Abstract
Nevirapine is a highly lipophilic and water insoluble non-nucleoside reverse transcriptase inhibitor used for the treatment of HIV-1 infection. Lymphoid tissue constitutes the major reservoir of HIV virus and infected cells in HIV-infected patients. Self-emulsifying drug delivery system, using long chain triglycerides, is a popular carrier of drugs due to their ability to transport lipophilic drugs into the lymphatic circulation. However, HIV/AIDS patients experience a variety of functional and anatomical abnormalities in gastrointestinal tract that result in diarrhoea and nutrient malabsorption. Medium chain triglycerides are readily absorbed from the small bowel under conditions in which the absorption of long chain triglycerides is impaired. Therefore, nevirapine self-emulsifying drug delivery system containing medium chain fatty acid, caprylic acid and a solubilizer, Soluphor® P (2-pyrrolidone) was developed and found to be superior to the marketed conventional suspension with respect to in vitro diffusion and ex vivo intestinal permeability. This self-emulsifying drug delivery system has now been further investigated for in vivo absorption in an animal model. The contribution of caprylic acid and Soluphor® P on in vivo absorption of nevirapine was also studied in the present study. The bioavailability of nevirapine from self-emulsifying drug delivery system, after oral administration, was 2.69 times higher than that of the marketed suspension. The improved bioavailability could be due to absorption of nevirapine via both portal and intestinal lymphatic routes. The study indicates that medium chain or structured triglycerides can be a better option to develop self-emulsifying drug delivery system for lipophilic and extensively metabolised drugs like nevirapine for patients with AIDS-associated malabsorption.
Keywords: Medium chain triglycerides, nevirapine, self-emulsifying drug delivery system, 2-pyrrolidone; pharmacokinetics
Nevirapine is a non-nucleoside reverse transcriptase inhibitor of human immunodeficiency virus, type 1 (HIV-1). The drug is practically insoluble in water (0.1 mg/ml), belongs to Class II as per Biopharmaceutical Classification System[1] with a log octanol-water partition coefficient (log P) of 2.5 and a pKa of 2.8[2]. HIV enters the human host via mucosal surfaces and is subsequently disseminated throughout the lymphatic tissues, a major reservoir of virus throughout the course of infection[3,4]. A self-emulsifying drug delivery system (SEDDS) is defined as an isotropic mixtures of oil, surfactant, co-surfactant and the drug substance. Upon mixing with water the system has an ability to form fine colloidal droplets with very high surface area. The oil component of SEDDS is an important constituent, not only because it can solubilise lipophilic drug or facilitate self emulsification but it can increase the fraction of lipophilic drug transported via the intestinal lymphatic system, thereby increasing absorption from the gastrointestinal tract depending on the nature of the triglyceride[5,6]. Both long and medium chain triglyceride oils with different degrees of saturation have been used for the design of self-emulsifying formulations. Advantages of this drug delivery to intestinal lymphatics include avoidance of hepatic first pass metabolism and potential to target specific disease states known to spread via the lymphatic system, for example, certain lymphomas and HIV[7]. The self-emulsification process was shown to be dependent on the lipid/surfactant pair, the surfactant concentration, the ratio between lipid and surfactant[8]. However, only specific combinations can lead to an efficient self-emulsifying system[9,10]. It is advantageous to use medium chain triglycerides (MCT) SEDDS due to their higher fluidity, better solubility properties and self-emulsification ability compared with long chain triglycerides (LCT)[9,10], as well as a better chemical stability of drug substance in MCT due to the purity of the lipid and the lack of double bonds, that can catalyse oxidation. The two lipids are differently transported in the body: MCT is directly transported by the portal blood to the systemic circulation[11], whereas LCT is transported in the intestinal lymphatics. However, some of the medium-chain saturated fatty acids are resynthesized or re-esterified to triacylglycerols or neutral lipids, packaged within apoprotein surface layers and transported via the lymphatic system[5]. Recently, Sun et al. has reported that, the lymphatic transport of sirolimus from SMEDDS formulation containing ≥25% MCT was a major contributor to its oral bioavailability[12].
