Abstract
Aims:
The aim of the study was to study the clinical profile of the patients with Parkinson's disease (PD) and psychosis.
Settings and Design:
This was a prospective, cross sectional, hospital-based study done at the Department of Neurology, National Institute of Mental Health and Neurosciences, Bangalore, India from September 2009 to January 2011. All patients with PD, diagnosed by United Kingdom PD Society Brain Bank criteria, having with features of psychosis as diagnosed by the neuropsychiatric inventory (NPI) were included. Patients without a caregiver who could validate the patient's symptoms were excluded.
Results:
A total of 40 patients (5 women, 35 men) with PD with psychosis (mean age: 54.2 ± 11.5 years, mean duration of illness: 6.5 ± 4.5 years, and mean duration of psychosis: 4.3 ± 4.3 years) were included in the study. The Global NPI score was 19.1 ± 11.5. Majority of the patients had pure hallucinations (85%), while the rest had either pure delusions (7.5%) or a combination of delusions and hallucinations (7.5%). In those with hallucinations, visual hallucinations were the commonest (60%) (pure only in 22.5%), followed by auditory (45%), minor hallucinations (45%), and tactile (20%). Only one person reported having olfactory hallucinations (2.5%). Loss of insight was most often observed during the visual hallucinations (52%), followed by tactile (44.4%), auditory (38.9 %), and minor hallucinations (33.3%).
Conclusions:
In patients with PD and psychosis, pure hallucinations are common and visual hallucinations are the commonest among the hallucinations. A large proportion of patients have minor hallucinations, which need to be recognized early for effective and early management. The limitations of the study were small sample size, use of a single scale to assess psychosis and subjective assessment of insight.
Keywords: Delusions, hallucinations, NPI, PD, psychosis
Introduction
Parkinson's disease (PD) is one of the commonest movement disorders in the world.[1] In spite of PD being a predominantly motor disorder, majority of patients develop a variety of non-motor manifestations. The most challenging nonmotor symptom is psychosis. The clinical spectrum of psychosis in PD includes visual hallucinations with or without retained insight, other hallucinations (auditory, tactile, olfactory, and minor hallucinatory phenomena) and delusions.[2] These psychotic symptoms have a major impact on the natural course and prognosis of the disease and are important risk factors for nursing home placement and mortality of PD.[3,4]
The prevalence of the psychotic symptoms have varied largely in most studies, mostly owing to a lack of uniform definition of psychosis, lack of disease-specific scales to measure psychosis, and also use of scales that are not validated to measure psychotic symptoms.[5,6,7] In addition, there are only limited data on the pattern of psychosis in PD, especially from India.[8] Therefore, the present study was undertaken to study the clinical profile of the patients with Parkinson's disease and psychosis using the neuropsychiatric inventory (NPI) for rating of psychotic symptoms. Although various scales have been used to assess psychosis in PD,[9,10,11] the NPI is a well-validated scale to screen for various psychotic symptoms in PD.[12]
Materials and Methods
This prospective, cross-sectional, hospital-based study was conducted at the Department of Neurology, National Institute of Mental Health and Neurosciences, Bangalore, Karnataka, India from September 2009 to January 2011. The study was approved by the institute's ethics committee and all the patients and their caregivers who were interviewed gave written informed consent. A diagnosis of PD was made according to the United Kingdom PD Society (UKPDS) Brain Bank criteria for PD[13] and all patients were evaluated by the UPDRS-part III (motor part) scale,[13] Hoehn and Yahr staging, the NPI,[12] the mini mental status examination (MMSE) score for cognitive screening.
The NPI evaluates 12 neuropsychiatric domains: delusions, hallucinations, agitation, depression, anxiety, apathy, irritability, euphoria, disinhibition, aberrant night time behavior, night time behavior, and appetite change. The frequency, severity, and distress of each domain is rated on the basis of scripted questions administered to the patient's caregiver. The product of frequency and severity of each domain and the total NPI score was calculated in all patients. The test-retest variability, interrater variability has been already established.[12] The patients were diagnosed to have psychosis based on diagnostic criteria for psychosis in PD.[14] The diagnosis of PD associated psychosis was made in a case of PD with symptoms starting after the onset of PD with the presence of at least one of the following symptoms (1) illusions, (2) false sense of presence, (3) hallucinations, and (4) delusions. The symptoms should last for at least 1 month and the other mimickers should be excluded.[14] The patients who were excluded were those as per UKPDS brain bank criteria, those who were unwilling to participate in the study or did not cooperate for the interview and those without a caregiver who could validate the history of patient's symptoms. The patients with cognitive deficits were not excluded.
