Abstract
Background:
Although Belgium was once a major international manufacturer of asbestos products, asbestos-related diseases in the country have remained scarcely researched.
Objectives:
The aim of this study is to provide a descriptive analysis of Belgian mesothelioma mortality rates in order to improve the understanding of asbestos health hazards from an international perspective.
Methods:
Temporal and geographical analyses were performed on cause-specific mortality data (1969–2009) using quantitative demographic measures. Results were compared to recent findings on global mesothelioma deaths.
Results:
Belgium has one of the highest mesothelioma mortality rates in the world, following the UK, Australia, and Italy. With a progressive increase of male mesothelioma deaths in the mid-1980s, large differences in mortality rates between sexes are apparent. Mesothelioma deaths are primarily concentrated in geographic areas with proximity to former asbestos industries.
Conclusions:
Asbestos mortality in Belgium has been underestimated for decades. Our findings suggest that the location of asbestos industries is correlated with rates of mesothelioma, underlining the need to avert future asbestos exposure by thorough screening of potential contaminated sites and by pursuing a global ban on asbestos.
Keywords: Asbestos, Belgium, Environmental exposure, Malignant mesothelioma, Mortality, Occupational exposure
Introduction
Asbestos-related diseases are a growing public health concern worldwide. The inhalation of asbestos fibers can lead to a number of fatal diseases, which typically only become apparent after long latency periods.1
One of the most hazardous asbestos-related diseases is malignant mesothelioma, a tumor that develops in the protective linings that cover the lungs, chest wall, abdomen, and heart.2,3 Mesothelioma has an average latency period of 37–45 years and even low levels of exposure can induce a tumor, making mesothelioma a sensitive indicator for the adverse health effects of asbestos.4–6
The incidence of mesothelioma has increased substantially throughout the industrialized world. In 2011, Delgermaa and colleagues published a comparative study of mesothelioma deaths in 83 countries and found that age-adjusted mortality rates (AAMRs) for all mesothelioma deaths have more than doubled between 1994 and 2008.7 Belgium was not included in this study. Although asbestos has a long history of use in Belgium, the cause-specific mortality data covering these years were not reported to the World Health Organization and not available for analysis until 2012.
There is currently a dearth of research about the impact of asbestos in Belgium. Previous studies on the prevalence of asbestos-related diseases have relied primarily on biomedical data or on information delivered by victim compensation funds.8–12 Selection bias, differences in diagnostic criteria, and low civil awareness of compensation measures make the representativeness of these data sources questionable.
While asbestos does not occur naturally in Belgium, pathological research and consumption data suggest that the general population has been exposed to asbestos fibers. Asbestos consumption data indicate that Belgium became a major international asbestos manufacturer in the early 1900 and, one of Europe’s largest asbestos groups, Eternit, started operating in Belgium in 1905.13 The popularity of the ‘magic mineral’ culminated during the 1960s and 1970s, with Belgium having one of the highest consumption levels per capita in the world.14 Following an extensive period of legislative attempts to reduce health risks, the use and transaction of all types of asbestos were finally banned in 2001. A study of a post-mortem population in an urban Brussels hospital found pleural plaques, an asbestos-related disease, in 14% of the 150 consecutive necropsies in patients. The patients were not selected by occupation or previous asbestos-related conditions, indicating the widespread exposure to asbestos in the general public.15 However, there are no studies that describe the asbestos-related mortality in Belgium on a population level, which is fundamental for evaluating the public health ramifications of asbestos.
This paper provides a descriptive analysis of mesothelioma mortality rates in Belgium in order to improve the understanding of the impact of asbestos exposure. It is the first study of its kind to present Belgian data on mesothelioma mortality in an international context, alongside temporal and geographical analyses.
Methods
Cause-specific mortality data for Belgium were obtained from the WHO Mortality Database (1969–1986) and from the Scientific Institute of Public Health (1987–2009).16,17 Both data sources derive mortality information from death certificates. For the period 1969–2009, the number of mesothelioma deaths by sex and 5-year age category was extracted. Mesothelioma was defined using the International Classification of Diseases (ICD) coding system. In 1998, the ICD coding system was modified to include a specific code for mesothelioma (ICD-10 C45). Prior to this modification, pleural cancer was used as an indirect indicator for mesothelioma. For the periods 1969–1978 and 1979–1997, the codes ICD-8 163.0 and ICD-9 163 were used to identify cases of mesothelioma.
There were 4 years of missing data in the dataset. In 1985, Belgium did not report detailed cause-specific mortality data to the WHO. In 1998, the responsibility to collect and report mortality statistics was shifted from the national level to the regional level and due to problems in this regionalization process, cause-specific mortality data for the years 2000–2002 are still not available. To complete the data series, we used a linear interpolation to obtain estimates for 1985, 2000, 2001, and 2002.