HIV/AIDS patients often have severe fat malabsorption and thus their quality of life is greatly diminished. If they could ingest a diet containing a readily absorbable fat source, such as MCTs, their steatorrhea and stool nitrogen excretion could be diminished and their nutritional status may be improved[13,14]. Craig et al. have demonstrated that the MCT-containing formula to be better tolerated by AIDS patients, with fat malabsorption, compared to control liquid formulas (relative to solid food diet) and suggested as a source of dietary fat for such patients[13].
With a view to improve the solubility of the poorly water-soluble drug nevirapine, we have reported a SEDDS using caprylic acid, Soluphor® P and Transcutol® P[15]. Soluphor® P has been much explored as permeation enhancer for topical[16] and parenteral application[17]. In recent years, Soluphor® P has been tried by many researcher groups for solubility and permeability improvement of poorly-soluble drugs for oral human use. Shende et al. were successful to solubilise lamotrigene, an anti-epileptic drug, by virtue of microemulsion for nasal delivery using Soluphor® P as a co-surfactant[18]. Dharap et al. tried several solvents and solubilizers to solubilise ibuprofen and nifedipine and reported that Soluphor® P and Solutol® HS15 (Polyoxyl 15 hydroxystearate) improved solubility and were compatible for use in hard gelatine capsules[19]. Ruchatz and Kotler[20], studied the properties of 2-pyrrolidone (Soluphor® P) as a co-solvent for the dissolution enhancement of water insoluble drugs like clotrimazole, 17-β-estradiole, sulfathiazole, riboflavin, PHB-ester, acetaminophen in comparison to other commonly used solvents. It was found that the efficacy of Soluphor® P was considerably higher than other often used co-solvents. To date, pharmaceutical formulations developed using Soluphor® P for human oral use have been confined to in vitro characterization. In our laboratory, we have made an attempt to check its role in development of a self-emulsifying drug delivery system for hydrophobic drug, nevirapine for human oral use, carried out in vitro and ex vivo characterisation and in vivo performance in a rat model.
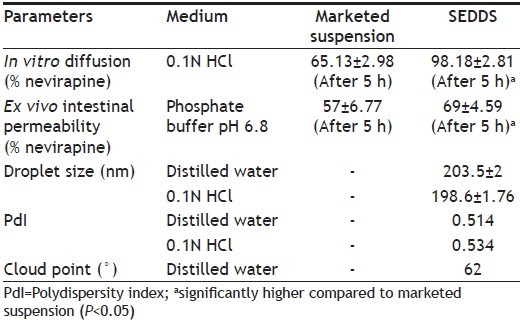
Our study[15] indicated that the developed nevirapine SEDDS showed greater diffusion and intestinal permeability than the marketed suspension (Nevimune®, CIPLA, Mumbai). However the previous studies focused on solubility of nevirapine, optimization of self-emulsifying drug delivery system, its characterization by transmission electron microscopy (TEM), droplet size determination, cloud point measurement, in vitro diffusion and ex vivo intestinal permeability studies. Such characterizations as shown in Table 1 form the fundamental basis for developing SEDDS. In the present study, we investigated the pharmacokinetic profile of nevirapine after oral administration of developed nevirapine SEDDS and compared with the marketed conventional suspension. The in vivo investigation confirmed the solubilising and permeation enhancing property of Soluphor® P. The attempt was also made to understand the role of medium chain fatty acid, caprylic acid and solubilizer, Soluphor® P in absorption of lipophilic drug in an animal model. We reported earlier that LCT (oleic acid) was next to MCT (caprylic acid) in solubilising nevirapine, but the latter was selected due to its specific advantages, especially in formulating the lipid-based formulation for HIV patients, where medication last for longer duration. Our results indicate that nevirapine SEDDS results in improved bioavailability of the poorly-water soluble drug nevirapine.
TABLE 1.
IN VITRO CHARACTERIZATION OF NEVIRAPINE MARKETED SUSPENSION AND SEDDS
MATERIALS AND METHODS
Nevirapine was a generous gift from Matrix Laboratories Ltd., Hyderabad, India, caprylic acid (MCT) was obtained from Soofi Traders, Mumbai, India, 2-pyrrolidone (Soluphor® P) was obtained from BASF Corp., Mumbai, India. Diethylene glycol monoethyl ether (Transcutol® P) was provided by Gattefosse, France. Diethyl ether was purchased from S. D. Fine-Chem Ltd., Mumbai. All other chemicals and solvents used were of analytical grade.