A predesigned proforma was used to record comprehensively all the information pertaining to PD and the psychotic symptoms. Hallucinations, when present, were classified into visual, auditory, tactile, olfactory, minor, or mixed. Further characterization of hallucinations included frequency, threatening or nonthreatening, presence or absence of insight, time of occurrence, and so on. All effort was made to collect accurate information of the past and current medications for Parkinsonian symptoms as well for other disorders, if any. Details of anti-Parkinsonian drugs, especially dosage, duration of treatment, and temporal association between medications and onset of psychosis were ascertained. The total levodopa equivalent dosage (TLED) was calculated after converting all drug dosages patients were currently taking into their levodopa equivalent dosages.[15] These were then added up and the mean TLED calculated.
Statistical analysis
Statistical analysis was done using SPSS 16.0 package. Data were expressed using descriptive statistics, that is, mean, standard deviation (SD) for continuous variables and frequency, percentages for categorical variables. Comparisons between groups were done using independent Student's t-test for continuous variables and chi-square test for categorical variables. Correlation between continuous variables was done using Pearson's correlation coefficient. A P < 0.05 was considered statistically significant.
Results
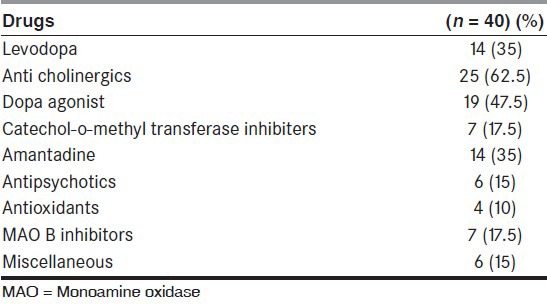
A total of 40 patients were recruited for this study. The mean TLED was 683.36 ± 404.33 mg/day [Table 1]. Majority of the patients had prior use of anticholinergics (62.5%), followed by use of dopamine agonists (47.5%), levodopa (35%), and amantadine (35%). Approximately, 15% of patients had prior exposure to antipsychotics [Table 2]. Six patients had an MMSE <24.
Table 1.
Demographic variables of patients with Parkinson's disease and Psychosis (n = 40)
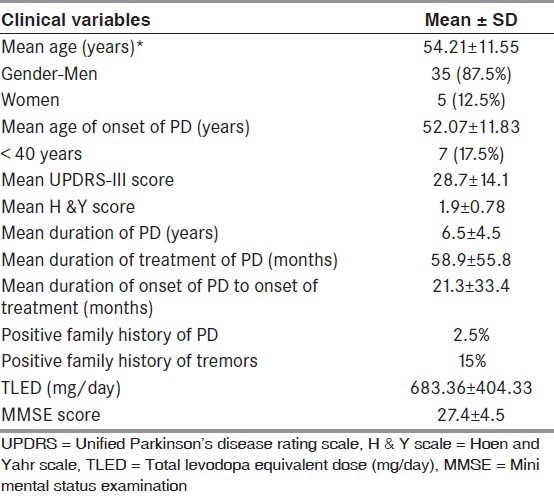
Table 2.
Pharmacotherapy in patients with Parkinson's disease and psychosis
Characteristics of psychosis
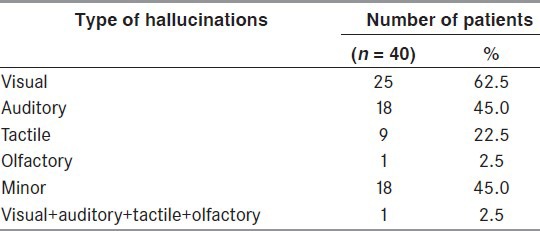
The mean duration of psychotic symptoms (hallucinations/delusions) was 4.34 ± 4.26 years. The psychotic symptoms included pure hallucinations in 34 patients (85%), pure delusions in 3 patients (7.5%), and a combination of delusions and hallucinations in 3 patients (7.5%) [Tables 3-6]. Visual hallucinations were the commonest type of hallucinations, followed by other hallucinations, such as auditory, minor, tactile, and olfactory [Table 5]. Majority of the patients had a mixed type of hallucinations (45%). Nine (22.5%) had pure visual hallucinations, five (12.5%) had pure auditory, and only two patients (5%) had minor hallucinations. A combination of visual and auditory hallucinations was seen in six patients (15%).