To compare Belgian mortality data to recent findings on global mesothelioma deaths, the same method was used as detailed by Delgermaa et al.7 Consequently, only observed mesothelioma deaths (ICD-10 C45) between 1994 and 2008 were used. Age- and sex-specific mortality rates and 95% confidence intervals were calculated through direct standardization using the world population from the year 2000. Annual per cent changes in AAMRs were calculated and log-transformed to construct a linear regression using calendar year as an independent variable. The annual per cent change was calculated using the formula 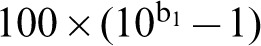 , where b1 is the regression coefficient from the linear regression. The 95% confidence intervals and P-values for the annual per cent changes were calculated using IBM SPSS Statistics (version 22).
, where b1 is the regression coefficient from the linear regression. The 95% confidence intervals and P-values for the annual per cent changes were calculated using IBM SPSS Statistics (version 22).
For the temporal analysis of mesothelioma deaths, the European standard population was used as reference. Owing to Belgium’s relatively old age structure, this method provided a more accurate assessment of Belgian mesothelioma mortality than using the world population as the reference. Annual per cent changes, 95% confidence intervals, and P-values were also calculated.
To examine the most recent geographical distribution of mesothelioma mortality, standardized mortality ratios (SMRs) were analyzed for all 43 Belgian districts for the years 2005–2009. Expected deaths were calculated using the age- and sex-specific Belgian mortality rates as reference. Owing to the small number of deaths at the district level, SMRs for 5-year blocks of data were analyzed. Data from 2007 were used as an estimator for the population at the midpoint of the study period. Owing to the mathematical link between the Poisson and the χ2 distributions, 95% confidence intervals were calculated using tables from the χ2.18 The limits were obtained using the following formulas, where x is the number of observed deaths.
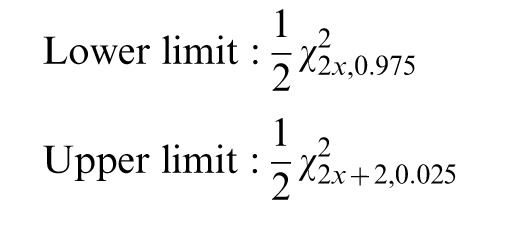 |
Information on the Belgian asbestos industry is scarce. We performed a comprehensive review of academic literature, government documents, and news articles to identify Belgian asbestos manufacturers. For this study, only companies with documented asbestos use were taken into account. Information about asbestos exposure in specific companies was not available. Territorial maps showing the distribution of SMRs and locations of the asbestos industries were made using Quantum GIS software (version 1.8.0).
Results
A total of 3425 Belgian mesothelioma deaths (2646 males and 779 females) were identified for the period 1969–2009. The annual number of mesothelioma deaths increased from 22 deaths in 1969 to 219 deaths in 2009.
Belgium in international perspective(1994–2008)
In 2011, Delgermaa et al. published a study that analyzed 92 253 global mesothelioma deaths between 1994 and 2008.7 They found that 88% of all deaths were reported in developed countries. Comparing our results with the findings from this study, we found that the Belgian mesothelioma mortality rate is the fourth highest in the world (Table 1). The global baseline rate of mesothelioma mortality is 9.0 deaths per million males and 1.9 deaths per million females. In Belgium, the AAMRs for asbestos are 18.0 deaths per million for males and 3.1 deaths per million for females.
Table 1. Belgian mesothelioma deaths compared to the 10 counties reporting the highest number of mesothelioma deaths (1994–2008)7*.
| Country | No. of deaths | AAMR† per million | M∶F ratio‡ |
| United Kingdom (9)§ | 13 517 | 17.8 | 5.7∶1 |
| Australia (8) | 3747 | 16.5 | 5.4∶1 |
| Italy (3) | 3706 | 10.3 | 2.4∶1 |
| Belgium (8) | 1467 | 9.6 | 4.7∶1 |
| France (8) | 6608 | 7.6 | 3.4∶1 |
| Germany (9) | 9569 | 6.8 | 3.2∶1 |
| South Africa (12) | 2322 | 6.7 | 3.3∶1 |
| Netherlands (13) | 5141 | 6.4 | 4.0∶1 |
| United States of America (7) | 17 062 | 5.0 | 4.2∶1 |
| Spain (7) | 1840 | 3.9 | 2.9∶1 |
| Japan (14) | 11 212 | 3.2 | 3.3∶1 |
Compared to the findings from the Delgermaa study, Belgium has the fourth highest AAMR of the 10 countries reporting the highest number of mesothelioma deaths. With almost 10 deaths per million people, Belgium is behind the two countries with the highest mesothelioma burdens: United Kingdom (AAMR 17.8) and Australia (AAMR 16.5), and is almost on par with Italy (AAMR 10.3).
