Abstract
Background:
Factors that influence exposure to silver particles from the use of textiles are not well understood.
Objectives:
The aim of this study was to evaluate the influence of product treatment and physiological factors on silver release from two textiles.
Methods:
Atomic and absorbance spectroscopy, electron microscopy, and dynamic light scattering (DLS) were applied to characterize the chemical and physical properties of the textiles and evaluate silver release in artificial sweat and saliva under varying physiological conditions. One textile had silver incorporated into fiber threads (masterbatch process) and the other had silver nanoparticles coated on fiber surfaces (finishing process).
Results:
Several complementary and confirmatory analytical techniques (spectroscopy, microscopy, etc.) were required to properly assess silver release. Silver released into artificial sweat or saliva was primarily in ionic form. In a simulated “use” and laundering experiment, the total cumulative amount of silver ion released was greater for the finishing process textile (0.51±0.04%) than the masterbatch process textile (0.21±0.01%); P<0.01.
Conclusions:
We found that the process (masterbatch vs finishing) used to treat textile fibers was a more influential exposure factor than physiological properties of artificial sweat or saliva.
Keywords: Nanoparticles, Silver, Skin, Dermal exposure, Textiles
Introduction
Textiles are derived from both natural resources (e.g. cotton) and synthetic materials (e.g. polyester). Neither natural nor synthetic textile fibers possess strong antimicrobial properties and are therefore often treated with antimicrobial agents for such diverse products as wound bandages and garments. Silver metal and salts are used to treat textiles because of a desire to replace organic chemical agents with additives that can be used with a broader range of product matrices.1,2 Use of silver as an antimicrobial treatment for textiles is based on the premise that biocidal potency is a function of ion release.2 The exact bactericidal mechanism is not fully understood, but may be a result of an interaction of silver ions with enzymes or generation of free radicals that damage bacteria cell membranes.3 Application of nanotechnology for antimicrobial treatments is growing in interest because of the small scale of nanoparticles (defined here as a particle with all three dimensions in the range of 1–100 nm). The potential for metallic silver particles to release ions is predicted to increase as the size of the particles decrease from the micrometer scale to the nanoscale, due to the greater available specific surface area.2 Hence, the size-dependent dissolution kinetics of metallic silver nanoparticles in textiles can be exploited for controlled and sustained release of ions to impart antimicrobial properties.2 Treatment of textiles with silver nanoparticles is one of the most commercialized applications for this nanomaterial though concerns exist over product safety.4–10 Samberg et al.6 reported that unwashed silver nanoparticles caused a significant increase in pro-inflammatory cytokine release areas of focal inflammation and localization of silver nanoparticles on the surface and in the upper stratum corneum layers of porcine skin. Szmyd et al.7 exposed normal human primary keratinocytes in vitro to polyvinyl propylene-capped silver nanoparticles and observed decreases in cell viability, metabolism, proliferation, and migratory behavior. The use of silver nanoparticles in wound bandages also raises concern, as the product will be in contact with compromised skin. Arora et al.4 exposed human skin carcinoma cells in vitro to silver nanoparticles and observed changes in cell morphology and evidence of oxidative stress. When human skin burns were treated using silver-containing wound dressings, a transient increase in silver plasma and urine levels during product use has been reported.8,9 Further, Trop et al.8 reported that a case of a person with burned skin and treated with a silver-containing wound dressing developed hepatotoxicity and argyria (grayish discoloration of the skin). Hence, dermal exposure to silver nanoparticles may present a wide range of adverse health concerns and merits further investigation.
Strong bonds between the textile and silver nanoparticles are required to ensure long-term durability of the antimicrobial treatment and the type of treatment is a major determinant in the retention of silver on textiles and long-term efficacy of the additive to control microbial growth. In commercial practice, antimicrobials such as silver can be added to textile fibers during (also referred to as masterbatch) or after processing (also referred to as finishing). Masterbatch processing is used for synthetic materials and incorporates the silver into the thickness of the fiber polymer threads during manufacture. In contrast, finishing is used with natural and synthetic fabric types and involves coating the silver onto threads by immersing the thread or textile in a wet bath after manufacture.
Investigations of textiles containing silver nanoparticles have focused mainly on studying the release of silver into the ambient environment.1,11–19 More recently, investigators have begun to focus on release of silver into biological fluids and the exposure potential from contact with textile consumer products.20,21 These studies have reported that silver in textiles was released primarily in ionic form into artificial sweat at varying levels depending on the product tested. However, little is known about the factors that influence silver exposure from the use of textile consumer products. We hypothesized that relevant exposure factors may include the product itself and/or the physiological properties of the biological fluids that come into contact with the textile during use. Examples of product-related factors include fiber type (synthetic or natural) and how the silver treatment was applied during manufacture. Biological fluids that may come into contact with textiles include sweat and saliva; which because of their unique biological functions have different properties. Examples of relevant physiological factors of biological fluids include composition, pH, and temperature.
The surface of the skin is coated with a thin co-solvent film of sweat, containing electrolytes and ionic constituents (e.g. Cl−, S−,  ), organic acids (e.g. lactate), and sebum (a mix of lipids and vitamin E). In a previous study, Quadros et al. investigated the influence of individual chemical constituents of artificial sweat on silver release from a textile and found that only sodium chloride had a minor influence on silver release.20 Other studies of textiles treated with silver nanoparticles have demonstrated that the release of silver into artificial sweat varies with pH. Amounts of total silver (ionic and particulate) released are generally higher at pH 8 than at more biologically common skin pH values of 4–6.22–27 Finally, Larese-Filon et al. reported that more silver from a suspension of silver nanoparticles is absorbed through abraded skin compared to intact skin.28 Taken together, these studies suggest that properties of skin surface fluids and the skin barrier integrity are relevant considerations when assessing exposure potential from textiles that contact the skin.
), organic acids (e.g. lactate), and sebum (a mix of lipids and vitamin E). In a previous study, Quadros et al. investigated the influence of individual chemical constituents of artificial sweat on silver release from a textile and found that only sodium chloride had a minor influence on silver release.20 Other studies of textiles treated with silver nanoparticles have demonstrated that the release of silver into artificial sweat varies with pH. Amounts of total silver (ionic and particulate) released are generally higher at pH 8 than at more biologically common skin pH values of 4–6.22–27 Finally, Larese-Filon et al. reported that more silver from a suspension of silver nanoparticles is absorbed through abraded skin compared to intact skin.28 Taken together, these studies suggest that properties of skin surface fluids and the skin barrier integrity are relevant considerations when assessing exposure potential from textiles that contact the skin.
The purpose of this project was to investigate the product and physiological factors that influence the amount and form (ionic and particulate) of silver released from treated textiles exposed to artificial human sweat and saliva. We hypothesized that because finishing treatment results in silver on the surface of the fibers, the release of silver would be higher compared to textiles prepared by masterbatch treatment, which incorporates silver into the fibers. To test our hypothesis, we evaluated the potential for silver ions to interact with sweat constituents, for silver migration from textiles to a surrogate skin, leaching of silver from textiles exposed to sweat and saliva, and leaching of silver from textiles into artificial sweat in a simulated use scenario.
