Abstract
Aims:
In progressive supranuclear palsy (PSP) tissue damage occurs in specific cortical and subcortical regions. Voxel based analysis using T1-weighted images depict quantitative gray matter (GM) atrophy changes. Magnetization transfer (MT) imaging depicts qualitative changes in the brain parenchyma. The purpose of our study was to investigate whether MT imaging could indicate abnormalities in PSP.
Settings and Design:
A total of 10 patients with PSP (9 men and 1 woman) and 8 controls (5 men and 3 women) were studied with T1-weighted magnetic resonance imaging (MRI) and 3DMT imaging. Voxel based analysis of T1-weighted MRI was performed to investigate brain atrophy while MT was used to study qualitative abnormalities in the brain tissue. We used SPM8 to investigate group differences (with two sample t-test) using the GM and white matter (WM) segmented data.
Results:
T1-weighted imaging and MT are equally sensitive to detect changes in GM and WM in PSP. Magnetization transfer ratio images and magnetization-prepared rapid acquisition of gradient echo revealed extensive bilateral volume and qualitative changes in the orbitofrontal, prefrontal cortex and limbic lobe and sub cortical GM. The prefrontal structures involved were the rectal gyrus, medial, inferior frontal gyrus (IFG) and middle frontal gyrus (MFG). The anterior cingulate, cingulate gyrus and lingual gyrus of limbic lobe and subcortical structures such as caudate, thalamus, insula and claustrum were also involved. Cerebellar involvement mainly of anterior lobe was also noted.
Conclusions:
The findings suggest that voxel based MT imaging permits a whole brain unbiased investigation of central nervous system structural integrity in PSP.
Keywords: Magnetization transfer ratio, progressive supranuclear palsy, progressive supranuclear palsy classic subtype, progressive supranuclear palsy Parkinson subtype, voxel based morphometry
Introduction
Progressive supranuclear palsy (PSP) is a sporadic, progressive, neurodegenerative disease presenting as an akinetic rigid syndrome with postural instability, supranuclear gaze palsy and frontal dementia.[1] It was described as a separate clinicopathological entity in 1964.[2] Pathologically it is described as a taupathy with neuro fibrillary tangles in neurons, neuropil threads in neuritis, coiled bodies in oligodendrocytes, tufted astrocytes in gray matter (GM) in midrain, superior cerebellar peduncle and basal ganglia.[3] PSP has a slight male preponderance and a prevalence of 1.4 cases/1,00,000 population. PSP affects 4-6% of patients with parkinsonian symptoms.[4] PSP is usually fatal within 6 years of onset.[5] The major cause of concern in patients of PSP are dementia, dysphagia, immobility and visual symptoms.
Clinically patients with PSP present with slow walking with erect or retroflexed posture which evolves into lurching steps and a tendency to topple forward or backward. PSP patients typically have a stiff gait with the legs extended at the knee (not flexed, as is typical in PD patients) and pivoting (rather than en bloc shuffle) when turning. In PSP patient's postural instability is disproportionate to other parkinsonian signs. Gait difficulties progress at a faster rate in PSP patients as compared to the Parkinson's disease (PD) patients. Unlike typical PD, in PSP falls begin within the 1st year and by 3rd year falls are common unless precautions are taken to prevent them.
Diagnosis of this entity is based on clinical criteria.[6] The early diagnosis remains a challenge since the clinical criteria may be insensitive.[7] Hence there is a need for supportive imaging methods. Many studies on both conventional and advanced magnetic resonance imaging (MRI) techniques are available including magnetic resonance planimetry, voxel based morphometry (VBM) analysis, magnetization transfer (MT) imaging and diffusion tensor imaging (DTI) for evaluation of these patients.[8]
VBM is based on co registration of high-resolution 3D datasets as obtained by 3D magnetization-prepared rapid acquisition of gradient echo (MPRAGE) or 3DMT sequences, which are normalized to a study-specific template for detection of volume differences between 2 or more groups. Contrary to operator-dependent segmentation techniques including region of interest selection, VBM permits an operator-independent and automated detection of significant differences in different tissue types of the whole brain, involving voxel-wise statistical analysis of preprocessed structural MRI.[9]
Disadvantage of this technique is that it involves group wise comparisons and hence routine diagnostic work-up of individual patients is difficult. Varied methodological options during processing are available and are known for pitfalls.[10] Few studies do not account for correction of alignment inaccuracies and intracranial volume (ICV) correction with resulting misinterpretation of apparent results in voxel that do not correspond to same anatomical structures in all subjects.[11]
Conventional MRI has not proved to be sufficiently sensitive to PSP related cerebral tissue damage. Voxel based analysis of T1-weighted MRI has indicated widespread cortical and sub cortical atrophy in line with the distribution of micro structural damage in advanced stages of disease. GM loss in PSP patients has been found in frontotemporal cortical areas such as the prefrontal cortex, the insular region comprising the frontal opercula, the supplementary motor areas and the left mediotemporal area, subthalamic region and midbrain tegmentum[12] whereas white matter (WM) loss has been additionally reported in the midbrain including cerebellar peduncles and central midbrain.[13,14] Clinical utility of the VBM lies in the fact that it guides to allocate the subject to either PSP or “non-PSP” based on the presence or absence of the midbrain tissue loss on T1-weighted images with a sensitivity of 83% and a specificity of 79%.[12,14] Limitation of volumetric measurements is that they are sensitive to detect severe tissue damage as opposed to subtle reductions of tissue integrity that occurs without volume loss.