Preparation of self-emulsifying drug delivery system of nevirapine:
The developed formulation consisted of nevirapine:caprylic acid:Soluphor® P:Transcutol® P (4.5: 8.17: 71.16: 16.17, % w/w). The formulation was prepared by dissolving the nevirapine (50 mg) in the mixture of Transcutol® P (180 mg) and Soluphor® P (792 mg) at 50° in a water bath (Equitron, Chennai, India). Caprylic acid (91 mg) was then added. This mixture was mixed by vortexing (Cyclomixer, Remi Instruments Ltd., Mumbai, India) until a transparent preparation was obtained.
HPLC analysis:
The HPLC analysis was carried out on Jasco high performance liquid chromatography system with a BDS column (LCGC Qualisil C18, 250×4.6 mm, 5 μm). The mobile phase of 10 mM sodium phosphate buffer pH 5.0/acetonitrile (70:30) was pumped at a flow rate of 1.0 ml/min. The column temperature was kept at 25°. The detector was set at 245 nm. The method was validated with respect to accuracy and precision as per ICH guidelines[21].
Pharmacokinetic study:
Bioavailability studies were performed in male Wistar rats weighing 280 to 350 g. All experiments and protocols described in this study were approved by the Institutional Animal Ethics Committee of Sri Dhanvantary Pharmaceutical Analysis and Research Centre, Surat, India and were in accordance with guidelines of the Committee for Purpose of Control and Supervision of Experiments on Animals, Ministry of Social Justice and Empowerment, Government of India. Two groups were made for the study, and four rats were kept in each group. The animals were fasted overnight prior to the experiment but had free access to water. The developed SEDDS and marketed suspension were administered by oral snode in an equivalent dose of 20 mg/kg of nevirapine. The blood samples (approximately 500 μl) were collected from the retro-orbital vein using a heparinized needle (18-20 size) at 0, 1, 2, 4, 8, 12, 16 and 24 hours after oral administration. The blood samples were collected into a heparinized microcentrifuge tube, subjected to centrifugation on a laboratory centrifuge and supernatant plasma was collected and kept at –20° until analysis.
Frozen plasma samples were thawed just prior to extraction. A 100 μl of plasma sample was transferred in 2 ml centrifuge tube. To that 100 μl of carbamazepine (1μg/ml in methanol) was added as an internal standard and vibrated on cyclomixer for 10 s. Then, 25μl of 0.1M sodium hydroxide was added, vortex-mixed for 20 s and to it 1ml of ethyl acetate was added and vortexed for 2 min. The tube was centrifuged at 15,000 rpm for 20 min at 4°, the organic layer was collected (top layer) and was transferred to clean tube and dried under nitrogen stream at ambient temperature. The residue was reconstituted with a 100 μl aliquot of mobile phase, and 50 μl was injected directly onto the HPLC column at a flow rate of 1.0 ml/min.
Plasma concentration versus time data of nevirapine for rats was analyzed using standard non-compartment analysis. The area under the plasma concentration-time curve (AUC0→t) from zero to 24 h was estimated by the linear trapezoidal method. The relative bioavailability (F) of SEDDS to the suspension was calculated using the Eqn., F =(AUC0-∞(test)/AUC0-∞(reference))×100 %.
Statistical analysis:
Statistical analysis for the determination of differences in diffusion, permeability and in vivo absorption profiles of nevirapine SEDDS and the marketed preparation was assessed by the use of Student's t-test. The pharmacokinetic data between different formulations were compared for statistical significance by Student's t-test. Statistical probability (P) values less than 0.05 were considered significantly different.
RESULTS AND DISCUSSION
The linear range as shown in fig. 1 for the determination of nevirapine was 25-5000 ng/ml (correlation coefficient r2 =0.999). Intra-day and inter-day precision and accuracy of the developed method were assessed by analyzing three quality control samples containing a low, middle and high concentration of nevirapine, respectively 75, 500 and 3500 ng/ml. Precision is the degree of repeatability of a bioanalytical method under normal operational conditions. The precision was determined by repeatability (intra-day) and intermediate precision (inter-day) and reported as % RSD. At three quality control samples concentrations, intra-day precision was 5.12, 0.66 and 2.34 %; inter-day precision was 1.94, 0.75 and 2.34 %, respectively. Accuracy is the percent of analyte recovered by assay from a known added amount. The accuracy was calculated as the average percentage of the nominal concentration. Intra-day accuracy was found to be 94.58, 100.4 and 103.93%; inter-day accuracy was found to be 98.93, 101.09 and 102.55 %, respectively. A representative chromatogram is shown in fig. 2.