Table 3.
Neuropsychiatric inventory (NPI) domains of delusions and hallucinations in patients with Parkinson's disease and psychosis
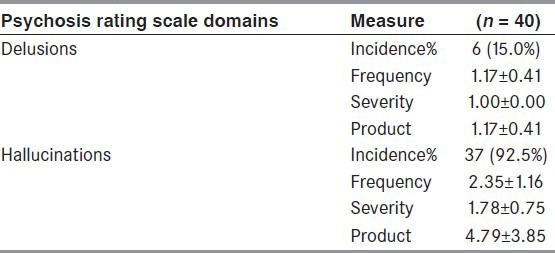
Table 6.
Pattern of visual and auditory hallucinations in patients with Parkinson's disease and psychosis
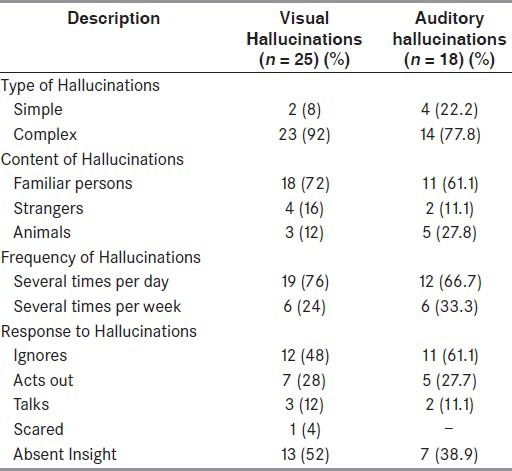
Table 5.
The types of hallucinations observed in patients of Parkinson's disease with psychosis
NPI
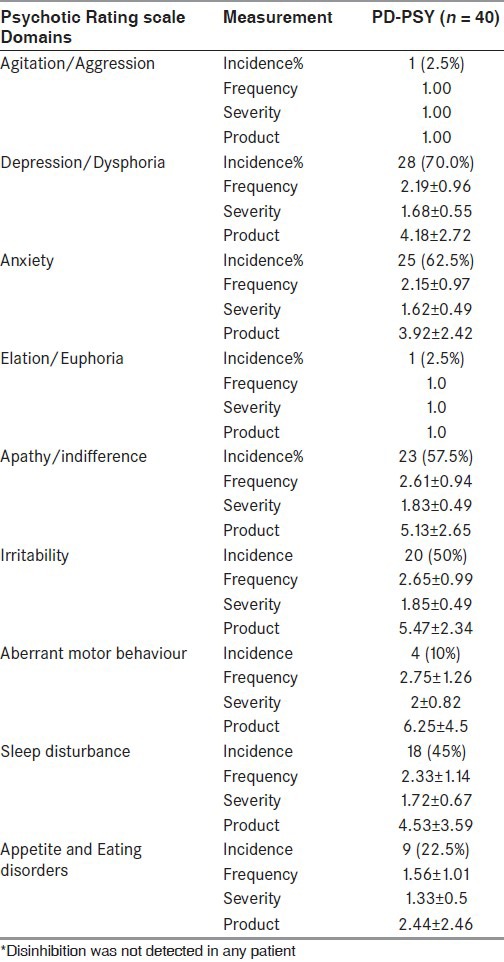
NPI consisted of 12 domains. The global NPI score was calculated by calculating the sum of products of all domains and then the mean NPI score was calculated [Tables 3 and 4]. The mean NPI score was 15.70 ± 11.25 in the study group (range: 1-55).
Table 4.
Details of NPI domains of agitation, depression, anxiety, elation, apathy, irritability, aberrant motor behaviour, sleep disturbance, appetite and eating disorder
Visual hallucinations
A total of 11 patients (44%) had hallucinations only during the ON-period after taking various Parkinsonian drugs and 3 patients (12%) had them only during the OFF-period [Tables 5 and 6]. A total of 11 patients (44%) reported no specific relation of hallucinations to drug intake.