MT imaging is a sensitive sequence to detect subtle reductions of tissue integrity that occurs before volume loss and relies on continuous interchange of magnetization between free protons and protons bound to macromolecules and thus permits indirect measurement of tissue integrity. It has been used to provide quantitative measurements of central nervous system damage in multiple sclerosis and other diseases.[15]
Our hypothesis is that MT imaging would be an additional marker and a more sensitive sequence to assess progression of PSP compared with T1-weighted imaging. We postulated that MT would reveal injury to existing tissue in areas already affected by atrophy and would indicate subtle damage in additional locations.
In the current study, we investigated the cerebral tissue changes in a group of patients with PSP with voxel based analyses of magnetization transfer ratio (MTR) images and T1-weighted images.
Materials and Methods
Patients were recruited from the out-patient Department of Neurology and the PD and Movement Disorder Clinic of the National Institute of Mental Health and Neurosciences, a Tertiary Referral Centre in Southern India, Bangalore. The inclusion criteria were patients diagnosed as probable/possible PSP using National Institute of Neurological Disorders and the Society for Progressive Supranuclear Palsy (NINDS-SPSP) clinical criteria for the diagnosis of PSP and exclusion criteria as per the NINDS-SPSP clinical criteria for the diagnosis of PSP. “Possible PSP” requires the presence of a gradually progressive disorder with onset at age 40 or later, either vertical supranuclear gaze palsy or both slowing of vertical saccades and prominent postural instability with falls in the 1st year of onset, as well as no evidence of other diseases that could explain these features. “Probable PSP” requires vertical supranuclear gaze palsy, prominent postural instability and falls in the 1st year of onset, as well as the other features of possible PSP. The exclusion criteria included any recent history of encephalitis, features of Alien limb syndrome, cortical sensory deficits, focal frontal or temporoparietal atrophy, Hallucinations or delusions unrelated to dopaminergic therapy, Cortical dementia of Alzheimer's type (severe amnesia and aphasia or agnosia, according to NINCDS-ADRA criteria), Prominent, early cerebellar symptoms or prominent, early unexplained dysautonomia (marked hypotension and urinary disturbances), Severe, asymmetric parkinsonian signs (i.e. bradykinesia)., Neuroradiologic evidence of relevant structural abnormality (i.e. basal ganglia or brainstem infarcts, lobar atrophy), Whipple's disease, confirmed by polymerase chain reaction, if indicated.
Assessments
All patients enrolled in the study underwent the following assessments: (a) A detailed neurological examination for the diagnosis of PSP and clinical classification of subtypes of PSP. (b) MRI of brain (B). Controls: All the controls underwent neurological examination and MRI of brain.
In all there were 10 patients with PSP (9 Men 1 woman) and 8 controls (5 men and 3 women). They were studied with T1-weighted MRI and MT imaging. Voxel based analysis of T1-weighted MRI was performed to investigate brain atrophy while MT was used to study qualitative abnormalities in the brain tissue.
Protocol
Patients and controls underwent MRI on 3T (Achieva Philips). To assess regional volume differences of cortical GM with VBM, T1-weighted images were acquired by using a MPRAGE sequence (165 sagittal sections, TR = 8.3 ms, TE = 3.9 ms, voxel size = 1 mm3, flip angle = 8°). Inversion pulse is used to provide good contrast of T1-weighted images in a MPRAGE sequence of high-resolution 3-dimensional datasets. To assess qualitative differences of cortical WM with VBM 3 Dimensional Magnetisation transfer images were acquired using the following parameters (TR 86, TE 3.8 NSA 12 dynamics MTC FA = 18 slices 24 voxel 1 mm). By selectively saturating the macromolecular proton pool, MT imaging permits quantification of the magnetization exchange between protons in macromolecules and unbound water. The MT effect can be measured as the ratio of voxel intensities known as MTR.