Fig. 1.
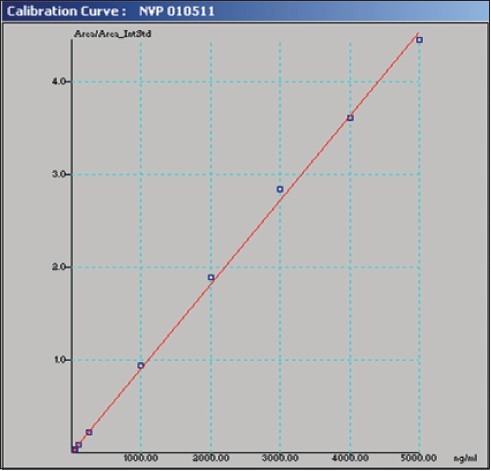
Calibration curve of nevirapine in rat plasma.
Fig. 2.
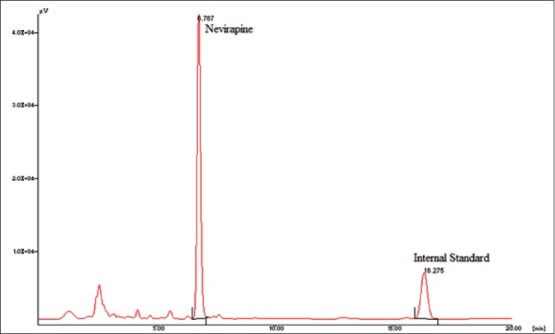
Typical chromatogram of nevirapine and internal standard.
Typical chromatogram showing the elution of nevirapine, Rt at 6.76 min and internal standard, carbamazepine with Rt at 16.27 min.
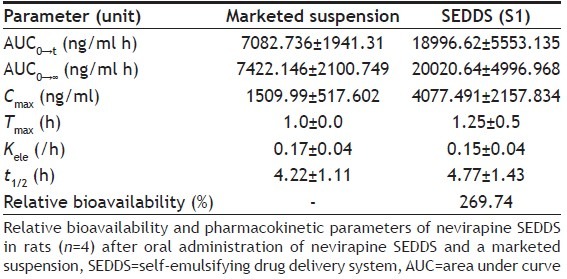
To investigate the oral bioavailability of nevirapine SEDDS, the pharmacokinetic parameters of nevirapine were determined after oral administration of nevirapine SEDDS and marketed conventional suspension to male Wistar rats. Fig. 3 shows the change of mean plasma concentration after oral administration of nevirapine in rats. The calculated relative bioavailability of the nevirapine SEDDS compared to the marketed suspension was 269%. The AUC0-t of nevirapine from SEDDS was found to be significantly higher (P<0.05) as compared to marketed conventional suspension. The Kele and t1/2 values of nevirapine from SEDDS were not much different from suspension. The Cmax of the SEDDS was much higher than the suspension (Table 2).
Fig. 3.
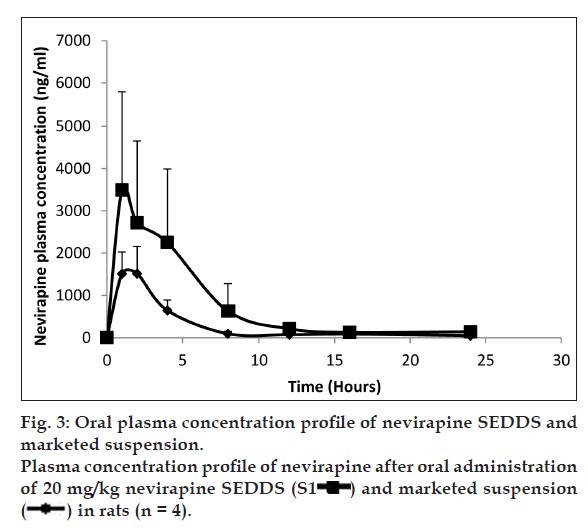
TABLE 2.