Materials and Methods
In this study, silver release was evaluated for two polyester textiles. The first textile was composed of 88% polyester/12% lycra fibers and produced using a masterbatch process (HVACTM glove). The second textile was 96% polyester fibers interwoven with 4% X-staticTM silver-coated fibers produced using a finishing process (SilverMaxTM underwear). Details of the analytical techniques used for characterization are reported separately.29
Artificial Biological Fluids
Silver release was assessed using a skin surface film liquid (SSFL) model, a simple human sweat, and simple saliva. Composition, pH, and temperature of biological fluids were evaluated. Metallic silver particles were used in the release studies as a benchmark for comparison with the textiles. The SSFL model consisted of an aqueous sweat component and a lipid sebum component. The simple sweat contained 61 different constituents, with the largest concentrations of chloride (33.2 mM), sodium (30.7 mM), lactic acid (14.0 mM), urea (10.0 mM), potassium (6.1 mM), and ammonium (5.3 mM).30 This model contained low concentrations of sulfur (2.3 mM) and sulfate (0.4 mM), which can react with free silver ions to form precipitates.31–33 The SSFL was prepared as described by Harvey et al. and buffered to a pH 5.3 (median value for human sweat) or 4.5 (acidic sweat).30 Artificial sebum (saturated and unsaturated lipids and vitamin E) was prepared as described by Stefaniak et al.34 The simple artificial sweat formulation consisted of 5 g/l (85.6 mM) sodium chloride and 0.5 g/l (8.3 mM) urea and had pH 5.8.35 The simple artificial saliva consisted of 0.1667 g/l magnesium chloride hexahydrate (0.82 mM), 0.1470 g/l calcium chloride dehydrate (1.0 mM), 0.5748 g/l dipotassium hydrogen phosphate (3.3 mM), 0.5252 g/l potassium carbonate (3.8 mM), 0.3272 g/l sodium chloride (5.6 mM), and 0.7454 g/l potassium chloride (10.0 mM) and had pH 6.8.36
All experiments were performed in the dark to prevent photo-oxidation of the silver. All glassware was soaked overnight in dilute nitric acid, rinsed in deionized water, and air dried prior to use. A silver-free polyester textile was used as a control.
Experiment 1: interaction of silver with artificial sweat fluid constituents
We conducted an experiment to evaluate the silver–ligand complex formation in artificial sweat. The purpose of this experiment was to determine whether silver ions released into biological fluids would form particulate compounds by interacting with sweat constituents. If formation of silver salt precipitates occurs, it would reduce the amount of free silver ions available to penetrate into or through skin. Ten milligrams of silver in the form of 0.1 N silver nitrate (Sigma-Aldrich, St. Louis, MO, USA) or nanoparticles (<30 nm, PN: AG-M-02M-NP.030N, American Elements, Los Angeles, CA, USA) were added to separate clean glass scintillation vials. Next, 20 ml of SSFL (without sebum) buffered to pH 5.3 was added to each vial. The contents were briefly subjected to ultrasonic agitation using a 3 mm probe tip (delivered energy 1000 J), capped, and placed on a rotisserie in an incubator at 36°C. Three vials of each form of silver were removed from the incubator after 5, 30, and 60 minutes and at 2, 8, and 24 hours. The pH of the artificial sweat was 5.30±0.03 throughout the experiment.
At each time point, an aliquot of SSFL was removed from each vial for determination of hydrodynamic diameter (DH) by dynamic light scattering (DLS) using cumulants analysis and zeta potential of particles in suspension by laser Doppler electrophoresis (Nano ZS90, Malvern Instruments, Worcestershire, UK). Details of these analytical methods are summarized in //dx.doi.org/10.1179/2049396714Y.0000000070.S1>Supplementary Material 1. A separate aliquot of SSFL (diluted 3 : 1 in deionized water) from each sample was analyzed using ultraviolet-visible (UV-Vis) spectroscopy (Lambda 35, Perkin-Elmer, Beaconsfield, UK) to measure the absorbance signal due to the surface Plasmon resonance (SPR) of molecules on the surfaces of metallic nanoparticles (silver oxide and salts do not exhibit a SPR). An SPR peak absorbance in the wavelength range 390–420 nm by Mie scattering is indicative of unagglomerated silver nanoparticles.37,38 Finally, a 1 ml aliquot of the undiluted SSFL solution was passed through a 0.2 μm pore-size track-etched polycarbonate filter and analyzed using a Hitachi S-4800 field-emission scanning electron microscopy (FE-SEM) with an energy dispersive X-ray analysis (EDX) detector. Images were acquired using a 20 kV accelerating voltage at ×1K to ×25K magnification.
Experiment 2: barrier migration test
Next, we performed an experiment to assess the form and amount of silver that could migrate from a textile to the skin using a “barrier migration test” method.39 Also referred to as “contact-blotting extraction,” this test was originally developed to evaluate flame-retardant migration from fabrics and provides information on exposure potential.40 We used SSFL at pH 5.3 to test the potential for migration of silver from a textile to a filter paper (i.e. surrogate skin). A 5×5 cm textile swatch was placed in a clean Petri dish, covered with a 300-mesh copper transmission electron microscopy (TEM) grid with lacey carbon support, followed by a sebum-coated 47-mm filter (Whatman 541 ashless circle, Maidstone, Kent, England) that was pre-wetted with artificial SSFL. To evaluate the influence of skin liquid composition, tests were performed using filters coated with artificial sebum prepared with and without the antioxidant vitamin E. To evaluate the influence of skin contact pressure with a textile on silver migration, a 5.1 cm diameter stainless steel weight that delivered the equivalent of 1 pound per square inch pressure, mimicking the peak pressure exerted by an adult while lying or sitting, was placed on half of the samples.41 Triplicate samples of each textile product were prepared for each combination of experimental conditions.
The wetted filters remained in contact with the textiles until dry (approximately 5 hours with weights and 3 hours without weights). After drying, the grids were removed and analyzed using TEM (JEM-1220, JEOL, Tokyo, Japan) with an EDX detector for the presence of silver-containing particles. Both the filters and textiles were analyzed using UV-Vis spectroscopy immediately after the experiment and again at 4 and 7 days post-exposure in order to evaluate whether metallic silver nanoparticles formed via precipitation of silver ions.42 Following UV-Vis analysis, all filters were subjected to acid-assisted microwave digestion using a modified Environmental Protection Agency method 6010 and masses of total silver quantified using inductively coupled plasma atomic emission spectroscopy (ICP-AES) with a limit of detection = 0.1 μg silver/filter. Details of the analytical methods are described in //dx.doi.org/10.1179/2049396714Y.0000000070.S2>Supplementary Material 2.
Experiment 3: aggressive extraction test
The purpose of this test was to assess the influence of sweat composition, pH, and temperature on silver release from textiles in a head-over-heels (HOH) test.39,40 These physiological factors were evaluated to determine their relative importance on silver release from textiles. This test consists of immersing a sample (textile or particles) in 20 ml of artificial biological fluid in a sealed glass vial, clamping the vial in place on a motor assembly (Model 24A4BEPM, Bodine Electric Company, Chicago, IL, USA), and rotating end-over-end at 60 rpm (i.e. head-over-heels) in a temperature-controlled incubator for 30 minutes. The SSFL formulation was used to investigate the potential influence of sweat pH (4.5 vs 5.3), sweat temperature (36 vs 45°C), and sebum vitamin E (with and without) on silver release. Temperature values were selected to span the range from skin at rest to heavy exercise.26 Simple artificial sweat was used to investigate differences between sweat models; the formulation did not contain vitamin E, had a pH of 5.8, and the temperature was set to 36°C. Simple artificial saliva was used to investigate whether silver could be released if a textile was mouthed (e.g. removing a glove by biting on the fingertip and pulling out the hand); the formulation had a pH of 6.8, and the temperature was set to 37°C. Three independent samples were prepared for each experimental combination of textile, fluid, pH, temperature, and vitamin E.