We found significant differences in mesothelioma mortality rates by gender in Belgium. Between 1994 and 2008, male mesothelioma deaths were 4.7 times higher than female mesothelioma deaths. Only the United Kingdom (5.7 : 1) and Australia (5.4 : 1) have a higher male-to-female ratio of mesothelioma deaths.
The annual per cent changes show global mesothelioma mortality rates have increased significantly over time. Delgermaa et al. found a 5.85% annual rise in age-adjusted mesothelioma mortality rates for males and 3.48% annual rise for females between 1994 and 2008.7 In Belgium, mesothelioma mortality rates also increased during this time, albeit at a slower pace. During the same time period, Belgian male mortality rates increased 3.23%. There was no significant change in mesothelioma mortality rates among Belgian women between 1994 and 2008.
National analysis(1969–2009)
Figure 1 shows the magnitude and evolution of the age-adjusted mesothelioma mortality rates for men and women between 1969 and 2009 in Belgium. Male AAMRs increased nearly tenfold, from 2.5 to 26.3 deaths per million during the 40-year period. A sharp increase in male mesothelioma mortality is apparent in 1984. Data for 1985 were linearly interpolated, possibly masking a more fluctuating transition. Nonetheless, the trend indicates a strong increase in male deaths due to mesothelioma during the mid-1980s. Female mesothelioma mortality rates increased from 1.6 deaths per million in 1969 to 4.1 deaths per million in 2009. With the rising mortality rates for men, a sharp divergence between sexes appears in the mid-1980s. The male-to-female ratio tripled from 2.1 in 1983 to 6.4 in 2009.
Figure 1.
Age-adjusted mortality rates (AAMRs) for mesothelioma: Belgium (1969–2009). Note: Reference European standard population (1976).
Belgian males experienced an increase in mesothelioma mortality at an annual rate of 6.65% between 1969 and 2009. For Belgian women, there was an annual increase of 3.04% during the same period. Since 1983, male mesothelioma mortality rates increased 5.44% annually, whereas female mortality rates increased 2.80% annually. The annual rise in mesothelioma mortality rates is significant for both men and women at the P = 0.01 level.
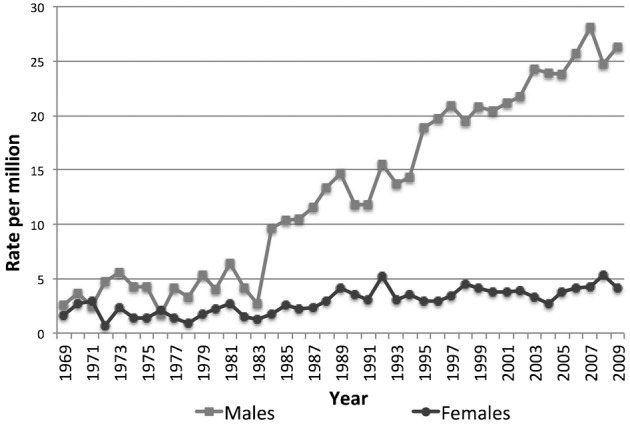
The results from the geographical analysis of mesothelioma mortality at the Belgian district level are shown in Figs 2 and 3. The male and female SMRs for 2005–2009 are presented in conjunction with the location of former asbestos industries in Belgium. Table 2 (see Appendix) presents the number of deaths, SMRs, and 95% confidence intervals for all 43 districts.
Figure 2.
Asbestos industry and standardized mortality ratios (SMRs) for Belgian males at the district level (2005–2009). Reference: Belgium male population. Categories: Natural Breaks Jenks.
Figure 3.
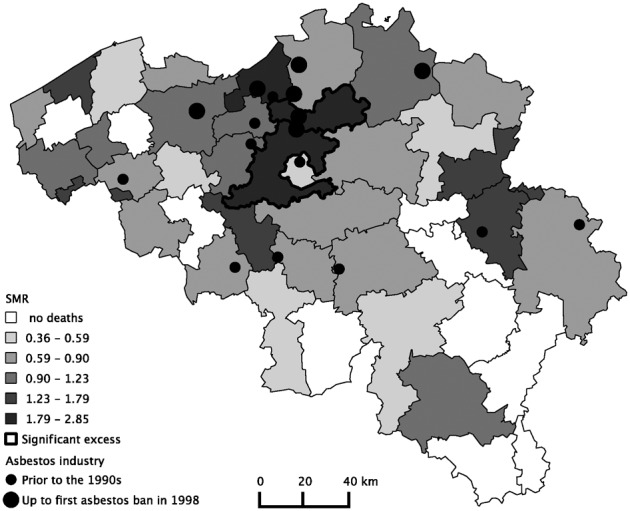
Asbestos industry and standardized mortality ratios (SMRs) for Belgian females at the district level (2005–2009). Reference: Belgium female population. Categories: Natural Breaks Jenks.