RELATIVE BIOAVAILABILITY AND PHARMACOKINETIC PARAMETERS OF NEVIRAPINE SEDDS
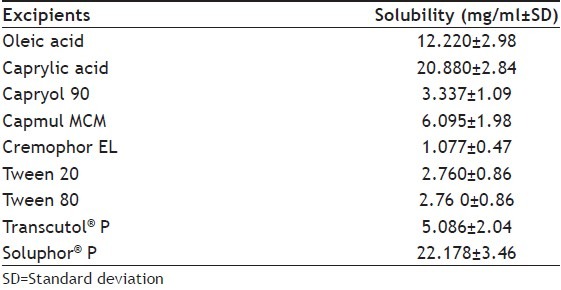
Our earlier study[15] has indicated that among the various surfactants and solubilizers, Soluphor® P has shown the maximum solubility of nevirapine (Table 3). The in vitro diffusion study across dialysis membrane and ex vivo intestinal permeation study across intestine of male wistar rat showed better results (P<0.05) compared to marketed conventional suspension. The present investigation of in vivo absorption in animal model confirms the solubilising and permeation enhancing property of Soluphor® P.
TABLE 3.
SOLUBILITY OF NEVIRAPINE IN VARIOUS EXCIPIENTS
A certain amount of lipid is needed to initiate the biochemical processes involved in the lymphatic transport of drugs[5,22]. Fifty microliters of lipid dosed orally has been shown to be the borderline values[22,23]. In the present study, the amount of fatty acid (caprylic acid) dosed per rat was approximately 90 μl, when the rats were administered with formulation including 8.56% MCT. The amount of oil dosed has been confirmed as being above the threshold. Khoo et al. have demonstrated that formulation approaches using a small lipid volume either long chain or medium chain lipids can stimulate the endogenous release of triglycerides, the formation of lipoproteins and the drug transport through the lymphatic transport[24]. The dependence of portal or lymphatic transport on chain length of the fatty acid is less clear, as there are reports of lymphatic transport of medium chain fatty acids and portal blood absorption of long chain fatty acids[25,26].
Our in vivo results using, caprylic acid having lipid number C8:0 (fig. 4) is in agreement with those obtained with similar medium chain fatty acids reported earlier for several lipophilic drugs[12,27,28,29,30,31,32,33]. During the absorption phase, the drug molecules are usually exposed to the activity of major phase I drug metabolizing enzyme, cytochrome P450 3A4 (CY3A4), present at high concentrations in enterocytes located at the villus tip of the small intestine. The lipid component of the nevirapine SEDDS may shield the drug molecule from the enzymes and/or attenuates the expression and activity of these enzymes[34].
Fig. 4.
Caprylic acid.
The medium chain triglycerides, as reported are hydrolyzed rapidly and help in immediate absorption of lipophilic drugs via portal vein[35]. The higher amount of caprylic acid, a medium chain fatty acid may initially give rise to plasma nevirapine concentration and there after there may be a stimulation of endogenous lipid secretion leading to lipoprotein synthesis and subsequent lymphatic absorption[36,37].
The enhanced oral bioavailability of nevirapine may be due to cumulative effects of medium chain fatty acid and solubilizer. Main mechanism may include transient effect of fatty acid on membrane fluidity[38], transport either via portal route or both portal and lymphatic routes[5,25,39,40] and permeation enhancing property of Soluphor® P[17].