Auditory hallucinations
Most (11; 61.1%) patients heard human voices either talking among themselves or talking to the patient, two patients heard inanimate or animal sounds like bullet sounds, dogs, barking, or tigers and lions roaring, tinkling of bells [Tables 5 and 6]. Four patients had hallucinations during the ON-period of various Parkinsonian drugs (22.2%) and two patients had them during the OFF-period (11.1%). A total of 12 patients reported no specific relation of hallucinations to drug intake (66.7%).
Minor hallucinations
There were 18 patients (45%) with minor hallucinations, which included passage hallucinations in 7 (38.6%), sense of presence in 6 (33.3%), and a combination of both in 5 (27.8%) [Table 5]. Majority of patients reported a perception of seeing people (94.4%), one person reported a feeling of seeing a white cloth passing by him in the peripheral field of vision. Most of these hallucinations were stereotyped (83.3%) and nonthreatening in nature (77.8%). Insight into the hallucinations was absent in 6 patients (33.3%) and present in 12 patients (66.7%). Four patients had hallucinations during the ON-period of various Parkinsonian drugs (22.2%) and three patients had them during the OFF-period (16.7%). In 11 patients, there was no specific temporal relationship of the occurrence of hallucinations to drug intake (61.1%).
Tactile hallucinations
There were nine patients with tactile hallucinations. Five patients reportedly felt contact with insects and small animals (ants, mosquitoes crawling on hands and legs, one person reportedly felt snakes crawling on his legs), four patients reported contact with small insects [Table 5]. Most of the hallucinations were stereotyped (88.9%) and nonthreatening in nature (88.9%).
Olfactory hallucinations
A 66-year-old man was the only patient who reported presence of olfactory hallucinations [Table 5]. He used to perceive smell of oils and spices used during cooking.
The global NPI score correlated significantly with UPDRS (r = 0.43, P = 0.006) and H&Y Stage (r = 0.32, P = 0.05).
Discussion
The clinical features of psychosis in PD have been studied and there are various clinicoradiological and clinicopathological correlations showing the involvement of parahippocampus and amygdala and multiple neurotransmitter pathways.[16] Our study, which focussed on the clinical characterisation of psychosis in patients with PD, had several significant observations.
We found a higher mean age of onset of PD at presentation, younger age of onset of PD, and longer duration of illness in patients with PD and psychosis compared with previous studies.[17,18] This was due to a higher number of younger-onset patients with PD (17.5%) in our study.
Papapetropoulos et al.,[17] compared 31 patients of PD with psychosis with 39 patients without psychosis. They reported a similar age of onset of 54.4 ± 12.5 years in hallucinators and 55.8 ± 11.8 years in nonhallucinators. They also reported a duration of 9.5 ± 5.6 years in hallucinators and 8.5 ± 5.3 years in nonhallucinators.[17] Our study also revealed a significantly longer duration of illness (6.5 ± 4.5 years) in psychotic patients, though the mean age of onset of psychosis (54.21 ± 11.55 years) was slightly less as compared with the above study.[17]
Even though PD is viewed as a homogeneous group, some studies have tried to divide PD into subgroups: younger onset, tremor-dominant, nontremor dominant, and rapid disease progression.[19,20] It has also been found that younger-onset patients and tremor-dominant patients have a slower rate of progression and a lesser cognitive impairment than the nontremor dominant subgroup.[21] But whether these subgroups of PD patients have any relation to the future development of psychosis has not been studied till now. The higher incidence of cognitive impairment in patients of PD with psychosis suggests that the presence of psychosis may itself be a risk factor for future development of dementia. Even though PD has been traditionally thought to spare the intellectual functions, dementia has been reported to range from 40% to 78%.[22,23,24]
Determinants of psychosis
The severity of PD as measured by modified H&Y stage was high in the study group (1.9 ± 0.78). A study by Aarsland et al.,[18] reported higher UPDRS “ON” scores in patients with hallucinations (44.7 ± 15.4) compared with patients without hallucinations (24.0 ± 13.4). The authors also reported higher H&Y stage in patients with hallucinations (stages 3-5) as compared with patients without hallucinations (stages 2-3).[18] Duration of illness and Hand Y stage were also found significant determinants of psychosis by Gupta et al.,[8] from India. Our patients with psychosis also had a higher stage of severity of PD as measured by H&Y staging.