A total of 24 known asbestos companies were identified in 17 districts. The first known manufacturer of asbestos products in Belgium opened in 1885. Seven companies used asbestos until the first major asbestos ban in 1998. Of the identified asbestos companies, 9 of 24 were asbestos–cement manufacturers. Other asbestos industries included shipyards and manufacturers of automobile parts, electrical material, aluminum foil, textile, and paper.
The distribution of SMRs shows a significant excess in male mesothelioma mortality in five adjacent districts: Sint-Niklaas (SMR: 3.4 CI: 2.7–4.4), Mechelen (SMR: 2.5, CI: 2.0–3.2), Dendermonde (SMR: 1.8, CI: 1.2–2.5), Halle-Vilvoorde (SMR: 1.4, CI: 1.1–1.8), and Antwerp (SMR: 1.3, CI 1.1–1.6). Large asbestos industries were located in these districts. The three largest Belgian asbestos–cement manufacturers were an important source of employment in Sint-Niklaas, Mechelen, and Halle-Vilvoorde. There were also shipbuilding industries in three out of the five districts with excess in male mesothelioma mortality. Small but substantial shipbuilding industries were active in Sint-Niklaas and Dendermonde. Antwerp had an international port and a large shipbuilding industry as well as an asbestos production center.
For women, the risk of mesothelioma is not as straightforward as it is for men. In 10 districts, there were no female mesothelioma deaths between 2005 and 2009. There were low mesothelioma mortality ratios in districts with textile asbestos industries, which traditionally employed a female workforce. The small number of employees working in the asbestos textile manufacturing units is one possible explanation for the low mesothelioma mortality ratios. Only two districts had higher than expected observed death rates compared to Belgian women: Mechelen (SMR: 2.8, CI 1.6–4.6) and Halle-Vilvoorde (SMR: 2.5, CI 1.6–3.7).
Discussion
Findings from this study indicate that among countries with data available, Belgium has the fourth highest rate of mesothelioma mortality, behind the United Kingdom, Australia, and Italy. Prior research has found that the high mortality rates in these countries are related to a history of industrial asbestos use.19–21
Our findings indicate a similar influence of industrial activities on Belgian mesothelioma mortality rates. Between 1969 and 2009, mesothelioma deaths increased progressively. The burden of mesothelioma has been consistently higher among men than women. The gender gap in mesothelioma mortality has also changed, becoming three times larger between 1984 and 2009. Changes in mortality registration are an unlikely explanation, since any differences in registration would also affect female mesothelioma deaths. Revisions of the ICD-codes occurred in 1979 and 1998 and do not appear to influence mesothelioma mortality rates. The large differences between sexes may be explained by the traditional low participation of women in industrial work.22 Male employees typically dominated the occupations most associated with asbestos exposure in Belgium. The sharp increase in male deaths in the mid-1980s may be the result of the expansion of the asbestos industry some 40 years ago. Asbestos was extensively used in the economic recovery of Europe after WWII and the manufacturing of asbestos products on a large scale increased dramatically in the post-war period.13 Belgian consumption of asbestos peaked in 1975, and by the 1990s had dropped below the pre-war level.13 Owing to the small proportion of women working in Belgian asbestos industries, the gradual rise in female mortality is more likely affected by increased secondary (e.g. indirect contact via family members) and environmental exposure (e.g. air pollution).23–25
The geographical distribution of mesothelioma deaths supports the hypothesis that mesothelioma deaths are correlated with the location of asbestos industries. We found that mesothelioma deaths were concentrated in areas with a history of industrial asbestos use, with a significant excess in male mortality in five districts associated with shipbuilding and asbestos–cement production. Two of these districts also had significantly higher mortality rates for women than expected. Due to the close vicinity of the two largest asbestos–cement factories in Belgium, the lack of sufficient health safety measures during production years, secondary and environmental exposure may have played an important role in these areas.26
There are several limitations of the study to acknowledge. Data about mesothelioma deaths from Belgium during the years 1994–1999 and 2003–2008 were compared to international data from heterogeneous periods. The average AAMRs may be influenced by differences in reported years. Additionally, the under-reporting of mesothelioma deaths is always a concern and caution should be exercised when comparing results from different countries. Differences in diagnostic awareness, statistical knowledge, and economic motivations may all influence the registration of mesothelioma mortality.27 Park and colleagues estimate one in four to five mesothelioma cases is overlooked globally.28
The use of multiple revisions of the ICD-codes in the temporal analysis may have caused some degree of misclassification at the individual level. ICD-8 163.0 and ICD-9 163 only capture the most frequent form of this disease: pleural mesothelioma. The correspondence between reported pleural cancer and actual pleural mesothelioma cases has been a point of discussion in previous studies.29,30 It has been suggested that misclassified pleural cancers might compensate peritoneal and pericardial mesothelioma to some degree at the population level.21,31 We consider pleural cancer to be the most appropriate indicator for trends in Belgian mesothelioma mortality before 1998.