Data analysis
The MTR was calculated using Image J software and voxel based MTR analysis was done in SPM8 software and MATLAB R2009a. The MT ratio images were calculated with the formula below using Image J software and later this image was co registered with the corresponding MPRAGE. Later segmentation was done and used separately for GM and WM analysis.
An MTR image was calculated according to the following equation.
MTR = (without MT–with MT)/without MT
Where without MT and with MT images are voxel intensities of the MT images with and without the saturation pulse.
VBM with SPM 8
The image processing was performed on Statistical Parametric Mapping 8 software (SPM8) using VBM tools 8.1 toolbox (Welcome Department of Imaging Neuroscience, London; http://www.fil.ion.ucl.ac.uk/spm). This enables spatial normalization of images acquired using a wider range of sequences and accurate results are achieved by combining tissue classification, bias correction and nonlinear warping into the same generative of forward model. The VBM tools segmentation algorithm in SPM8 additionally warps the prior images to the data and tries to minimize the impact of the template and the prior images. The image pre-processing as integrated in VBM tools 8 involves a number of defined stages. The original T1 images were spatially normalized to Montreal Neurological Institute template image given in the SPM8 to generate optimally normalized whole brain template. The process of smoothing conditioned the residuals to conform more closely to the Gaussian random field model underlying the statistical process used for adjusting P values.[16] The normalized, segmented, modulated and smoothed GM images with a voxel size of 1 mm3 were used for further statistical analysis. The GM, WM and ICV were also generated as part of VBM tools analysis and total brain volume was calculated as sum of GM and WM. The statistical analysis was performed using the R cran Statistical Package (http://www.Rproject.org). Univariate comparisons of demographic characteristics between PSP patients and controls were done using Pearson's Chi-square test and continuous variables were analyzed using the independent samples t-test. Statistical significance was noted at P < 0.001 false discovery rate uncorrected and cluster of 5.
SPM: VBM analysis between groups
Group comparisons for cerebral volume and MT differences were performed using two sample t-test within the framework of general linear model in SPM8. The GM morphometric differences were compared between patients and controls. The GM images of PSP patients (n = 10) and controls (n = 8) were compared using two samples t-test and statistical parametric maps were generated at significance level of P < 0.001, uncorrected for multiple corrections at voxel level with the age, gender and ICV as nuisance regressors (confounding covariates) in the design matrix.
The coordinates of the voxels showing reduction at a significance of P < 0.001 were converted into Talairach space (http://imaging.mrc.bu.cam.ac.uk/imaging/MNI Talairach). The coordinates were mapped using Tailairich client version 2.4.3. The Talairach software, a Java application created and developed at the Research Imaging Institute of the University of Texas Health Science Center San Antonio (UTHSCSA).
Results
MTR analysis (Table 1)
Table 1.
Location of peaks reduced regional MTR values in PSP group as compared to controls
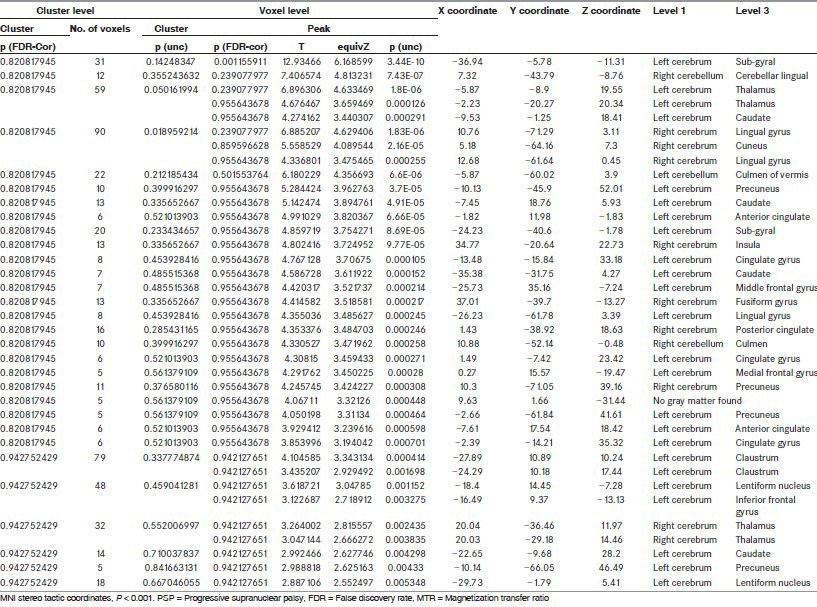
In our study, MTR analysis showed that the areas showing reduction of MI values at a significance level of P < 0.001 in PSP patients when compared with controls were in the limbic lobe specifically involving the anterior cingulate, cingulate gyrus, parahippocampal gyrus and lingual sulcus and in the left frontal lobe involving the MFG and IFG. The subcortical structures were also involved and included the bilateral thalami, left caudate and left claustrum. Furthermore the anterior lobe of cerebellum was involved [Figure 1 and Table 1].