After 30 minutes, an aliquot of artificial biological fluid was used for determination of DH and zeta potential (as described in //dx.doi.org/10.1179/2049396714Y.0000000070.S1>Supplementary Material 1. A separate aliquot of artificial biological fluid was pipetted into a 1-cm plastic cuvette for UV-Vis analysis (additionally, each textile piece was air dried overnight and analyzed as described in //dx.doi.org/10.1179/2049396714Y.0000000070.S2>Supplementary Material 2). Another aliquot of the fluid was used to prepare filter samples for analysis of particle morphology and elemental composition by FE-SEM-EDX as described above. Finally, the biological fluid was analyzed to quantify forms (ionic and particulate) of silver. We used cloud point extraction (CPE) to separate all dissolved silver ion (freely dissolved, matrix associated, and particle adsorbed) from particulate silver followed by analysis using ICP-AES (limit of detection = 0.001 mg/l).43 Details of the CPE method are summarized in //dx.doi.org/10.1179/2049396714Y.0000000070.S3>Supplementary Material 3.
Experiment 4: simulated use
Skin surface film liquid buffered to pH 5.3 was used to assess silver release from textiles during a simulation that consisted of wetting samples with artificial sweat once per day on three consecutive days to mimic use, laundering the samples in detergent and bleach, and wetting the samples with sweat again once per day for 3 days. This scenario was intended to simulate typical occupational and consumer activities including contact with a textile (e.g. using gloves for a task or heat-induced sweating while using performance underwear) and care for a textile between uses. Triplicate 5×5 cm samples of each textile (SilverMax, HVAC, and silver-free polyester control) were coated with artificial sebum containing vitamin E and their baseline UV-Vis absorption spectra were measured. For the first use, each textile swatch was placed in separate clean glass scintillation vials, saturated with SSFL (approximately 8–10 ml), and placed in an incubator at 36°C for 60 minutes without agitation. Immediately afterward, aliquots of the liquid were drawn for determination of DH, zeta potential, and separation of silver particles from ions by CPE. This protocol was repeated daily for three consecutive days. To simulate laundering, 2.5 ml of bleach and 5 ml of a fragrance-free detergent were mixed in 250 ml tap water (pH = 10.1). Each textile swatch was placed in 25 ml of wash water and stirred gently at 40°C on a hot plate for 15 minutes, followed by a 5-minute rest, and stirred for an additional 15 minutes. The wash water from each textile sample was analysed to determine DH, zeta potential, UV-Vis absorbance, and separation of dissolved from particulate silver by CPE. After laundering, each textile sample was air dried and UV-Vis absorbance measured as described previously. Each textile was subjected to one use simulation per day for three additional days. After the final use, each textile piece was air dried and UV-Vis absorbance measured.
We used the measured concentrations of silver ion in artificial sweat from the use scenario and the freely available GEOCHEM-EZ software program to model silver ion complex formation in artificial sweat (accounting for the pH of each sample).44 The sweat organic acids and vitamins (except ascorbic acid) were not in the GEOCHEM-EZ ligand database and were therefore omitted from the models.
Data Analysis
All statistical analyses were performed using SAS® version 9.1 (SAS Institute, Cary, NC, USA). Summary statistics, including mean and standard deviation, were calculated for measurements with multiple samples. To investigate the influence of solvent type, pH, temperature, and the presence of vitamin E on silver release from textile materials, analysis of variance (ANOVA) models were developed for the mass fraction of silver dissolved using the generalized linear model procedure in SAS. Specifically, the ANOVA models were used to investigate the fixed effects of: (1) pH, temperature, and vitamin E on dissolved silver for the different textiles; and (2) textile material on dissolved silver for the different solvents controlling for pH, temperature, and vitamin E. Tukey’s test option was specified for multiple comparisons.
Results
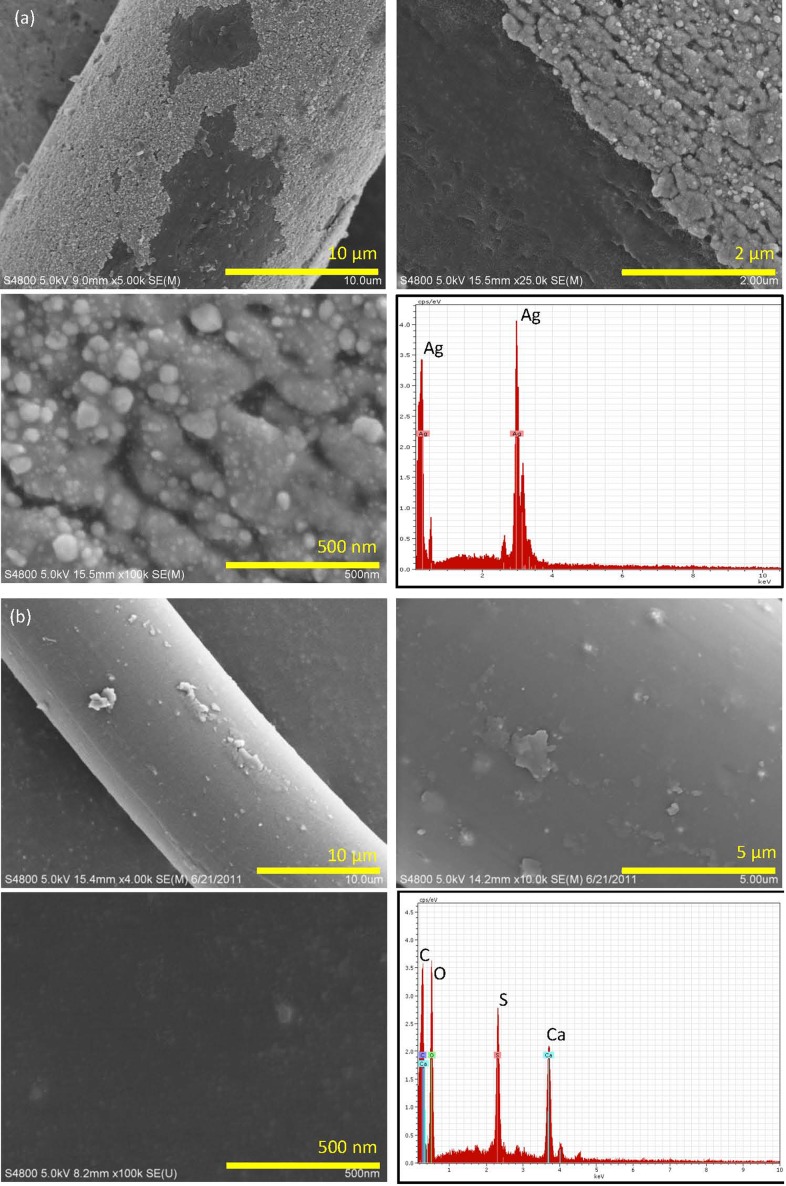
The total silver concentration of the textiles, as determined by mass spectroscopy, was homogeneous throughout the SilverMax textile (1095±292 mg Ag/kg textile) but was heterogeneous for the HVAC textile (range: 3.8±1.5 (cuffs) to 1070±140 mg/kg (palms)).29 Figure 1 shows FE-SEM micrographs and EDX spectra of the two textile products. The surfaces of fibers from the SilverMax textile were partially coated with a flaky material that appears light gray in the image. This light gray coating contained many nanoparticles. Energy dispersive X-ray analysis yielded a strong signal for silver; however, it is important to note that the spatial resolution of the electron beam used in EDX analysis was not sufficient to distinguish whether the signal was generated from the nanoparticles, the gray coating, or both. Energy dispersive X-ray analysis of the darker uncoated areas of the polymer fibers did not identify silver (data not shown). The average measured nanoparticle size was 83±37 nm (geometric mean 79 nm, geometric standard deviation 1.5). Fibers from the HVAC glove textile (masterbatch process) had a smooth appearance and particles were not visible on surfaces; analysis by EDX identified calcium and sulfur, but not silver. The ICP-AES results coupled with the EDX spectra of the smooth HVAC glove fibers indicated that the silver was incorporated in the fibers. To investigate further, we ashed a sample of the HVAC glove material and analyzed the residue using FE-SEM-EDX. The micrograph and corresponding EDX spectra demonstrated the presence of silver particles with size >100 nm (see Figure S1 in //dx.doi.org/10.1179/2049396714Y.0000000070.S4>Supplementary Material 4); however, it is possible that heating could have sintered smaller silver particles to form larger particles. Hence, the image demonstrates the presence of silver particles but the size may not be representative of the particles in situ.