The current study uses deaths based on the district of residence at the time of death. This raises two issues. First, the geographical distribution of mortality rates may be influenced by labor commute. Although commuting in Belgium typically occurs within the district of residence, the large production centers may have promoted increased labor mobility between districts.32–34 Second, individuals may have changed residence during the long latency period associated with the development of mesothelioma. Information on migration, work history, or asbestos exposure that may have occurred abroad is not included in this study.
There is little transparency in asbestos products manufacturing in Belgium and more detailed information on asbestos companies is not available. Therefore, many sources of occupational exposure cannot be pinpointed. The geographical analysis provides an overview of asbestos exposure hotspots. This is only a fraction of the actual industrial volume, since only large and documented production facilities were identified for this study.
The future development of malignant mesothelioma remains unclear. Although some countries have experienced a decrease or deceleration, mesothelioma mortality rates in most Western countries are expected to peak during the next decade.6,35–37 The task at hand is to efficiently manage a possible epidemic of asbestos-related diseases. Belgium should consider establishing a national surveillance system such as the British or Italian mesothelioma registries.38,39 Mesothelioma mortality is only one indicator of asbestos exposure and further research on the progress of other asbestos-related diseases in Belgium is needed. In order to identify the source, type, and duration of asbestos exposure, future research should incorporate information on migration and work history for individuals. Finally, additional information on the historical exposure in industries is vital to comprehend the Belgian asbestos problem.
In conclusion, this study provides a descriptive analysis of mesothelioma mortality rates to improve the understanding of asbestos health hazards in Belgium. The magnitude and progress of Belgian mesothelioma mortality has long been underestimated and our findings indicate that Belgium has one of the highest mesothelioma mortality rates in the world. With a progressive increase of male mesothelioma deaths in the mid-1980s, large differences in mortality between sexes are apparent. Furthermore, we found that mesothelioma deaths are concentrated in specific geographic areas related to former asbestos industries, indicating the link between asbestos industries and disease. As asbestos production shifts to economically developing countries, a global ban on asbestos is imperative to prevent further occupational and environmental exposure worldwide.
Disclaimer statements
Contributors No contributors/guarantor to declare.
Funding The funding for this study is provided by the Research Council of Vrije Universiteit Brussel.
Conflicts of interest The authors declare no conflicts of interest.
Ethics approval Ethical approval was not required.
Appendix
Table 2. Geographical distribution of observed deaths, expected deaths, and standardized mortality ratios (SMRs) with the 95% confidence interval, by gender, by district, 2005–2009.
| Males | Females | |||||||||
| District | O* | E† | SMR‡ | LL§ | UL | O | E | SMR | LL | UL |