Treatment history
All the available dopaminergic drugs may produce adverse psychotic reactions, there being a higher incidence with dopamine receptor agonists than with levodopa.[25] In PD, the psychotic symptoms may reduce after a decrease in dopaminergic medication. However, there is no simple dose-response relationship between dopaminergic treatment and development of hallucinations.[25] Moreover, two prospective studies by Sanchez-Ramos et al., and Graham et al.,[26,27] have reported that hallucinations were not associated with the dosage of dopaminergic medication (levodopa or dopaminergic agonists). However in the present study, PD patients with psychosis had high TLED which indicates that our group had advanced PD as shown by H & Y stage, therefore required higher doses. A large proportion of our patients had prior exposure to anticholinergics (62.5%) and dopaminergic agonists (47.5%), reflecting the prescription patterns to treat PD in the community. Anticholinergic drug usage has been found to be a predictor of severe psychosis by Sawada et al.,[28] although it was not considered significant in another study.[29]
The mean TLED in our study was 683.4 ± 404.3 mg. Sanchez-Ramos et al., reported mean levodopa dosage of 426 ± 216 mg in psychosis patients and 443 ± 310 mg in nonpsychosis group while Graham et al., reported a dosage of 614 ± 433 mg in late hallucinators group and 667 ± 286 mg in the non-hallucinators group.[26,27] In addition, Fenelon et al., reported a levodopa dose of 367 ± 154 mg in patients with visual hallucinations (disease duration of <5 years) and 431 ± 204 mg in patients without hallucinations (disease duration of >5 years).[29] Therefore, the dose-response relationship of levodopa with psychosis in PD is still not clear.
Clinical profile of psychosis in PD
Visual hallucination was the most frequent type of hallucination observed in our patients. Molho et al.,[30] reported visual hallucinations in about 30% of patients. They described the presence of animals and inanimate objects as a part of visual hallucinations. These authors also reported that 28% of the hallucinations were threatening to the patients. Another study by Fenelon et al.,[25] also reported visual hallucinations which consisted of complex hallucinations, involving familiar persons, single or few in number, seen usually in dim light, superimposed on a normal background. The findings of our study are comparable to these previous studies. The threatening visual hallucinations were reported by three (8%) patients in our study.
Auditory hallucinations were seen in 45% of patients in our study. Most of the previous studies have reported auditory hallucinations occurring as a part of visual hallucinations[25,27,30,31] which was also seen in our study. Pure auditory hallucinations are rare in PD and there has been no systematic report of auditory hallucinations in PD. Other studies have reported an incidence varying from 25% to 40%.[18,25,31 Molho et al.,[30] reported auditory hallucinations which consisted of musical tunes of a particular type which the patient could not identify. Fenelon et al.,[25] also reported auditory hallucinations occurring as a “sound-track” of visual hallucinations.
In our study, minor hallucinations were seen in a large number of patients (45%). These hallucinations have been largely ignored in most studies. Fenelon et al.,[7,25] reported that if these types of hallucinations were taken into account, the prevalence of hallucinations would increase to about 40%-75%. The authors reported minor hallucinations which consisted of seeing an inanimate object as a living being and passage hallucinations consisting of a brief vision of a person or an animal passing sideways.[7]
The importance of minor hallucinations like presence and passage is significant in view of the new diagnostic criteria given by Ravina et al.,[14] where it is incorporated as one of the four characteristic features for diagnosis of PD psychosis.
Tactile hallucinations were seen in 22.5% of patients in our study. Fenelon et al.,[25] reported tactile hallucinations consisting of contact with small animals or a feeling of being touched by someone. They also reported that tactile hallucinations are usually combined with visual hallucinations which increase their realistic experience, which was not seen in our study. One patient in our study reported having olfactory hallucinations. He reported having a sense of smell of oily and spicy foods, occurring in the afternoons, during the off periods with a retained insight. Olfactory hallucinations are extremely rare and reported in 2.1%-10% of study populations in different studies.[32,33] [Table 6]
There were several limitations in our study namely, small sample size, chance of investigator bias, lack of follow-up data that could have given information of treatment response in the patients with psychosis and the limitation of NPI as an instrument to assess psychosis in PD. Insight was assessed only subjectively; hence, this was also a limitation of the study.