Figure 1.
Field-emission scanning electron micrographs (FE-SEM) and corresponding energy dispersive X-ray (EDX) spectra of (A) SilverMax polyester textile and (B) HVAC textile. The SilverMax fibers were treated by a finishing process (light gray coating on fibers in images) that was incomplete in some places. The higher magnification images show numerous nanoparticles adhered to the surface of the light gray coating. The EDX spectra of the nanoparticles and surrounding light gray coating identified silver. HVAC polyester/lycra textile was prepared by a masterbatch process in which the silver was incorporated into the polymer fibers during production. The HVAC fibers had a smooth appearance and no nanoparticles were visible on fiber surfaces. The EDX spectra identified carbon, calcium, oxygen, and sulfur; silver was not detected.
Experiment 1: formation of silver complexes in artificial human sweat
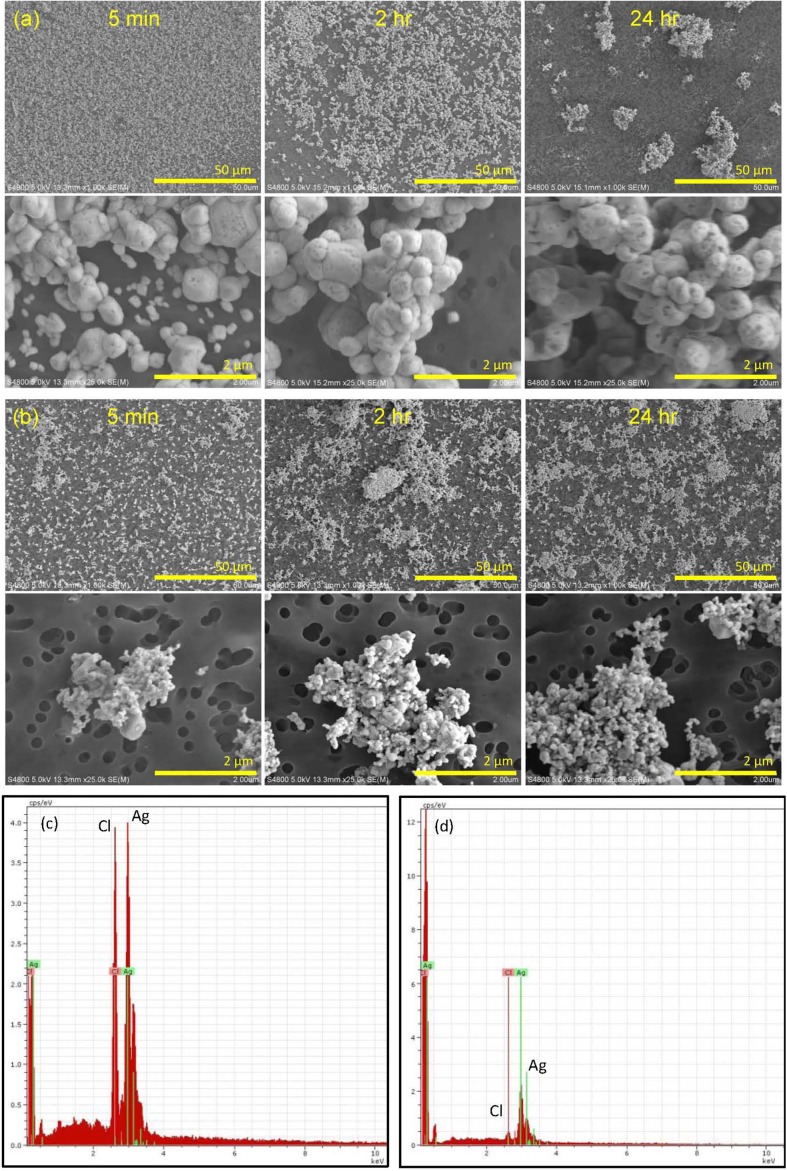
Figure 2 shows FE-SEM images that illustrate the morphology and elemental composition of silver-containing solids formed from the interaction of silver nitrate with artificial human sweat constituents. Silver nitrate rapidly (<5 minutes) formed sub-micron to micron-scale particles and EDX analysis confirmed that these particles were composed of silver and chlorine. Morphology of these particles was distinctly different from that of the commercially available silver nanoparticles. Over time, a reduction in the number of individual particles was observed, concomitant with the formation of cluster particles. The rapid formation of silver chloride particles from silver nitrate indicates that free silver ions may not remain in a soluble form on the skin surface for an appreciable enough time to permit absorption. There was no drastic change in the morphology of individual commercially available metallic silver nanoparticles over time in artificial sweat, although cluster size appeared to increase slightly with time. Energy dispersive X-ray spectra of the silver nanoparticles identified both silver and chlorine.
Figure 2.
Field-emission scanning electron microscopy (FE-SEM) micrographs and Energy dispersive X-ray (EDX) spectra illustrating: (A) formation of solids by interaction of silver nitrate with artificial human sweat constituents; (B) no appreciable change in the morphology of individual commercially available metallic silver nanoparticles; (C) EDX spectra demonstrating that solids formed from reactions of silver nitrate in artificial sweat were silver and chlorine; and (D) EDX spectra demonstrating that following immersion of silver nanoparticles in artificial human sweat, particles were silver and chlorine.
No SPR absorbance peak in the wavelength range 390–420 nm was observed for the solids formed by the interaction of silver nitrate with sweat chloride. This confirms the FE-SEM-EDX results, because silver salts (e.g. AgCl) do not exhibit a SPR. Surprisingly, no SPR absorbance peak was observed at any time point for the commercially available metallic silver nanoparticles dispersed in artificial human sweat (data not shown). After exposure to artificial sweat, EDX analysis identified both silver and chlorine in these particles. Hence, the absence of an SPR absorbance peak for the commercially available particles is attributed to the adsorption of chloride ions onto their surfaces, though it is possible that the surfaces of silver nanoparticles could have been oxidized.
Values of DH and zeta potential for solids formed by reaction of silver nitrate with sweat constituents and for silver nanoparticles in artificial sweat are summarized in //dx.doi.org/10.1179/2049396714Y.0000000070.S1>Supplementary Material 1 (see Table S1). For solids formed by the interaction of silver nitrate with sweat chloride, values of DH were stable up to 60 minutes, but thereafter, scattering intensity was too low to obtain a reliable signal for cumulants analysis. The values of zeta potential for the silver/chloride solids followed a nearly linear trend over time, indicating a reduction in the stability of the dispersions from 5 minutes to 24 hours. Using the categories developed by Riddick, the dispersion stability trended from fairly good to moderate to the threshold of agglomeration.45 The decrease in signal intensity and reduction in zeta potential for these solids are consistent with the FE-SEM images, which illustrate formation of particle clusters over time. For the commercially available silver nanoparticles immersed in artificial sweat, a reduction in DH values was observed over time, whereas the calculated zeta potential values remained essentially unchanged, indicating moderate to fairly good dispersion throughout the study. The observed reduction in DH over time may be due to individual nanoparticles forming larger clusters. These clusters would be removed when the liquid was passed through a membrane prior to analysis, or as a result of nanoparticle dissolution.