The pharmacokinetic data indicated that the nevirapine SEDDS have better in vivo absorption compared to marketed suspension. The higher bioavailability might be due to the enhanced solubility of nevirapine and the composition of the delivery system. The investigation also supports the wide application of Soluphor® P including dermal and parenteral in pharmaceuticals for poorly water-soluble drugs. In conclusion, the data obtained in the present study highlight the importance of medium chain triglycerides and solubilizer in the enhancement of lipophilic drug by self emulsifying drug delivery system. Despite the fact that MCT are absorbed primarily by portal route and nevirapine being extensively metabolised, our results suggest that the combined effect of rapid hydrolysis and efficient absorption of caprylic acid and permeation enhancing property of Soluphor® P may be responsible for improved bioavailability. It seems that oil component in the formulation may be sufficient enough to trigger endogenous release of triglycerides and resynthesis of medium chain triglycerides to triacylglycerol for formation of chylomicrons and subsequently intestinal lymphatic absorption[39]. However, a further investigation is needed for these observations. The results of the study suggest that both lymphatic transport and absorption via portal blood of nevirapine can be enhanced after co-administration of structured triglyceride vehicle comprising both medium- and long-chain fatty acids. The structured lipid may also provide additional pharmaceutical benefits in the solubility of lipophilic drugs such as nevirapine. As medium chain fatty acids are absorbed into intestinal cells without micellar solubilisation in intestinal lumen, the amount of surfactant/solubilizer may be reduced and will be more compliant for HIV/AIDS patients with debilitated gastrointestinal tract. Much work needs to be done in this area to assess the potential of medium chain triglycerides in development of lipid-based antiretroviral therapy for AIDS patients.
Footnotes
Chudasama, et al.: Nevirapine SEDDS
REFERENCES
- 1.Lindenberg M, Kopp S, Dressman JB. Classification of orally administered drugs on the World Health Organization Model list of Essential Medicines according to the biopharmaceutics classification system. Eur J Pharm Biopharm. 2004;58:265–78. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Pereira BG, Fonte-Boa FD, Resende JA, Pinheiro CB, Fernandes NG, Yoshida MI, et al. Pseudopolymorphs and intrinsic dissolution of nevirapine. Cryst Growth Des. 2007;7:2016–23. [Google Scholar]
- 3.Pantaleo G, Graziosi C, Butini L, Pizzo PA, Schnittman SM, Kotler DP, et al. Lymphoid organs function as major reservoirs for human immunodeficiency virus. Proc Natl Acad Sci U S A. 1991;88:9838–42. doi: 10.1073/pnas.88.21.9838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tenner-Racz K, Racz P, Schmidt H, Dietrich M, Kern P, Louie A, et al. Immunohistochemical, electron microscopic and in situ hybridization evidence for the involvement of lymphatics in the spread of HIV-1. Aids. 1998;2:299–309. doi: 10.1097/00002030-198808000-00010. [DOI] [PubMed] [Google Scholar]
- 5.Holm R, Porter CJ, Mullertz A, Kristensen HG, Charman WN. Structured triglyceride vehicles for oral delivery of halofantrine: Examination of intestinal lymphatic transport and bioavailability in conscious rats. Pharm Res. 2002;19:1354–61. doi: 10.1023/a:1020311127328. [DOI] [PubMed] [Google Scholar]
- 6.Charman WN, Stella VJ. Transport of lipophilic molecules by the intestinal lymphatic system. Adv Drug Deliv Rev. 1991;7:1–14. [Google Scholar]
- 7.O’Driscoll CM. Lipid-based formulations for intestinal lymphatic delivery. Eur J Pharm Sci. 2002;15:405–15. doi: 10.1016/s0928-0987(02)00051-9. [DOI] [PubMed] [Google Scholar]
- 8.Wakerly MG, Pouton CW, Meakin BJ. Evaluation of the selfemulsifying performance of a non-ionic surfactant-vegetable oil mixture. J Pharm Pharmacol. 1987;39:6. [Google Scholar]