To conclude, among our patients with PD and psychosis, visual hallucinations were most common, followed by auditory and tactile hallucinations. Keeping in mind the implications of psychosis in PD, this study highlights the common patterns of hallucinations and delusions observed in a cohort of Indian patients with PD. Minor hallucinations in patients with PD have been largely ignored in most previous studies. These are harbinger of psychosis in the future. By giving importance to these minor hallucinations, our study has attempted to characterize these hallucinations, the recognition of which is as important as other common types of hallucinations.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Zhang ZX, Roman GC. Worldwide occurrence of Parkinson's disease: An updated review. Neuroepidemiol. 1993;12:195–208. doi: 10.1159/000110318. [DOI] [PubMed] [Google Scholar]
- 2.Weintraub D, Hurtig HI. Presentation and management of psychosis in Parkinson's disease and dementia with Lewy bodies. Am J Psychiatry. 2007;164:1491–8. doi: 10.1176/appi.ajp.2007.07040715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz CG, Wuu J, Curgian L, Leurgans S. Age-related influences on the clinical characteristics of new-onset hallucinations in Parkinson's disease patients. Mov Disord. 2006;21:267–70. doi: 10.1002/mds.20701. [DOI] [PubMed] [Google Scholar]
- 4.Hassan A, Wu SS, Schmidt P, Dai Y, Simuni T, Giladi N, et al. High rates and the risk factors for emergency room visits and hospitalization in Parkinson's disease. Parkinsonism Relat Disord. 2013;19:949–54. doi: 10.1016/j.parkreldis.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 5.Rabey JM. Hallucinations and psychosis in Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 4):S105–10. doi: 10.1016/S1353-8020(09)70846-6. [DOI] [PubMed] [Google Scholar]
- 6.Fénelon G, Soulas T, Zenasni F, Cleret de Langavant L. The changing face of Parkinson's disease-associated psychosis: A cross-sectional study based on the new NINDS-NIMH criteria. Mov Disord. 2010;25:763–6. doi: 10.1002/mds.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fénelon G, Soulas T, Cleret de Langavant L, Trinkler I, Bachoud-Lévi AC. Feeling of presence in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2011;82:1219–24. doi: 10.1136/jnnp.2010.234799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta M, Singh G, Khwaja GA, Mehndiratta MM. Hallucinations in Parkinson's disease--a study of forty three patients. J Assoc Physicians India. 2004;52:703–6. [PubMed] [Google Scholar]
- 9.Martinez-Martin P, Frades-Payo B, Agüera-Ortiz L, Ayuga-Martinez A. A short scale for evaluation of neuropsychiatric disorders in Parkinson's disease: First psychometric approach. J Neurol. 2012;259:2299–308. doi: 10.1007/s00415-012-6490-x. [DOI] [PubMed] [Google Scholar]
- 10.Cargaleiro I, Serra M, Alves da Silva J, Neto B, Gonçalves-Pereira M, Bugalho P. Psychosis assessment in early-stage Parkinson's disease: Comparing Parkinson's psychosis questionnaire with the brief psychiatric rating scale in a portuguese sample. Parkinsons Dis 2012. 2012:469126. doi: 10.1155/2012/469126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Voss T, Bahr D, Cummings J, Mills R, Ravina B, Williams H. Performance of a shortened Scale for Assessment of Positive Symptoms for Parkinson's disease psychosis. Parkinsonism Relat Disord. 2013;19:295–9. doi: 10.1016/j.parkreldis.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JL, Mega M, Gray K, Rosenberg-Thompson S, Carusi DA, Gornbein J. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology. 1994;44:2308–14. doi: 10.1212/wnl.44.12.2308. [DOI] [PubMed] [Google Scholar]
- 13.Movement Disorder Society Task Force on Rating Scales for Parkinson's Disease. The Unified Parkinson's Disease Rating Scale (UPDRS): Status and recommendations. Mov Disord. 2003;18:738–50. doi: 10.1002/mds.10473. [DOI] [PubMed] [Google Scholar]
- 14.Ravina B, Marder K, Fernandez HH, Friedman JH, McDonald W, Murphy D, et al. Diagnostic criteria for psychosis in Parkinson's disease: Report of an NINDS, NIMH work group. Mov Disord. 2007;22:1061–8. doi: 10.1002/mds.21382. [DOI] [PubMed] [Google Scholar]