Experiment 2: barrier migration test of textile products
Ultraviolet-visible spectroscopy absorption spectra of textiles before and immediately after the barrier migration test did not indicate any shift in the maximum absorption peaks of the SilverMax (420–430 nm) or HVAC (415–425 nm) textiles (see Figure S1 in //dx.doi.org/10.1179/2049396714Y.0000000070.S2>Supplementary Material 2). This observation was true immediately after the test and at 4 and 7 days post-test, and results did not differ based on the use of the stainless steel weight or sebum that contained vitamin E. None of the filters in contact with the textiles exhibited an absorbance peak in the characteristic wavelength range of 390–420 nm for metallic silver nanoparticles immediately after the test or at 4 and 7 days post-test (data not shown). However, if the form of silver that migrated to the filters was a salt (e.g. AgCl), a metallic silver SPR absorption peak would not be observed in the UV-Vis absorbance spectra. Levels of total silver (regardless of chemical form) on all filters were below the analytical limit of detection for ICP-AES, indicating no migration. Using TEM, no particles were observed on any of the grid samples. Based on these data, it was concluded that silver did not migrate from the textiles to the filters during this test.
Experiment 3: aggressive extraction test of textile products
Using UV-Vis absorbance spectroscopy, there was no appreciable shift in the maximum absorption peaks of the SilverMax or HVAC textiles before and immediately after the HOH test (data not shown). Likewise, none of the spectra, from the artificial biological fluids that were exposed to the textiles, exhibited an absorbance peak maximum in the wavelength range 390–420 nm. This observation could mean that (1) metallic silver nanoparticles from textile fibers did not detach during the HOH test, (2) nanoparticles did detach but absorption of anions such as chloride to particle surfaces masked their SPR signal, or (3) the signal was below our instrument detection limit. Interestingly, the commercially available metallic silver nanoparticles exposed to the artificial biological fluids under the same conditions as the textiles did not exhibit a SPR maximum in the wavelength range 390–420 nm (data not shown).
To better understand the UV-Vis absorbance results, FE-SEM-EDX was used to characterize the particles on filters through which artificial biological fluids were passed after the HOH tests. Silver-containing particles were only observed for the samples from SilverMax textile exposed to SSFL sweat at pH 4.5 (at both 36 and 45°C); the particles were composed of silver and chlorine. For all other artificial biological fluids exposed to textiles, particles were either not observed on filters, or if present, were salt crystals composed of Na, Mg, and/or Cl (data not shown). No visible changes in morphology were observed for the commercially available nano- and micron-scale silver particles; however, EDX analysis identified Cl in all samples. We cannot say for certain if the silver/chlorine particles observed for the SilverMax textile exposed to sweat at pH 4.5 were formed by the release of silver ions into the fluid during the HOH test with subsequent precipitation with chloride, were released as particles and chloride ions adsorbed to their surfaces, or formed as an artifact of the drying process.
Within each sample type (e.g. SilverMax, HVAC), values of DH were generally constant for all artificial biological fluids used in the HOH test. The measured values of DH from textile samples in the FE-SEM data are attributed to salt crystals, not silver nanoparticles released into the artificial biological fluids. The exception is the artificial sweat having pH 4.5 at 36°C (DH = 164±14 nm) and 45°C (DH = 165±55 nm) exposed to the SilverMax textile; these DH values may reflect both silver/chlorine particles and salt crystals in the fluid. Values of zeta potential generally indicated that particles in suspension in the artificial biological fluids had moderate to fairly good dispersion stability, although under some conditions, particles were on the threshold of agglomeration (data not shown).
Table 1 summarizes the mass fraction of silver dissolved by sample type under each experimental physiological factor condition. Levels of silver ions released into artificial biological fluids were below the analytical limit of detection for ICP-AES (0.001 mg/l) in 24% (5/21) of the HVAC textile samples and 5% (1/21) of the SilverMax samples. Silver was not detected in any of the artificial biological fluids exposed to the polyester control textile. The SSFL formulation was used to investigate the potential influence of sweat solvent pH (4.5 vs 5.3), temperature (36 vs 45°C), and composition (with and without sebum vitamin E) on silver release for each sample type (SilverMax, HVAC, micron particles, nanoparticles). Generally, neither SSFL pH nor temperature influenced silver ion release from any of the samples. The only exception was the HVAC textile. Significantly more silver ion was released in SSFL (36°C only) buffered to pH 5.3 compared to pH 4.5 (P<0.05). With regard to temperature, significantly more silver ion was released for SSFL (pH 4.5 only) maintained at 45°C compared to 36°C (P<0.05). Note that the amounts of silver ion released from two of the three replicate samples of HVAC textile in SSFL at 36°C and pH 4.5 were below our analytical detection limit. The presence of vitamin E in sebum only influenced dissolution of the micron-scale silver particles, with more silver ion released into SSFL prepared with sebum that contained vitamin E (P<0.05).
Table 1. Fraction of silver dissolved from textiles and particles immersed in artificial human biological fluids.
| Sample* | Fluid | Sebum | pH | T (°C) | Mass dissolved (%)† |
| SilverMax | SSFL | Vit E | 5.3 | 36 | 0.11±0.04 |
| SSFL | Vit E | 4.5 | 36 | 0.05±0.01 | |
| SSFL | Vit E | 5.3 | 45 | 0.08±0.02‡ | |
| SSFL | Vit E | 4.5 | 45 | 0.11±0.04 | |
| SSFL | w/o Vit E | 5.3 | 36 | 0.07±0.01 | |
| Simple sweat | N/A | 5.8 | 36 | 0.11±0.02 | |
| Simple saliva | N/A | 6.8 | 37 | 0.05±0.02 | |
| HVAC | SSFL | Vit E | 5.3 | 36 | 0.02±0.00 |
| SSFL | Vit E | 4.5 | 36 | 0.01§ | |
| SSFL | Vit E | 5.3 | 45 | 0.02±0.00 | |
| SSFL | Vit E | 4.5 | 45 | 0.02±0.00 | |
| SSFL | w/o Vit E | 5.3 | 36 | 0.02±0.00 | |
| Simple sweat | N/A | 5.8 | 36 | 0.03±0.01‡ | |
| Simple saliva | N/A | 6.8 | 37 | 0.02±0.00‡ | |
| Ag NP | SSFL | Vit E | 5.3 | 36 | 0.52±0.33 |
| SSFL | Vit E | 4.5 | 36 | 0.41±0.20 | |
| SSFL | Vit E | 5.3 | 45 | 0.36±0.09 | |
| SSFL | Vit E | 4.5 | 45 | 0.50±0.35 | |
| SSFL | w/o Vit E | 5.3 | 36 | 0.34±0.11 | |
| Simple sweat | N/A | 5.8 | 36 | 0.32±0.16 | |
| Simple saliva | N/A | 6.8 | 37 | 0.51±0.16‡ | |
| Ag MP | SSFL | Vit E | 5.3 | 36 | 0.23±0.09 |
| SSFL | Vit E | 4.5 | 36 | 0.33±0.12 | |
| SSFL | Vit E | 5.3 | 45 | 0.40±0.08 | |
| SSFL | Vit E | 4.5 | 45 | 0.33±0.06 | |
| SSFL | w/o Vit E | 5.3 | 36 | 0.47±0.04 | |
| Simple sweat | N/A | 5.8 | 36 | 0.29±0.04 | |
| Simple saliva | N/A | 6.8 | 37 | 0.45±0.21 |
* Ag NP = <30 nm (PN: AG-M-02M-NP.030N, American Elements); Ag MP = ≤2 μm (PN: AG-M-02M-MP.020UM, American Elements).
SSFL: skin surface film liquid; Vit E: vitamin E.
† Value is for n = 3 samples unless noted otherwise.
‡ One of three replicate samples below the limit of detection for ICP-AES (0.001 mg/l).
§ Two of three replicate samples below the limit of detection for ICP-AES (0.001 mg/l).
