokinetic studies. Pol J Pharmacol. 2004;56:179–85. [PubMed] [Google Scholar]
- 20.Khisti RT, Chopde CT. Serotonergic agents modulate antidepressant-like effect of the neurosteroid 3alpha-hydroxy-5alpha-pregnan-20-one in mice. Brain Res. 2000;26(865):291–300. doi: 10.1016/s0006-8993(00)02373-8. [DOI] [PubMed] [Google Scholar]
- 21.Patel DS, Anand IS, Bhatt PA. Evaluation of antidepressant and anxiolytic activity of phosphodiesterase 3 inhibitor-cilostazol. Indian J Psychol Med. 2012;34:124–8. doi: 10.4103/0253-7176.101776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu Y, Ku BS, Yao HY, Lin YH, Ma X, Zhang YH, et al. The effects of curcumin on depressive-like behaviors in mice. Eur J Pharmacol. 2005;518:40–6. doi: 10.1016/j.ejphar.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Xu T, Wang S, Yu L, Liu D, Zhan R, et al. Curcumin produces antidepressant effects via activating MAPK/ERK-dependent brain-derived neurotrophic factor expression in the amygdala of mice. Behav Brain Res. 2012;235:67–72. doi: 10.1016/j.bbr.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 24.Redrobe JP, Bourin M, Colombel MC, Baker GB. Psychopharmacological profile of the selective serotonin reuptake inhibitor, paroxetine: Implication of noradrenergic and serotonergic mechanisms. J Psychopharmacol. 1998;12:348–55. doi: 10.1177/026988119801200404. [DOI] [PubMed] [Google Scholar]
- 25.Artigas F, Celada P, Laruelle M, Adell A. How does pindolol improve antidepressant action? Trends Pharmacol Sci. 2001;22:224–8. doi: 10.1016/s0165-6147(00)01682-5. [DOI] [PubMed] [Google Scholar]
- 26.Chaouloff F. Sympathomimetic effects of pindolol in depression. Trends Pharmacol Sci. 2001;22:554–5. doi: 10.1016/s0165-6147(00)01830-7. [DOI] [PubMed] [Google Scholar]
- 27.Martinez D, Broft A, Laruelle M. Pindolol augmentation of antidepressant treatment: Recent contributions from brain imaging studies. Biol Psychiatry. 2000;48:844–53. doi: 10.1016/s0006-3223(00)00993-8. [DOI] [PubMed] [Google Scholar]
- 28.Rabiner EA, Bhagwagar Z, Gunn RN, Sargent PA, Bench CJ, Cowen PJ, et al. Pindolol augmentation of selective serotonin reuptake inhibitors: PET evidence that the dose used in clinical trials is too low. Am J Psychiatry. 2001;158:2080–2. doi: 10.1176/appi.ajp.158.12.2080. [DOI] [PubMed] [Google Scholar]
- 29.Dawson LA, Nguyen HQ, Smith DI, Schechter LE. Effects of chronic fluoxetine treatment in the presence and absence of (+/-)pindolol: A microdialysis study. Br J Pharmacol. 2000;130:797–804. doi: 10.1038/sj.bjp.0703378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fornal CA, Metzler CW, Jacobs BL. Pindolol, a putative 5-Hydroxytryptamine1A antagonist, does not reverse the inhibition of serotonergic neuronal activity induced by fluoxetine in awake cats: Comparison to WAY-1006351. J Pharmacol Exp Ther. 1999;291:220–8. [PubMed] [Google Scholar]
- 31.Wesołowska A, Tatarczyńska E, Nikiforuk A, Chojnacka-Wójcik E. Enhancement of the anti-immobility action of antidepressants by a selective 5-HT7 receptor antagonist in the forced swimming test in mice. Eur J Pharmacol. 2007;555:43–7. doi: 10.1016/j.ejphar.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 32.Page ME, Detke MJ, Dalvi A, Kirby LG, Lucki I. Serotonergic mediation of the effects of fluoxetine, but not desipramine, in the rat forced swimming test. Psychopharmacology (Berl) 1999;147:162–7. doi: 10.1007/s002130051156. [DOI] [PubMed] [Google Scholar]
- 33.Borsini F, Meli A. Is the forced swimming test a suitable model for revealing antidepressant activity? Psychopharmacology (Berl) 1988;94:147–60. doi: 10.1007/BF00176837. [DOI] [PubMed] [Google Scholar]
- 34.Rogóz Z, Skuza G, Maj J, Danysz W. Synergistic effect of uncompetitive NMDA receptor antagonists and antidepressant drugs in the forced swimming test in rats. Neuropharmacology. 2002;42:1024–30. doi: 10.1016/s0028-3908(02)00055-2. [DOI] [PubMed] [Google Scholar]
- 35.Li YC, Wang FM, Pan Y, Qiang LQ, Cheng G, Zhang WY, et al. Antidepressant-like effects of curcumin on serotonergic receptor-coupled AC-cAMP pathway in chronic unpredictable mild stress of rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:435–49. doi: 10.1016/j.pnpbp.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 36.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 37.Ballesteros J, Callado LF. Effectiveness of pindolol plus serotonin uptake inhibitors in depression: A meta-analysis of early and late outcomes from randomised controlled trials. J Affect Disord. 2004;79:137–47. doi: 10.1016/S0165-0327(02)00404-4. [DOI] [PubMed] [Google Scholar]
- 38.Brousse G, Schmitt A, Chereau I, Eschalier A, Dubray C, Llorca PM. Interest of the use of pindolol in the treatment of depression: Review. Encephale. 2003;29:338–50. [PubMed] [Google Scholar]
- 39.Antony B, Merina B, Iyer VS, Judy N, Lennertz K, Joyal S. A pilot cross-over study to evaluate human oral bioavailability of BCM-95CG (Biocurcumax), a novel bioenhanced preparation of curcumin. Indian J Pharm Sci. 2008;70:445–9. doi: 10.4103/0250-474X.44591. [DOI] [PMC free article] [PubMed] [Google Scholar]


