Abstract
Aromatic L-amino acid decarboxylase (AADC), a vitamin B6-requiring enzyme that converts L-dopa to dopamine and 5-hydroxytryptophan to serotonin. Deficiency of this enzyme results in developmental delay, muscular hypotonia, dystonia, involuntary movements, autonomic dysfunction, and oculogyric crises. We now report a 2-year-old Turkish boy with AADC deficiency confirmed by greatly reduced AADC activity in the plasma and by genetic studies. Mutation analysis revealed a homozygous mutation c.208C > T (p. His70Tyr) in exon 3 of the AADC gene which has not been described to date.
Keywords: Aromatic L-amino acid decarboxylase deficiency, case report, novel mutation
Introduction
Aromatic L-amino acid decarboxylase (AADC) is a vitamin B6-requiring enzyme that converts L-dopa to dopamine and 5-hydroxytryptophan to serotonin.[1] Deficiency of this enzyme results in developmental delay, muscular hypotonia, dystonia, involuntary movements, autonomic dysfunction, and oculogyric crises.[2] We now report a 2-year-old Turkish boy with AADC deficiency confirmed by greatly reduced AADC activity in the plasma and by genetic studies.
Case Report
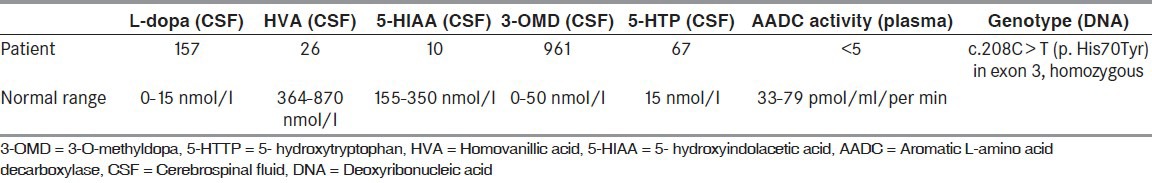
A 2-year-old male was born as the second child of healthy nonconsanguineous Turkish parents at term after an uncomplicated pregnancy and delivery. From the age of 4 months truncal muscular hypotonia, limb hypertonia, and irritability were noted by his caregivers. Physical examination showed weight loss (7.3 kg, below the third centile), length 65 cm (below the third centile), and head circumference 43 cm (below third percentile). A neurological examination revealed general hypotonia, exaggerated deep tendon reflexes with normal cranial nerve examination. He was not able to sit without assistance and did not grasp objects because of impaired eye-hand coordination. He also presented distinct extraneurological features such as hypersalivation, constipation, hyperhidrosis, and sleep disturbances. He had swallowing difficulty, recurrent vomiting and eventually evaluated for gastroeosophageal reflux disease. Cranial magnetic resonance imaging, which performed because of involuntary non-epileptic dystonic movements resembling epileptic spasms, was normal. The patient had admitted several times with the complaints of dystonic movements which were interpreted as tonic epileptic movements and were given antiepileptic medications despite the existing multiple normal electroencephalography (EEG) recordings. After a careful history and reevaluation of the patient with the interpretation of the video recordings of so-called epileptic movements; patient's tonic seizure like movements were diagnosed as dystonic movements with the lateral deviation of eyes. Routine clinical investigations for inborn errors of metabolism were negative. The diagnosis of AADC deficiency was established at the age of 11 months, by screening of the cerebrospinal fluid (CSF) for neurotransmitter metabolites. Examination of a CSF sample showed a strikingly abnormal pattern of biogenic amine metabolites indicating AADC deficiency. The relevant biochemical findings were elevated concentrations of L-dopa, 3-O-methyldopa (3-OMD), and 5-hydroxytryptophan together with decreased CSF concentrations of homovanillic acid (HVA), 5-hydroxyindolacetic acid (5-HIAA), and normal levels of 5-methyltetrahydrofolate (5MTHF) and pterins [Table 1]. Diagnosis was confirmed by measurement of AADC activity in plasma and found to be severely decreased as expected (AADC activity: <5 pmol/min/ml and normal value: 33-79). The AADC gene mutation was analyzed and sequencing results revealed a novel mutation in exon 3 of the AADC gene homozygous change c.208C > T (p. His70Tyr) which renders the patient clinical phenotype resistant to therapies. Treatment with a combination of the AADC cofactor pyridoxine and bromocriptine was started during the 1st year of life and showed only a moderate clinical improvement as decreasing the severity and duration of the dystonic episodes, but had no effect on the muscle tone and development. We added monoamine oxidase (MAO) inhibitor, melatonin, and folinic acid to drug regimes; but showed no favorable response. He had not yet obtained head control or rolling over. He did not suffer from hypoglycemia, hyperprolactinemia, and growth hormone deficiency.
Table 1.