We also investigated differences in silver ion release from each sample type by fluid (controlling pH and temperature). For each fluid tested (SSFL, simple sweat, simple saliva), the mass fractions of silver ions released from the SilverMax textile made by a finishing operation were three to five times higher than the HVAC textile made by a masterbatch process; however, differences were not significant (P>0.05). The mass fractions of silver ions dissolved from the commercially available metallic silver micron- and nanoscale particles were at least a factor of three greater than either textile material though differences were not significant for all fluids. In simple sweat, silver ion release from the micron- and nanoscale silver particles was significantly greater than from the HVAC textile, but not the SilverMax textile. In SSFL, the amount of silver ions released from micron-scale silver particles was significantly greater than from either textile, except for the fluid at pH 4.5/45°C and pH 5.3/36°C. Release of silver ions from silver nanoparticles was significantly greater than from either textile.
Experiment 4: release of silver during simulated use scenario
Silver was not detected in any of the polyester control samples. On a mass basis, the total cumulative amount of silver ion released from the SilverMax textile (2.12±0.28 μg) was similar to that measured for the HVAC textile (2.46±0.19 μg). However, when the masses of silver ion released were expressed as a fraction of the initial mass of total silver in a textile sample, the results exhibited the expected trend of more silver ions released from the SilverMax textile (0.51±0.04%) than the HVAC textile (0.21±0.01%); (P<0.01). The tabular data from all ICP analyses are summarized in //dx.doi.org/10.1179/2049396714Y.0000000070.S5>Supplementary Material 5 (see Table S1). The effect of treatment method is further highlighted by the data for silver particles released during the use scenario. The amount of silver released in particulate form represented 0.29% of the total mass of silver in SilverMax textile samples but almost no silver particulate was released from the HVAC textile.
For all use scenario samples, the GEOCHEM-EZ thermodynamic model predicted that the low levels of released silver ion would primarily form soluble complexes (no precipitates) with little free ion (<0.6%). Sweat sulfur compounds were excluded from the model because it was unstable and did not converge on a solution when these ligands were present. For SilverMax textile, the model predicted that silver ions would form soluble complexes with iodide, chloride, and histidine for the first three uses. The model predicted that 99.8% of silver ion in the laundering wash water (pH 10) was complexed with histidine with the balance bound to iodide. In the subsequent three uses, the model predicted formation of predominantly soluble silver chloride complexes, followed by silver iodide, and silver histidine. The same trend was observed for calculations using the HVAC textile sample data.
Figure 3 is a plot of the cumulative amounts of ionic and total (ionic and particulate) silver released from the SilverMax and HVAC textiles during simulated uses. The plot shows two trends. First, approximately three to five times more total silver was released from the SilverMax textile than the HVAC textile. Second, the slopes of the curves for the SilverMax and HVAC textile samples became steeper following the laundering procedure for both ionic and total silver release, though the trend is more pronounced for the SilverMax textile. For the plots of silver ion release, the average slopes for the first three uses were 0.0533 (SilverMax) and 0.0261 (HVAC) and for the last three uses they were 0.0978 (SilverMax) and 0.0368 (HVAC). The increased slopes indicate that the simplified laundering procedure promoted subsequent dissolution and weakened the interface between the silver nanoparticles and the textile fiber surface.
Figure 3.
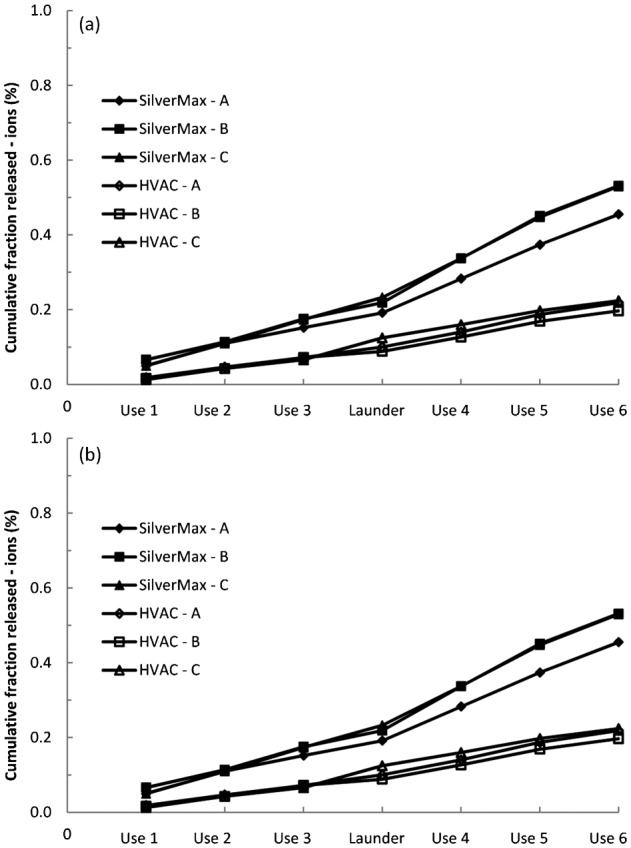
Cumulative fractions of (A) ionic and (B) total (ionic and particulate) silver released from the SilverMax and HVAC textiles during simulated uses and laundering.
Values of DH and zeta potential for the artificial sweat samples from the use and laundering simulations are summarized in //dx.doi.org/10.1179/2049396714Y.0000000070.S5>Supplementary Material 5 (see Table S2). For the SilverMax textile, values of DH are generally stable through the first three washes, increase during the laundering step, and decrease over subsequent wash simulations. The observed increase in DH during laundering tracked with the increased release of silver in the form of particles from the textile (see Table S1 in //dx.doi.org/10.1179/2049396714Y.0000000070.S5>Supplementary Material 5). In contrast, for the HVAC textile, nanoscale particles were present in the sweat fluids at all times, but silver particles were only detected by ICP in sweat from the last two washes of replicate sample C. Values of zeta potential for the sweat samples exposed to SilverMax textile were between −30 and −35 mV during the initial three use scenarios but increased to −51 mV during laundering and remained at this level throughout the final three wash scenarios. For sweat samples exposed to HVAC textile, values of zeta potential increased from −22 mV after the first wash, to a maximum of −53 mV during laundering, and declined to −30 mV by the last wash. Neither measurement of DH nor zeta potential is chemical specific. As such, we cannot rule out that some measured values of DH and zeta potential were due to surfactant micelles from the detergent.46 None of the sweat fluid samples from the SilverMax or HVAC textiles analyzed by UV-Vis spectroscopy yielded a SPR absorption maximum consistent with the presence of metallic silver particles (data not shown).
Discussion
Product-related and physiological factors that could influence exposure to silver from contact with two commercially available antimicrobial textiles were evaluated in this study. Regardless of manufacturing process, masterbatch or finishing, dissolution of silver particles to form ions was the primary release mechanism. This observation is consistent with Quadros et al. who reported that more silver was released as ion than particulate from textiles in children’s products and von Goetz et al. who reported the same trend for adult clothing in artificial sweat (pH 5.5).20,21
Quadros et al. noted that the fraction of silver released from an infant blanket was lower than from the interior foam of a plush toy despite a higher total silver concentration in the blanket.20 The authors suggested that the discrepancy in silver release between the products could be attributed to the method used to treat the fibers of the product with silver. Our simulated use scenario (Fig. 3) demonstrated that the amounts of silver ions released from the HVAC textile were significantly lower than SilverMax textile. This observed difference in silver release could be from factors such as differences in the physicochemical properties of silver used in the textiles (size, size distribution, crystallinity, coatings, etc.) or the treatment process used to apply silver to the fibers. Inspection of fiber surfaces following the sixth simulated use did not reveal appreciable changes in morphology (see Figure S2 in //dx.doi.org/10.1179/2049396714Y.0000000070.S4>Supplementary Material 4). The absence of appreciable disruption to HVAC fiber surfaces means that silver particles incorporated into the polymer generally remained within the fibers and that observed dissolution was likely the result of silver ions dissolving within the fibers and diffusing to the bulk sweat liquid. Hence, we interpret our data to mean that for these two products, the treatment process was an important factor that influenced release of silver ions into artificial sweat and saliva for these textiles. It is important to note that polymer surrounding silver particles in HVAC fibers may act as a rate-limiting barrier to incursion of sweat or diffusion of silver to the bulk liquid. As such, it is possible that if the silver particles from the SilverMax and HVAC textiles were exposed to sweat independent of their textile matrices any differences in physicochemical properties could influence dissolution.