- 9.Shah NH, Carvajal MT, Patel CI, Infeld MH, Malick AW. Self-emulsifying drug delivery systems (SEDDS) with polyglycolyzed glycerides for improving in vitro dissolution and oral absorption of lipophilic drugs. Int J Pharm. 1994;106:15–23. [Google Scholar]
- 10.Charman SA, Charman WN, Rogge MC, Wilson TD, Dutko FJ, Pouton CW. Self-emulsifying drug delivery systems: Formulation and biopharmaceutics evaluation of an investigational lipophilic compound. Pharm Res. 1992;9:87–93. doi: 10.1023/a:1018987928936. [DOI] [PubMed] [Google Scholar]
- 11.Porter CJ, Charman WN. Uptake of drugs into the intestinal lymphatics after oral administration. Adv Drug Deliv Rev. 1997;25:71–89. [Google Scholar]
- 12.Sun M, Zhai X, Xue K, Hu L, Yang X, Li G, et al. Intestinal absorption and intestinal lymphatic transport of sirolimus from self-microemulsifying drug delivery systems assessed using the single-pass intestinal perfusion (SPIP) technique and a chylomicron flow blocking approach: Linear correlation with oral bioavailabilities in rats. Eur J Pharm Sci. 2011;43:132–40. doi: 10.1016/j.ejps.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 13.Craig CB, Darnell BE, Weinsier RL, Saag MS, Epps L, Mullins L, et al. Decreased fat and nitrogen losses in patients with AIDS receiving medium-chain-triglyceride-enriched formula vs those receiving long-chain-triglyceride-containing formula. J Am Diet Assoc. 1997;97:605–11. doi: 10.1016/s0002-8223(97)00155-7. [DOI] [PubMed] [Google Scholar]
- 14.Wanke CA, Pleskow D, Degirolami PC, Lambl BB, Merkel K, Akrabawi S. A medium chain triglyceride-based diet in patients with HIV and chronic diarrhea reduces diarrhea and malabsorption: A prospective, controlled trial. Nutrition. 1996;12:766–71. doi: 10.1016/s0899-9007(96)00221-3. [DOI] [PubMed] [Google Scholar]
- 15.Chudasama A, Patel V, Nivsarkar M, Vasu K, Shishoo C. A novel lipid-based oral drug delivery system of nevirapine. Int J PharmTech Res. 2011;3:1159–68. [Google Scholar]
- 16.Femenia-Font A, Padula C, Marra F, Balaguer-Fernandez MV, Lopez-Castellano A, Nicoli S, et al. Bioadhesive monolayer film for the in vitro transdermal delivery of sumatriptan. J Pharm Sci. 2006;95:1561–9. doi: 10.1002/jps.20638. [DOI] [PubMed] [Google Scholar]
- 17.Shah AK, Agnihotri SA. Recent advances and novel strategies in pre-clinical formulation development: An overview. J Control Release. 2011;156:281–96. doi: 10.1016/j.jconrel.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Shende AJ, Patil RR, Devrajan PV. Microemulsion of lamotrigine for nasal delivery. Indian J Pharm Sci. 2007;69:721–2. [Google Scholar]
- 19.Dharap S, Quadir A, Ali S. Solubilization of water insoluble drugs in pharmaceutically accepted solvents and solubilizers. 2005. Available from: http://www.aapsj.org/abstracts/AM_2005/AAPS2005-002930.pdf .
- 20.Ruchatz F, Kotler K. Properties of Soluphor® P as an effective cosolvent in pharmaceutical dosage forms. 1998. Available from: http://www.aapsj.org/abstracts/AM_1998/3780.html .
- 21.ICH Q2 (B) Guidelines: Validation of Analytical Procedure: Methodology Q2(B) 2003 [Google Scholar]
- 22.Porter CJ, Charman SA, Charman WN. Lymphatic transport of halofantrine in the triple-cannulated anesthetized rat model: Effect of lipid vehicle dispersion. J Pharm Sci. 1996;85:351–6. doi: 10.1021/js950221g. [DOI] [PubMed] [Google Scholar]
- 23.Charman WN, Stella VJ. Effects of lipid class and lipid vehicle volume on the intestinal lymphatic transport of DDT. Int J Pharm. 1986;33:165–72. [Google Scholar]
- 24.Khoo SM, Shackleford DM, Porter CJ, Edwards GA, Charman WN. Intestinal lymphatic transport of halofntrine occurs after oral administration of a unit-dose lipid-based formulation to fasted dogs. Pharm Res. 2003;20:1460–5. doi: 10.1023/a:1025718513246. [DOI] [PubMed] [Google Scholar]
- 25.McDonald GB, Weidman M. Partitioning of polar fatty acids into lymph and portal vein after intestinal absorption in the rat. Q J Exp Physiol. 1987;72:153–9. doi: 10.1113/expphysiol.1987.sp003059. [DOI] [PubMed] [Google Scholar]