| Antwerpen | 110 | 83.68 | 1.31 | 1.08 | 1.59 | 15 | 17.14 | 0.88 | 0.49 | 1.44 |
| Mechelen | 70 | 27.88 | 2.51 | 1.96 | 3.17 | 16 | 5.63 | 2.84 | 1.62 | 4.61 |
| Turnhout | 32 | 35.36 | 0.90 | 0.62 | 1.28 | 8 | 6.62 | 1.21 | 0.52 | 2.38 |
| Hasselt | 24 | 32.32 | 0.74 | 0.48 | 1.10 | 3 | 6.41 | 0.47 | 0.10 | 1.37 |
| Maaseik | 15 | 18.15 | 0.83 | 0.46 | 1.36 | 3 | 3.33 | 0.90 | 0.19 | 2.63 |
| Tongeren | 15 | 16.18 | 0.93 | 0.52 | 1.53 | 5 | 3.15 | 1.59 | 0.51 | 3.70 |
| Aalst | 30 | 23.33 | 1.29 | 0.87 | 1.84 | 6 | 4.93 | 1.22 | 0.45 | 2.65 |
| Dendermonde | 28 | 16.01 | 1.75 | 1.16 | 2.53 | 4 | 3.30 | 1.21 | 0.33 | 3.10 |
| Eeklo | 8 | 7.64 | 1.05 | 0.45 | 2.06 | 1 | 1.49 | 0.67 | 0.02 | 3.74 |
| Gent | 37 | 42.85 | 0.86 | 0.61 | 1.38 | 9 | 8.96 | 1.00 | 0.46 | 1.90 |
| Oudenaarde | 7 | 10.34 | 0.68 | 0.27 | 1.40 | 1 | 2.19 | 0.46 | 0.01 | 2.54 |
| Sint-Niklaas | 67 | 19.50 | 3.44 | 2.66 | 4.36 | 7 | 3.90 | 1.79 | 0.72 | 3.70 |
| Brugge | 16 | 26.72 | 0.60 | 0.34 | 0.97 | 2 | 5.43 | 0.37 | 0.04 | 1.33 |
| Diksmuide | 2 | 4.47 | 0.45 | 0.05 | 1.62 | 0 | 0.88 | 0.00 | 0.00 | 3.40 |
| Ieper | 7 | 9.39 | 0.75 | 0.30 | 1.54 | 2 | 1.94 | 1.03 | 0.13 | 3.73 |
| Kortrijk | 21 | 24.59 | 0.85 | 0.53 | 1.31 | 3 | 5.11 | 0.59 | 0.12 | 1.72 |
| Oostende | 13 | 15.69 | 0.83 | 0.44 | 1.42 | 4 | 3.25 | 1.23 | 0.34 | 3.15 |
| Roeselare | 7 | 13.02 | 0.54 | 0.22 | 1.11 | 3 | 2.61 | 1.15 | 0.24 | 3.36 |
| Tielt | 2 | 7.93 | 0.25 | 0.03 | 0.91 | 0 | 1.57 | 0.00 | 0.00 | 1.91 |
| Veurne | 1 | 6.85 | 0.15 | 0.00 | 0.81 | 1 | 1.36 | 0.73 | 0.02 | 4.08 |
| Halle-Vilvoorde | 69 | 49.11 | 1.40 | 1.09 | 1.78 | 25 | 10.03 | 2.49 | 1.61 | 3.68 |
| Leuven | 35 | 40.72 | 0.86 | 0.60 | 1.20 | 7 | 8.18 | 0.86 | 0.34 | 1.76 |
| Brussels Capital | 29 | 67.34 | 0.43 | 0.29 | 0.62 | 9 | 16.20 | 0.56 | 0.25 | 1.05 |
| Nivelles | 24 | 28.05 | 0.86 | 0.55 | 1.27 | 4 | 6.00 | 0.67 | 0.18 | 1.71 |
| Ath | 2 | 6.32 | 0.32 | 0.04 | 1.14 | 0 | 1.45 | 0.00 | 0.00 | 2.07 |
| Mons | 15 | 18.04 | 0.83 | 0.47 | 1.37 | 4 | 4.46 | 0.90 | 0.24 | 2.30 |
| Mouscron | 5 | 5.70 | 0.88 | 0.29 | 2.05 | 2 | 1.31 | 1.53 | 0.19 | 5.52 |
| Charleroi | 25 | 31.36 | 0.80 | 0.52 | 1.18 | 6 | 7.47 | 0.80 | 0.29 | 1.75 |
| Soignies | 10 | 13.51 | 0.74 | 0.36 | 1.36 | 5 | 3.06 | 1.63 | 0.53 | 3.81 |
| Thuin | 15 | 11.66 | 1.29 | 0.72 | 2.12 | 1 | 2.63 | 0.38 | 0.01 | 2.12 |
| Tournai | 6 | 11.10 | 0.54 | 0.20 | 1.18 | 2 | 2.60 | 0.77 | 0.09 | 2.78 |
| Huy | 9 | 7.74 | 1.16 | 0.53 | 2.21 | 0 | 1.71 | 0.00 | 0.00 | 1.75 |
| Liège | 44 | 48.21 | 0.91 | 0.66 | 1.23 | 15 | 10.95 | 1.37 | 0.77 | 2.26 |
| Verviers | 16 | 21.18 | 0.76 | 0.43 | 1.23 | 4 | 4.56 | 0.88 | 0.24 | 2.25 |
| Waremme | 10 | 5.52 | 1.81 | 0.87 | 3.33 | 0 | 1.23 | 0.00 | 0.00 | 2.43 |
| Arlon | 6 | 3.89 | 1.54 | 0.57 | 3.36 | 0 | 0.84 | 0.00 | 0.00 | 3.56 |
| Bastogne | 1 | 3.07 | 0.33 | 0.01 | 1.82 | 0 | 0.65 | 0.00 | 0.00 | 4.61 |
| Marche-en-Famenne | 2 | 4.07 | 0.49 | 0.06 | 1.78 | 0 | 0.86 | 0.00 | 0.00 | 3.47 |
| Neufchâteau | 3 | 4.61 | 0.65 | 0.13 | 1.90 | 1 | 0.97 | 1.03 | 0.03 | 5.74 |
| Virton | 5 | 3.86 | 1.30 | 0.42 | 3.02 | 0 | 0.82 | 0.00 | 0.00 | 3.65 |
| Dinant | 1 | 8.38 | 0.12 | 0.00 | 0.67 | 1 | 1.80 | 0.55 | 0.01 | 3.09 |
| Namur | 15 | 21.31 | 0.70 | 0.39 | 1.16 | 4 | 4.91 | 0.81 | 0.22 | 2.08 |
| Philippeville | 3 | 5.12 | 0.59 | 0.12 | 1.71 | 0 | 1.09 | 0.00 | 0.00 | 2.74 |
* Observed deaths.
† Expected deaths.
‡ Standardized mortality ratios.