Figure 1.
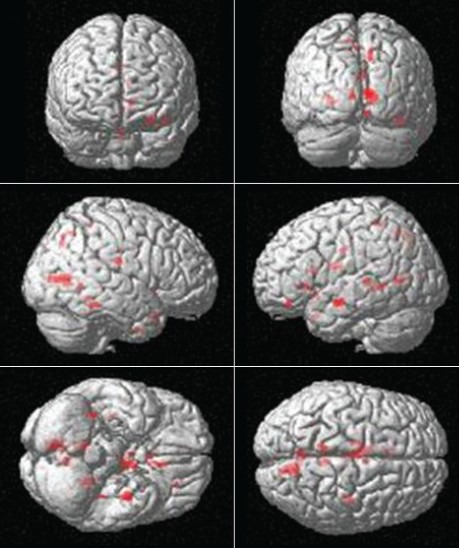
Location of peaks of decreased magnetization transfer ratio values in progressive supranuclear palsy group as compared to controls. Montreal Neurological Institute stereo tactic coordinates, P < 0.001 false discovery rate uncorrected
MPRAGE analysis (Supplementary Table 1)
On VBM analysis of MPRAGE data areas showing reduction in GM volume at a significance level of P < 0.001 were the thalamus, pre and post central gyrus, frontal gyri bilaterally, anterior cingulate and cerebellum. Inferior frontal and mesial frontal cortex including orbitofrontal, insular cortex, cingulate gyrus, pars opercularis and also the superior cerebellar peduncles as compared with the controls. Reduction in the volume of the diencephalic structures i.e., thalamus and hypothalamus was noted. The involvement was more extensive on left dominant side. In the thalamus pulvinar and ventral anterior and lateral posterior nucleus was more involved [Figure 2].
Figure 2.
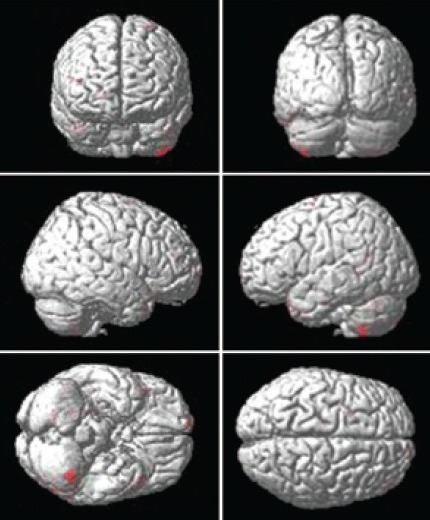
Location of peaks of decreased gray matter volume in progressive supranuclear palsy group when compared to controls. Montreal Neurological Institute stereo tactic coordinates, P < 0.001 false discovery rate uncorrected
On comparing the results of VBM analysis of MPRAGE and MTR maps the areas involved were similar although the number of voxels showing reduction in magnetization transfer values were greater when compared with GM volume reduction [Figures 1 and 2].
Discussion
In this study, we carried out voxel based analysis of MTR and T1 volume images to examine the detection of WM and GM tissue damage in patients with PSP. MTR is a sensitive tool to examine the qualitative changes in WM architecture.
VBM analysis of MTR helps us examine the whole brain analysis in an unbiased way and the areas showing significant decrease in MT ratio can be demonstrated. The fact that MTR images indicated structural changes in the existing brain tissue of patients of PSP presumably reflects the sensitivity of MTR to decreases in axonal and dendritic density as well to secondary myelin degeneration. Neuronal loss occurs in PSP following accumulation of tau protein.