Quadros et al. observed that silver release from an infant blanket was slightly lower when sodium and chloride were omitted from the artificial sweat formulation and that lactic acid and urea did not influence results.20 In our studies, there was no difference in dissolution between the simple artificial sweat formulation and the more complex SSFL formulation. Kulthong et al. reported that the total (ion and particulate) amount of silver released from textiles increased as the pH of artificial sweats increased.23 In that study, silver release from a commercially available textile was 0.1% (pH 4.3) to 0.5% (pH 5.5) and our results using SSFL at similar pH values (4.5 and 5.3) were consistent with their observation. Lazić et al. reported that total silver release from cotton fabrics treated with metallic silver nanoparticles by a finishing process in the laboratory was 7–50% depending on dyeing and artificial sweat pH (5.5–8.0).24,25 The relatively high release rates observed in their study are attributed to weak binding of the nanoparticles to the textile threads during the laboratory finishing treatment process. It should also be noted that release amounts may vary with the volume of wash water used in these studies, which limits extrapolating these data to what may occur in laundry machine appliances. The above mentioned studies did not differentiate between ionic and particulate silver released into artificial sweat. Yan et al. fractionated silver ions from particles that were released from a textile treated by a finishing process and concluded that silver was primarily in particulate form in acidic artificial sweat (pH 3) but in ionic form in alkaline sweat (pH 8).27 In contrast, von Goetz et al. reported that more silver was released in ionic form than in particulate form in acidic artificial sweat (pH 5.5).21 Our results are in agreement with von Goetz et al., we observed that more silver was released in ionic form than in particulate form in SSFL at pH 5.3 (see //dx.doi.org/10.1179/2049396714Y.0000000070.S5>Supplementary Material 5, Table S1). We did not observe as pronounced influences of sweat pH on dissolution of silver as those in previous reports, though in our study the pH difference between formulations was only 0.8 units.
Wet laundering is a common means to care for textile products. Quadros et al. reported that silver release from an infant blanket did not increase with sequential washings in water.20 In our study, laundering with hypochlorite (bleach) promoted dissolution and weakened the interface between the silver nanoparticles and the textile fiber surface. Impellitteri et al. exposed a sock textile that contained silver nanoparticles to a bleach/detergent solution with agitation and reported that a significant portion of the silver nanoparticles was converted to AgCl.14 This transformation of silver nanoparticles to AgCl proceeded rapidly in the presence of hypochlorite (an oxidizer). Impellitteri et al. acknowledged that their results cannot discern whether AgCl formed independently in solution from the reaction of oxidized silver ion with chloride or a surface oxidation reaction involving hypochlorite was responsible for the AgCl coating on silver nanoparticles.14 Similarly, our data do not permit us to state with certainty whether the particles released from the textiles during the use scenario were precipitates of AgCl or a surface coating of adsorbed chloride/silver complexes. Kent and Vikesland used atomic force microscopy to monitor silver nanoparticle dissolution.47 Thermodynamic modeling by these authors suggested that silver ions did not react with chloride ion to form precipitate particles or a precipitate coating that adsorbed to particle surfaces. Further studies using X-ray photoelectron spectroscopy to evaluate surface chemistry revealed that the binding states of chloride were not consistent with silver chloride precipitates. Rather, the authors suggest that silver and chloride ions form soluble complexes that prevent adsorption of silver back onto particle surfaces. Our thermodynamic modeling also indicated that silver ion would form soluble chloride complexes. Consistent with the conclusion by Kent and Vikesland that silver chloride complexes bind to particle surfaces, no SPR signal was measured by UV-Vis absorption spectroscopy for textiles or metallic silver particles after exposure to artificial biological fluids containing high levels of chloride.
Release is only a precursor to external skin exposure and the latter is not equivalent to internal dose. Using their release data for a silver-treated T-shirt or pair of trousers, von Goetz et al. estimated external exposure to silver for adult male and females.21 For silver ion release in acidic artificial sweat (pH 5.5), von Goetz et al. calculated that the external exposure for males using a T-shirt was 5 μg Ag/kg body weight and for trousers was 9 μg Ag/kg body weight.21 The external exposure for females using a T-shirt was 2 μg Ag/kg body weight and for trousers was 6 μg Ag/kg body weight. To permit more direct comparison between studies, we used the same exposure calculation equation and standard inputs values (mass of textile garment, human sweat rate, exposure time, area of skin exposed, fraction of skin in contact with textile, and body weight) as von Goetz et al. but our release rate data from the first use of the simulation experiment with the SilverMax textile (worst case scenario). Though it was used to produce adult underwear, for purposes of these calculations we assumed that the same textile could be used to make other garments such as a T-shirt or trousers. For silver ion release in artificial SSFL (pH 5.3), we calculated that the external exposure for males using a T-shirt was 4 μg Ag/kg body weight and for trousers it was 6 μg Ag/kg body weight. The external exposure for females using a T-shirt was 2 μg Ag/kg body weight and for trousers it was 4 μg Ag/kg body weight. These simple calculations are within the same order of magnitude for the two independent studies and provide useful insights to external exposure; however, it is the penetration of released silver through the stratum corneum that will influence internal dose.