§ Lower limit 95% confidence interval.
Upper limit 95% confidence interval; Reference: Belgian sex-specific population.
References
- 1.Jamrozik E, de Klerk N, Musk AW. Asbestos-related disease. Intern Med J. 2011;41:372–80. doi: 10.1111/j.1445-5994.2011.02451.x. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–71. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kishimoto T, Gemba K, Fujimoto N, Aoe K, Kato K, Takeshima Y, et al. Clinical study on mesothelioma in Japan: relevance to occupational asbestos exposure. Am J Ind Med. 2010;53:1081–7. doi: 10.1002/ajim.20868. [DOI] [PubMed] [Google Scholar]
- 4.Marinaccio A, Scarselli A, Binazzi A, Altavista P, Belli S, Mastrantonio M, et al. Asbestos related diseases in Italy: an integrated approach to identify unexpected professional or environmental exposure risks at municipal level. Int Arch Occup Environ Health. 2008;81:993–1001. doi: 10.1007/s00420-007-0293-x. [DOI] [PubMed] [Google Scholar]
- 5.Hillerdal G. Mesothelioma: cases associated with non-occupational and low dose exposures. Occup Environ Med. 1999;56:505–13. doi: 10.1136/oem.56.8.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weill H, Hughes JM, Churg AM. Changing trends in US mesothelioma incidence. Occup Environ Med. 2004;61:438–41. doi: 10.1136/oem.2003.010165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delgermaa V, Takahasi K, Park EK, Le GV, Hara T, Sorahan T. Global mesothelioma deaths reported to the World Health Organization between 1994 and 2008. Bull World Health Organ. 2011;89:716–724C. doi: 10.2471/BLT.11.086678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moulin E, Yourassowsky N, Dumortier P, De Vuyst P, Yernault JC. Electron microscopic analysis of asbestos body cores from the Belgian urban population. Eur Respir J. 1988;1(9):818–22. [PubMed] [Google Scholar]
- 9.Dumortier P, Thimpont J, de Maertelaer V, De Vuyst P. Trends in asbestos body counts in bronchoalveolar lavage fluid over two decades. Eur Respir J. 2003;22:519–24. doi: 10.1183/09031936.03.00001903. [DOI] [PubMed] [Google Scholar]
- 10.Vande Weyer R. Bilan de l’indemnisation de l’asbestose. Acta Tuberc Pneumol Belg. 1973;64:304–51. [PubMed] [Google Scholar]
- 11.Vande Weyer R. Pathologie respiratoire de l’amiante en Belgique. Rev Méd Brux. 1981;2(2):69–81. [PubMed] [Google Scholar]
- 12.Asbestfonds/Fonds amiante. Het Asbestfonds. 5-jarig bestaan (2007–2012). Brussel; 2012. p. 1–44. [Google Scholar]
- 13.Virta RL. Worldwide asbestos supply and consumption trends from 1900 through 2003. Reston, VA: U.S. Department of the Interior – U.S. Geological Survey Report No.: Circular 1298; 2006. p. 80. [Google Scholar]
- 14.Nawrot TS, Van Kersschaever G, Van Eycken E, Nemery B. Belgium: historical champion in asbestos consumption. Lancet. 2007;369(9574):1692. doi: 10.1016/S0140-6736(07)60776-4. [DOI] [PubMed] [Google Scholar]
- 15.Mitchev K, Dumortier P, De Vuyst P. ‘Black spots’ and hyaline pleural plaques on the parietal pleura of 150 urban necropsy cases. Am J Surg Pathol. 2002;26(9):1198–206. doi: 10.1097/00000478-200209000-00010. [DOI] [PubMed] [Google Scholar]
- 16.World Health Organization (WHO) Global Health Observatory. WHO Mortality Database: World Health Organization Statistical Information System (WHOSIS). [Internet]. ICD8-ICD9. 2013 [cited 2013 Mar 5]. Available from: http://www.who.int/healthinfo/statistics/mortality_rawdata/en/index.html. [Google Scholar]
- 17.Scientific Institute of Public Health, Public Health and Surveillance. Standardized Procedures for Mortality Analysis [Internet]. Brussels; 2013 [cited 2013 November 21]. Available from: https://www.wiv-isp.be/epidemio/spma. [Google Scholar]
- 18.Liddell FD. Simple exact analysis of the standardised mortality ratio. J Epidemiol Community Health. 1984;38:85–8. doi: 10.1136/jech.38.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McElvenny DM, Darnton AJ, Price MJ, Hodgson JT. Mesothelioma mortality in Great Britain from 1968 to 2001. Occup Med (Lond). 2005;55:79–87. doi: 10.1093/occmed/kqi034. [DOI] [PubMed] [Google Scholar]