T1-weighted imaging and MT are equally sensitive to the progression of brain pathology of the neocortex. MTR images and MPRAGE revealed extensive bilateral volume and qualitative changes in the orbitofrontal, prefrontal cortex, limbic lobe and sub cortical GM. The prefrontal structures involved were the rectal gyrus, medial, IFG and MFG. The anterior cingulate, cingulate gyrus and lingual gyrus of limbic lobe and subcortical structures such as caudate, thalamus, insula and claustrum were also involved. Cerebellar involvement mainly of anterior lobe was noted.
The involvement of prefrontal structures correlates with clinical presentation with dysexecutive syndrome in PSP. Subcortical structures such as basal ganglia and thalamus are involved in different neuronal functions. Histopathology studies have shown tufted astrocytes in the thalamus in PSP. The thalamus is a major relay station and is involved in cognition and sensory pathways. The thalamus is the structure involved in arousal and determines the sensitivity to sensory stimuli whereas claustrum is involved in the sensory association. This may explain the lack of this sensory feedback mechanism in patients and presentation with gait abnormality.[17] Region specific atrophy of thalamus is known.[4,18,19,20] Structural abnormalities in PSP have been described in thalamus and frontal cortex using DTI and VBM studies.[4,6] In addition, functional connectivity analysis has revealed reduction in the frontothalamic connectivity which was further supported by structural changes depicted using DTI and VBM.[21] Thalamic metabolic disturbances have also been noted on positron emission tomography (PET) in PSP.[22] In our study, we were able to replicate the results of previous studies and in addition show areas of reduced MT values in this substrate.
The involvement of limbic lobe explains the clinical presentation of dementia, hallucination and delirium in patients with PSP. The PET studies in PSP have shown hypometabolism in limbic lobe in the anterior cingulate, orbitofrontal cortex and midbrain and subcortical structures in the caudate, thalamus, claustrum and putamen.
Involvement of cerebellum is known in PSP with patients presenting with cerebellar ataxia. In accordance to clinical presentation extensive cerebellar involvement in the anterior lobe and posterior lobe was noted in PSP when compared with controls on VBM analysis of MT ratio.
On VBM analysis of MTR and MPRAGE similar results with extensive changes in limbic and subcortical structures was noted.
The limitation in our study was Classification was based purely on clinical criteria and histopathological correlation was not available. A detailed study on the role of MT in early PSP needs to be done, whereas in our study we enrolled all cases at different stages of PSP. This type of study will highlight the role of MT as an early marker in subclinical or early cases of PSP.
Conclusions
Voxel based MT imaging permits whole brain unbiased investigation for brain tissue structural integrity in PSP and is a valuable tool for identifying structural damage before atrophy is detected. Since our study group included PSP of varying severity the involvement of structures in both structural and MT imaging were similar. It could also be due to rapid clinical progression and severity of this pathology. Nevertheless this analysis constitutes a potentially effective method to track early PSP related pathology and also differentiate between subtypes of PSP. In the current study, it confirmed involvement of limbic lobe, prefrontal cortex and subcortical structures. Whether MTR measures of cerebral tissue damage can be used as predictors of clinical progression and detecting early PSP remains to be investigated.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Litvan I, Agid Y, Calne D, Campbell G, Dubois B, Duvoisin RC, et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology. 1996;47:1–9. doi: 10.1212/wnl.47.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Steele JC, Richardson JC, Olszewski J. Progressive supranuclear palsy: A heterogeneous degeneration involving the brain stem, basal ganglia and cerebellum with vertical gaze and pseudobulbar palsy, nuchal dystonia and dementia. Am Med Assoc. 1964;10:333. doi: 10.1001/archneur.1964.00460160003001. [DOI] [PubMed] [Google Scholar]