The laboratory findings of the patient
Discussion
Aromatic L-AADC deficiency is a rare autosomal recessive congenital metabolic disorder of neurotransmitter biosynthesis which is very intractable to treat.[3,4,5] In general, most of the signs and symptoms with AADC deficiency can be assigned to deficiencies of dopamine, norepinephrine, epinephrine, and serotonin. In 1990, Hyland et al., diagnosed the first case of AADC deficiency by screening CSF samples in patients with psychomotor retardation.[6] Main clinical features are muscular hypotonia, dystonia, oculogyric crises, developmental delay, and additional extraneurological symptoms. Onset of symptoms is typically in the first months of life. Although almost all patients reported so far presented oculogyric crises, current patient had not upward but lateral gaze deviation. It was difficult to discriminate seizures from nonepileptic spasms. Brain imaging and EEG revealed normal findings in most patients that are not helpful in diagnosing AADC deficiency. MRI findings that could be seen in this disorder were atrophy, reduced myelination, and white matter changes.[5,6,7] The first step in reaching the diagnosis is the investigation of neurotransmitters in CSF. In addition to typical pattern with a distinct reduction of HVA, 5-HIAA, and 3-OMD as well as an elevation of levo-dopa and 5-hydroxytryptophan; endocrinological findings could be found, for example, elevated prolactin, hypoglycemia, and growth hormone deficiency. In general, most of the signs and symptoms described in patients with AADC deficiency can be assigned to deficiencies of dopamine, norepinephrine, epinephrine, and serotonin.[8,9] Drug therapy aims at correcting the central and peripheral deficiency of serotonin and catecholamines. Medical treatment is challenging and first choice medications appear to be dopamine agonists in combination with pyridoxine and MAO inhibitors, anticholinergics, melatonin, and others are thought in the second step.[10] While some children have demonstrated some benefit, the overall outcome is unsatisfactory. He was started on pyridoxine, pyridoxal phosphate, folinic acid, MAO inhibitor, and trihexyphenidyl; but without any clinical improvement. He was extremely hypotonic, with no head control and never achieved verbal output and any developmental milestones. As far as we now, the mutation that was found in this patient has not been described earlier and unfortunately, the response to treatment was very poor. Cerebrospinal fluid neurotransmitter metabolite profile remains a critical tool in identifying patients with suspected neurotransmitter deficiency state. Treatment options are limited, in many cases not beneficial, and prognosis is uncertain. In the literature, the majority of cases showed no or poor response despite different protocols and a combination of different drugs.[9,10] From the clinical point of view in order to diagnose those patients as early and correctly as possible, a thorough evaluation in terms of medical history and physical and neurological examination should be done in order to rule out epileptic disorders. For now, we expect the development of new strategies and more effective treatments.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.Abdenur JE, Abeling N, Specola N, Jorge L, Schenone AB, van Cruchten AC, et al. Aromatic l-aminoacid decarboxylase deficiency: Unusual neonatal presentation and additional findings in organic acid analysis. Mol Genet Metab. 2006;87:48–53. doi: 10.1016/j.ymgme.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 2.Abeling NG, van Gennip AH, Barth PG, van Cruchten A, Westra M, Wijburg FA. Aromatic L-amino acid decarboxylase deficiency: A new case with a mild clinical presentation and unexpected laboratory findings. J Inherit Metab Dis. 1998;21:240–2. doi: 10.1023/a:1005307919767. [DOI] [PubMed] [Google Scholar]
- 3.Brun L, Ngu LH, Keng WT, Ch’ng GS, Choy YS, Hwu WL, et al. Clinical and biochemical features of aromatic L-amino acid decarboxylase Deficiency. Neurology. 2010;75:64–71. doi: 10.1212/WNL.0b013e3181e620ae. [DOI] [PubMed] [Google Scholar]
- 4.Fiumara A, Bräutigam C, Hyland K, Sharma R, Lagae L, Stoltenborg B, et al. Aromatic L-amino acid decarboxylase deficiency with hyperdopaminuria. Clinical and laboratory findings in response to different therapies. Neuropediatrics. 2002;33:203–8. doi: 10.1055/s-2002-34497. [DOI] [PubMed] [Google Scholar]
- 5.Hsieh HJ, Lin SH, Liu HM. Visualisation of impaired dopamine biosynthesis in a case of aromatic L-amino acid decarboxylase deficiency by co-registered 18F-FDOPA PET and magnetic resonance imaging. Eur J Nucl Med Mol Imaging. 2005;32:517. doi: 10.1007/s00259-004-1618-6. [DOI] [PubMed] [Google Scholar]
- 6.Hyland K, Surtees RA, Rodeck C, Clayton PT. Aromatic L-amino acid decarboxylase deficiency: Clinical features, diagnosis, and treatment of a new inborn error of neurotransmitter amine synthesis. Neurology. 1992;42:1980–8. doi: 10.1212/wnl.42.10.1980. [DOI] [PubMed] [Google Scholar]
- 7.Ito S, Nakayama T, Ide S, Ito Y, Oguni H, Goto Y, et al. Aromatic L-amino acid decarboxylase deficiency associated with epilepsy mimicking non-epileptic involuntary movements. Dev Med Child Neurol. 2008;50:876–8. doi: 10.1111/j.1469-8749.2008.03094.x. [DOI] [PubMed] [Google Scholar]
- 8.Korenke GC, Christen HJ, Hyland K, Hunneman DH, Hanefeld F. Aromatic L-amino acid decarboxylase deficiency: An extrapyramidal movement disorder with oculogyric crises. Eur J Paediatr Neurol. 1997;1:67–71. [PubMed] [Google Scholar]
- 9.Maller A, Hyland K, Milstien S, Biaggioni I, Butler IJ. Aromatic L-amino acid decarboxylase deficiency: Clinical features, diagnosis, and treatment of a second family. J Child Neurol. 1997;12:349–54. doi: 10.1177/088307389701200602. [DOI] [PubMed] [Google Scholar]
- 10.Pons R, Ford B, Chiriboga CA, Clayton PT, Hinton V, Hyland K, et al. Aromatic L-amino acid decarboxylase deficiency: Clinical features, treatment, and prognosis. Neurology. 2004;62:1058–65. doi: 10.1212/wnl.62.7.1058. [DOI] [PubMed] [Google Scholar]