- 20.Leigh J, Driscoll T. Malignant mesothelioma in Australia, 1945–2002. Int J Occup Environ Health. 2003;9:206–17. doi: 10.1179/oeh.2003.9.3.206. [DOI] [PubMed] [Google Scholar]
- 21.Gatto MP, Tanna D. Distribution and trends in mesothelioma mortality in Italy from 1974 to 2006. Int Arch Occup Environ Health. 2013;86:489–96. doi: 10.1007/s00420-012-0780-6. [DOI] [PubMed] [Google Scholar]
- 22.Deschacht N, Baerts A, Guerry M-A. De m/v carrièrekloof: carrièreverschillen tussen vrouwen en mannen in België. In: Samenleving en toekomst. Federaal Wetenschapsbeleid, editor. Gent: Academia Press; 2011. p. 216. [Google Scholar]
- 23.Vianna NJ, Polan AK. Non-occupational exposure to asbestos and malignant mesothelioma in females. Lancet. 1978;52:1061–3. doi: 10.1016/s0140-6736(78)90911-x. [DOI] [PubMed] [Google Scholar]
- 24.McDonald JC. Health implications of environmental exposure to asbestos. Environ Health Perspect. 1985;62:319–28. doi: 10.1289/ehp.8562319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madkour MT, El Bokhary MS, Awad Allah HI, Awad AA, Mahmoud HF. Environmental exposure to asbestos and the exposure–response relationship with mesothelioma. East Mediterr Health J. 2009;15:25–38. [PubMed] [Google Scholar]
- 26.Nay SY. Asbestos in Belgium: use and abuse. Int J Occup Environ Health. 2003;9(3):287–93. doi: 10.1179/oeh.2003.9.3.287. [DOI] [PubMed] [Google Scholar]
- 27.Terracini B. Additional features of the worldwide double standards in the prevention of asbestos-related diseases. Ann Ist Super Sanità. 2006;42:174–7. [PubMed] [Google Scholar]
- 28.Park EK, Takahashi K, Hoshuyama T, Cheng TJ, Delgermaa V, Le GV, et al. Global magnitude of reported and unreported mesothelioma. Environ Health Perspect. 2011;119:514–8. doi: 10.1289/ehp.1002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bruno C, Comba P, Maiozzi P, Vetrugno T. Accuracy of death certification of pleural mesothelioma in Italy. Eur J Epidemiol. 1996;12:421–3. doi: 10.1007/BF00145308. [DOI] [PubMed] [Google Scholar]
- 30.Iwatsubo Y, Matrat M, Michel E, Boutin C, Galateau-Salle F, Jougla E, et al. Estimation of the incidence of pleural mesothelioma according to death certificates in France. Am J Ind Med. 2002;42:188–99. doi: 10.1002/ajim.10098. [DOI] [PubMed] [Google Scholar]
- 31.Fazzo L, Minelli G, De Santis M, Bruno C, Zona A. Mesothelioma mortality surveillance and asbestos exposure tracking in Italy. Ann Ist Super Sanità. 2012;48:300–10. doi: 10.4415/ANN_12_03_11. [DOI] [PubMed] [Google Scholar]
- 32.Roels O, Vanoverbeke L, Van Rompuy P. Theorie en empirie van de arbeidsmobiliteit in België. Leuven: Acco; 1975. p. 147. [Google Scholar]
- 33.Verhetsel A, Van Hecke E, Thomas I, Beelen M, Halleux J-M, Rixhon G, et al. Pendel in België. Sociaal-economische enquête 2001. Monografieën. Brussel: FOD Economie, K.M.O., Middenstand en Engergie; 2009. p. 214. [Google Scholar]
- 34.Ravenstein EG. The laws of migration. J R Stat Soc. 1885;48:167–235. [Google Scholar]
- 35.Montanaro F, Bray F, Gennaro V, Merler E, Tyczynski JE, Parkin DM, et al. Pleural mesothelioma incidence in Europe: evidence of some deceleration in the increasing trends. Cancer Causes Control. 2003;14:791–803. doi: 10.1023/a:1026300619747. [DOI] [PubMed] [Google Scholar]
- 36.Hemminki K, Hussain S. Mesothelioma incidence has leveled off in Sweden. Int J Cancer. 2008;122:1200–1. doi: 10.1002/ijc.23230. [DOI] [PubMed] [Google Scholar]
- 37.Peto J, Decarli A, La Vecchia C, Levi F, Negri E. The European mesothelioma epidemic. Br J Cancer. 1999;79(3–4):666–72. doi: 10.1038/sj.bjc.6690105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Greenberg M, Davies TA. Mesothelioma register 1967–68. Br J Ind Med. 1974;31(2):91–104. doi: 10.1136/oem.31.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nesti M, Marinaccio A, Silvestri S. The national mesothelioma registry (ReNaM) First report. Rome: ISPESL National Institute of Occupational Safety and Prevention; 2001. p. 117. [Google Scholar]