- 3.Liao K, Wagner J, Joshi A, Estrovich I, Walker MF, Strupp M, et al. Why do patients with PSP fall? Evidence for abnormal otolith responses. Neurology. 2008;70:802–9. doi: 10.1212/01.wnl.0000304134.33380.1e. [DOI] [PubMed] [Google Scholar]
- 4.Jackson JA, Jankovic J, Ford J. Progressive supranuclear palsy: Clinical features and response to treatment in 16 patients. Ann Neurol. 1983;13:273–8. doi: 10.1002/ana.410130308. [DOI] [PubMed] [Google Scholar]
- 5.Golbe LI. Progressive supranuclear palsy. Curr Treat Options Neurol. 2001;3:473–7. doi: 10.1007/s11940-001-0010-0. [DOI] [PubMed] [Google Scholar]
- 6.Litvan I, Mangone CA, McKee A, Verny M, Parsa A, Jellinger K, et al. Natural history of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome) and clinical predictors of survival: A clinicopathological study. J Neurol Neurosurg Psychiatry. 1996;60:615–20. doi: 10.1136/jnnp.60.6.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Williams DR, Lees AJ. Progressive supranuclear palsy: Clinicopathological concepts and diagnostic challenges. Lancet Neurol. 2009;8:270–9. doi: 10.1016/S1474-4422(09)70042-0. [DOI] [PubMed] [Google Scholar]
- 8.Seppi K, Poewe W. Brain magnetic resonance imaging techniques in the diagnosis of parkinsonian syndromes. Neuroimaging Clin N Am. 2010;20:29–55. doi: 10.1016/j.nic.2009.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Ashburner J, Friston KJ. Voxel-based morphometry — The methods. Neuroimage. 2000;11:805–21. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 10.Henley SM, Ridgway GR, Scahill RI, Klöppel S, Tabrizi SJ, Fox NC, et al. Pitfalls in the use of voxel-based morphometry as a biomarker: Examples from huntington disease. AJNR Am J Neuroradiol. 2010;31:711–9. doi: 10.3174/ajnr.A1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: Voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 12.Price S, Paviour D, Scahill R, Stevens J, Rossor M, Lees A, et al. Voxel-based morphometry detects patterns of atrophy that help differentiate progressive supranuclear palsy and Parkinson's disease. Neuroimage. 2004;23:663–9. doi: 10.1016/j.neuroimage.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 13.Brenneis C, Seppi K, Schocke M, Benke T, Wenning GK, Poewe W. Voxel based morphometry reveals a distinct pattern of frontal atrophy in progressive supranuclear palsy. J Neurol Neurosurg Psychiatry. 2004;75:246–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Warmuth-Metz M, Naumann M, Csoti I, Solymosi L. Measurement of the midbrain diameter on routine magnetic resonance imaging: a simple and accurate method of differentiating between Parkinson disease and progressive supranuclear palsy. Am Med Assoc. 2001;58:1076. doi: 10.1001/archneur.58.7.1076. [DOI] [PubMed] [Google Scholar]
- 15.Filippi M, Rovaris M. Magnetisation transfer imaging in multiple sclerosis. J Neurovirol. 2000;6(Suppl 2):S115–20. [PubMed] [Google Scholar]
- 16.Louis ED, Borden S, Moskowitz CB. Essential tremor centralized brain repository: Diagnostic validity and clinical characteristics of a highly selected group of essential tremor cases. Mov Disord. 2005;20:1361–5. doi: 10.1002/mds.20583. [DOI] [PubMed] [Google Scholar]
- 17.Halliday GM, Macdonald V, Henderson JM. A comparison of degeneration in motor thalamus and cortex between progressive supranuclear palsy and Parkinson's disease. Brain. 2005;128:2272–80. doi: 10.1093/brain/awh596. [DOI] [PubMed] [Google Scholar]
- 18.Heiss WD, Hilker R. The sensitivity of 18-fluorodopa positron emission tomography and magnetic resonance imaging in Parkinson's disease. Eur J Neurol. 2004;11:5–12. doi: 10.1046/j.1351-5101.2003.00709.x. [DOI] [PubMed] [Google Scholar]
- 19.Manyam BV, Bhatt MH, Moore WD, Devleschoward AB, Anderson DR, Calne DB. Bilateral striopallidodentate calcinosis: Cerebrospinal fluid, imaging, and electrophysiological studies. Ann Neurol. 1992;31:379–84. doi: 10.1002/ana.410310406. [DOI] [PubMed] [Google Scholar]
- 20.Cordato NJ, Duggins AJ, Halliday GM, Morris JG, Pantelis C. Clinical deficits correlate with regional cerebral atrophy in progressive supranuclear palsy. Brain. 2005;128:1259–66. doi: 10.1093/brain/awh508. [DOI] [PubMed] [Google Scholar]
- 21.Saatci I, Topcu M, Baltaoglu FF, Köse G, Yalaz K, Renda Y, et al. Cranial MR findings in Wilson's disease. Acta Radiol. 1997;38:250–8. doi: 10.1080/02841859709172059. [DOI] [PubMed] [Google Scholar]
- 22.Sibon I, Tison F. Vascular parkinsonism. Curr Opin Neurol. 2004;17:49–54. doi: 10.1097/00019052-200402000-00009. [DOI] [PubMed] [Google Scholar]