- 15.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 16.Harding AJ, Broe GA, Halliday GM. Visual hallucinations in Lewy body disease relate to Lewy bodies in the temporal lobe. Brain. 2002;125(Pt 2):391–403. doi: 10.1093/brain/awf033. [DOI] [PubMed] [Google Scholar]
- 17.Papapetropoulos S, Katzen H, Schrag A, Singer C, Scanlon BK, Nation D, et al. A questionnaire-based (UM-PDHQ) study of hallucinations in Parkinson's disease. BMC Neurol. 2008;8:21. doi: 10.1186/1471-2377-8-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aarsland D, Larsen JP, Cummins JL, Laake K. Prevalence and clinical correlates of psychotic symptoms in Parkinson disease: A community-based study. Arch Neurol. 1999;56:595–601. doi: 10.1001/archneur.56.5.595. [DOI] [PubMed] [Google Scholar]
- 19.Lewis SJ, Foltynie T, Blackwell AD, Robbins TW, Owen AM, Barker RA. Heterogeneity of Parkinson's disease in the early clinical stages using a data driven approach. J Neurol Neurosurg Psychiatry. 2005;76:343–8. doi: 10.1136/jnnp.2003.033530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goetz CG, Tanner CM, Stebbins GT, Buchman AS. Risk factors for progression in Parkinson's disease. Neurology. 1988;38:1841–4. doi: 10.1212/wnl.38.12.1841. [DOI] [PubMed] [Google Scholar]
- 21.Paleacu D, Schechtman E, Inzelberg R. Association between family history of dementia and hallucinations in Parkinson disease. Neurology. 2005;64:1712–5. doi: 10.1212/01.WNL.0000161872.85903.8E. [DOI] [PubMed] [Google Scholar]
- 22.Cummings JL. The dementias of Parkinson's disease: Prevalence, characteristics, neurobiology, and comparison with dementia of the Alzheimer type. Eur Neurol. 1988;28(Suppl 1):15–23. [PubMed] [Google Scholar]
- 23.Cummings JL. Intellectual impairment in Parkinson's disease: Clinical, pathologic, and biochemical correlates. J Geriatr Psychiatry Neurol. 1988;1:24–36. doi: 10.1177/089198878800100106. [DOI] [PubMed] [Google Scholar]
- 24.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sørensen P. Prevalence and characteristics of dementia in Parkinson disease: An 8-year prospective study. Arch Neurol. 2003;60:387–92. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 25.Fenelon G. Psychosis in Parkinson's disease: Phenomenology, frequency, risk factors, and current understanding of pathophysiologic mechanisms. CNS Spectr. 2008;13(3 Suppl 4):18–25. doi: 10.1017/s1092852900017284. [DOI] [PubMed] [Google Scholar]
- 26.Sanchez-Ramos JR, Ortoll R, Paulson GW. Visual hallucinations associated with Parkinson disease. Arch Neurol. 1996;53:1265–8. doi: 10.1001/archneur.1996.00550120077019. [DOI] [PubMed] [Google Scholar]
- 27.Graham JM, Grünewald RA, Sagar HJ. Hallucinosis in idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry. 1997;63:434–40. doi: 10.1136/jnnp.63.4.434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawada H, Oeda T, Yamamoto K, Umemura A, Tomita S, Hayashi R, et al. Trigger medications and patient-related risk factors for Parkinson disease psychosis requiring anti-psychotic drugs: A retrospective cohort study. BMC Neurol. 2013;13:145. doi: 10.1186/1471-2377-13-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fénelon G, Mahieux F, Huon R, Ziégler M. Hallucinations in Parkinson's disease: Prevalence, phenomenology and risk factors. Brain. 2000;123(Pt 4):733–45. doi: 10.1093/brain/123.4.733. [DOI] [PubMed] [Google Scholar]
- 30.Molho ES, Factor SA. Parkinson's disease: The treatment of drug-induced hallucinations and psychosis. Curr Neurol Neurosci Rep. 2001;1:320–8. doi: 10.1007/s11910-001-0085-8. [DOI] [PubMed] [Google Scholar]
- 31.Inzelberg R, Kipervasser S, Korczyn AD. Auditory hallucinations in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1998;64:533–5. doi: 10.1136/jnnp.64.4.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bannier S, Berdagué JL, Rieu I, de Chazeron I, Marques A, Derost P, et al. Prevalence and phenomenology of olfactory hallucinations in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2012;83:1019–21. doi: 10.1136/jnnp-2012-302414. [DOI] [PubMed] [Google Scholar]
- 33.McAuley JH, Gregory S. Prevalence and clinical course of olfactory hallucinations in idiopathic Parkinson's disease. J Parkinsons Dis. 2012;2:199–205. doi: 10.3233/JPD-2012-012086. [DOI] [PubMed] [Google Scholar]