- 26.McDonald GB, Saunders DR, Weidman M, Fisher L. Portal venous transport of long-chain fatty acids absorbed from rat intestine. Am J Physiol. 1980;239:G141–50. doi: 10.1152/ajpgi.1980.239.3.G141. [DOI] [PubMed] [Google Scholar]
- 27.Singh AK, Chaurasiya A, Singh M, Upadhyay SC, Mukherjee R, Khar RK. Exemestane loaded self-microemulsifying drug delivery system (SMEDDS): Development and optimization. AAPS PharmSciTech. 2008;9:628–34. doi: 10.1208/s12249-008-9080-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peltier S, Oger JM, Lagarce F, Couet W, Benoit JP. Enhanced oral paclitaxel bioavailability after administration of paclitaxel-loaded lipid nanocapsules. Pharm Res. 2006;23:1243–50. doi: 10.1007/s11095-006-0022-2. [DOI] [PubMed] [Google Scholar]
- 29.Dahan A, Hoffman A. Use of a dynamic in vitro lipolysis model to rationalize oral formulation development for poor water soluble drugs: Correlation with in vivo data and the relationship to intra-enterocyte processes in rats. Pharm Res. 2006;23:2165–74. doi: 10.1007/s11095-006-9054-x. [DOI] [PubMed] [Google Scholar]
- 30.Kurowski M, Sternfeld T, Sawyer A, Hill A, Mocklinghoff C. Pharmacokinetic and tolerability profile of twice-daily saquinavir hard gelatine capsules and saquinavir soft gelatine capsules boosted with ritonavir in healthy volunteers. HIV Med. 2003;4:94–100. doi: 10.1046/j.1468-1293.2003.00143.x. [DOI] [PubMed] [Google Scholar]
- 31.Odeberg JM, Kaufmann P, Kroon KG, Hoglund P. Lipid drug delivery and rational formulation design for lipophilic drugs with low oral bioavailability, applied to cyclosporine. Eur J Pharm Sci. 2003;20:375–82. doi: 10.1016/j.ejps.2003.08.005. [DOI] [PubMed] [Google Scholar]
- 32.Greenberger NJ, Rodgers JB, Isselbacher KJ. Absorption of medium and long chain triglycerides: Factors influencing their hydrolysis and transport. J Clin Invest. 1966;45:217–27. doi: 10.1172/JCI105334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Porte D, Jr, Entenman C. Fatty acid metabolism in segments of rat intestine. Am J Physiol. 1965;208:607–14. doi: 10.1152/ajplegacy.1965.208.4.607. [DOI] [PubMed] [Google Scholar]
- 34.Wasan KM. The biological functions of lipid excipients and the implications for pharmaceutical products development. J Pharm Sci. 2009;98:379–82. doi: 10.1002/jps.21435. [DOI] [PubMed] [Google Scholar]
- 35.Jandacek RJ, Whiteside JA, Holcombe BN, Volpenhein RA, Taulbee JD. The rapid hydrolysis and efficient absorption of triglycerides with octanoic acid in the 1 and 3 positions and long-chain fatty acid in the 2 position. Am J Clin Nutr. 1987;45:940–5. doi: 10.1093/ajcn/45.5.940. [DOI] [PubMed] [Google Scholar]
- 36.Kishi T, Carvajal O, Tomoyori H, Ikeda I, Sugano M, Imaizumi K. Structured triglycerides containing medium-chain fatty acids and linoleic acid differently influence clearance rate in serum of triglycerides in rats. Nutr Res. 2002;22:1343–51. [Google Scholar]
- 37.Bernard A, Carlier H. Absorption and intestinal catabolism of fatty acids in the rat: Effect of chain length and unsaturation. Exp Physiol. 1991;76:445–55. doi: 10.1113/expphysiol.1991.sp003511. [DOI] [PubMed] [Google Scholar]
- 38.Mu H, Hoy CE. Effects of different medium-chain fatty acids on intestinal absorption of structured triacylglycerols. Lipids. 2000;35:83–9. doi: 10.1007/s11745-000-0498-x. [DOI] [PubMed] [Google Scholar]
- 39.Caliph SM, Charman WN, Porter CJ. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailbility and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci. 2000;89:1073–84. doi: 10.1002/1520-6017(200008)89:8<1073::aid-jps12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 40.Shiau YF, Popper DA, Reed M, Umstetter C, Capuzzi D, Levine GM. Intestinal triglycerides are derived from both endogenous and exogenous sources. Am J Physiol. 1985;248:G164–9. doi: 10.1152/ajpgi.1985.248.2.G164. [DOI] [PubMed] [Google Scholar]