If silver is released as ions it will primarily form water soluble complexes with sweat constituents. Silver ions in biological media may react with chloride ions, sulfur compounds (ion, sulfates), including thiol-containing amino acids such as methionine, and other ligands.18,32,48,49 According to Liu et al. the amount of free silver ion in biological fluids will be extremely low due to complex formation and possible precipitation with chloride ions.18 In our initial experiment using 5 mM silver ion (in the form of silver nitrate) concentration, the silver rapidly reacted with sweat chloride ions to form poorly soluble silver chloride particles (Fig. 2). In contrast, at the much lower silver ion concentrations measured in the use scenario experiment, silver was primarily in the ionic fraction. Our modeling using GEOCHEM-EZ predicted that silver ions in SSFL would primarily form soluble complexes with iodide, chloride, and histidine with <0.6% present as free ion. Modeling by Liu et al. indicates that sulfates and thiol-containing amino acids play an important role in the chemical transformation of silver nanoparticles in biological environments.18 They reported that sulfates have higher binding affinities than thiols but are present at lower concentrations in some biological fluids. Therefore, binding kinetics strongly favor formation of silver ion complexes with thiols. We cannot state for certain what influence thiol-containing amino acids would have on our results as they were omitted from our SSFL model because there is limited quantitative data on their concentrations in human sweat. Additionally, when sulfur and sulfate were included the GEOCHEM-EZ program with our dissolved silver data, the model did not converge. Hence, depending on the binding competition between chloride and sulfur compounds (sulfur, sulfate, thiols) with silver ions, the distribution of silver complexes on the skin surface may differ from our modeling results. However, it is still expected that the fraction of free silver ions will be less than that of soluble complexes. Importantly, water soluble forms (complexes, free ion) could penetrate the stratum corneum, and once across, precipitate to form particles in the viable skin layers or bind to proteins and be translocated throughout the body. Ultimately, the relative penetration efficiencies of different water soluble complexes through the stratum corneum layer of the skin, and the integrity of this skin barrier, will influence dose.28 If silver is released in the form of nanoscale particulate, penetration through skin may be an exposure pathway. However, this pathway is controversial; some studies indicate that penetration through the stratum corneum occurs and others suggesting that it is not a legitimate pathway.50,51 It is important to note that these studies often utilize skin with an intact outer stratum corneum layer, which by design, serves as a highly efficient barrier to the external environment. If the skin barrier integrity is compromised by scratches, its efficacy against penetration is impaired or if it is compromised by major trauma such as burns, this barrier is greatly reduced.8,28
Several studies, including ours, have demonstrated that textiles treated with silver particles have capacity to release silver primarily in ionic form when in contact with biological fluids such as sweat, saliva, and urine.20,21,23–25,27 Release of silver from products in contact with the skin is important because of the risk of inflammation and localization of silver nanoparticles in the upper stratum corneum of skin and known decreases in cell viability, morphology, and migratory behavior. Their contact with compromised skin may results in skin discoloration and translocation to other organs such as the kidney, and transient increases in biological burden of silver in the body.4–9 In the present study, we evaluated product-related and physiological factors that could influence release of silver from treated textiles. We observed that physiological factors (composition, pH, and temperature) representative of human biological fluids had a minor influence on release. Rather, the major factor that influenced the release of silver from these two textiles into biological fluids was the manner by which the textile fibers were treated (masterbatch vs finishing). Importantly, our observation of the importance of product type on dermal exposure potential complements the findings of Geranio et al. who observed that product type was an important factor for silver release during laundering.13 For efficacious performance, a silver-treated textile product must release ions at a rate that is sufficient to kill odor-causing microbes, though for health protection the release rate should not pose a dermal (or environmental) exposure hazard (though this rate is currently not known). As noted by Leavens et al., the current U.S. Environmental Protection Agency reference dose (RfD) for silver is 0.005 mg/kg/day, a value based on the risk for argyria ingestion. The study did not use silver nanoparticles. Furthermore, there is no good RfD for dermal exposure to silver.52 Hence, the observed influence of fiber treatment technique is significant because it indicates that, in the absence of scientifically defined allowable limits for dermal exposure to silver, existing production techniques could be modulated to control silver ion release to ensure product efficacy and minimize human and environmental exposure. More studies are needed to evaluate the generalizability of our data and that of other investigators from a limited number of products to migration from silver-treated textiles in general to better protect worker and consumer health.
Disclaimer Statements
Contributors ABS conceived and designed the experiments; acquired, analyzed, and interpreted associated data; and drafted the manuscript. EEW, MGD, and RBL acquired and analyzed data and drafted the manuscript. RFL developed spectroscopic approaches for analyses. MAV contributed to the study design; acquired, analyzed, and interpreted associated data; and drafted the manuscript. TAT contributed to the study design and drafted the manuscript. All authors read and approved the final manuscript.
Funding U.S. Consumer Product Safety Commission.
Conflicts of interest No conflicts of interest, financial or otherwise.
Ethics approval Not applicable.
Acknowledgments
This work was funded by the U.S. Consumer Product Safety Commission (CPSC) under Inter-Agency Agreement CPSC-I-10-006. Mention of a specific product or company does not constitute endorsement by the Centers for Disease Control and Prevention. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of NIOSH or CPSC. The authors wish to thank Dr. F. Selcen Kilinc-Balci at NIOSH and Drs. D. Holbrook and R. I. MacCuspie at NIST for critical review of this manuscript.
References
- 1.Lee HJ, Yeo SY, Jeong SH. Antibacterial effect of nanosized silver colloidal solution on textile fabrics. J Mat Sci. 2003;38(10):2199–204. [Google Scholar]
- 2.Nowack B, Krug HF, Height M. 120 years of nanosilver history: implications for policy makers. Environ Sci Technol. 2011;45(4):1177–83. doi: 10.1021/es103316q. [DOI] [PubMed] [Google Scholar]
- 3.Morones JR, Elechiguerra JL, Camacho A, Holt K, Kouri JB, Ramirez JT, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16(10):2346–53. doi: 10.1088/0957-4484/16/10/059. [DOI] [PubMed] [Google Scholar]
- 4.Arora S, Jain J, Rajwade JM, Paknikar KM. Cellular responses induced by silver nanoparticles: in vitro studies. Toxicol Lett. 2008;179(2):93–100. doi: 10.1016/j.toxlet.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Korani M, Rezayat SM, Gilani K, Bidgoli SA, Adeli S. Acute and subchronic dermal toxicity of nanosilver in guinea pig. Int J Nanomedicine. 2011;6:855–62. doi: 10.2147/IJN.S17065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samberg ME, Oldenburg SJ, Monteiro-Riviere NA. Evaluation of silver nanoparticle toxicity in skin in vivo and keratinocytes in vitro. Environ Health Perspect. 2010;118(3):407–13. doi: 10.1289/ehp.0901398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szmyd R, Goralczyk AG, Skalniak L, Cierniak A, Lipert B, Filon FL, et al. Effect of silver nanoparticles on human primary keratinocytes. Biol Chem. 2013;394(1):113–23. doi: 10.1515/hsz-2012-0202. [DOI] [PubMed] [Google Scholar]
- 8.Trop M, Novak M, Rodl S, Hellbom B, Kroell W, Goessler W. Silver coated dressing acticoat caused raised liver enzymes and argyria-like symptoms in burn patient. J Trauma. 2006;60(3):648–52. doi: 10.1097/01.ta.0000208126.22089.b6. [DOI] [PubMed] [Google Scholar]
- 9.Vlachou E, Chipp E, Shale E, Wilson YT, Papini R, Moiemen NS. The safety of nanocrystalline silver dressings on burns: a study of systemic silver absorption. Burns. 2007;33(8):979–85. doi: 10.1016/j.burns.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Wijnhoven SWP, Peijnenburg W, Herberts CA, Hagens WI, Oomen AG, Heugens EHW, et al. Nano-silver – a review of available data and knowledge gaps in human and environmental risk assessment. Nanotoxicology. 2009;3(2):109–38. [Google Scholar]
- 11.Benn T, Cavanagh B, Hristovski K, Posner JD, Westerhoff P. The release of nanosilver from consumer products used in the home. J Environ Qual. 2010;39(6):1875–82. doi: 10.2134/jeq2009.0363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benn TM, Westerhoff P. Nanoparticle silver released into water from commercially available sock fabrics. Environ Sci Technol. 2008;42(11):4133–9. doi: 10.1021/es7032718. [DOI] [PubMed] [Google Scholar]
- 13.Geranio L, Heuberger M, Nowack B. The behavior of silver nanotextiles during washing. Environ Sci Technol. 2009;43(21):8113–8. doi: 10.1021/es9018332. [DOI] [PubMed] [Google Scholar]
- 14.Impellitteri CA, Tolaymat TM, Scheckel KG. The speciation of silver nanoparticles in antimicrobial fabric before and after exposure to a hypochlorite/detergent solution. J Environ Qual. 2009;38(4):1528–30. doi: 10.2134/jeq2008.0390. [DOI] [PubMed] [Google Scholar]
- 15.Kim J, Kim S, Lee S. Differentiation of the toxicities of silver nanoparticles and silver ions to the Japanese medaka (Oryzias latipes) and the cladoceran Daphnia magna. Nanotoxicology. 2011;5(2):208–14. doi: 10.3109/17435390.2010.508137. [DOI] [PubMed] [Google Scholar]
- 16.Li GH, Liu H, Zhao HS, Gao YQ, Wang JY, Jiang HD, et al. Chemical assembly of TiO2 and TiO2@Ag nanoparticles on silk fiber to produce multifunctional fabrics. J Colloid Interface Sci. 2011;358(1):307–15. doi: 10.1016/j.jcis.2011.02.053. [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Hurt RH. Ion release kinetics and particle persistence in aqueous nano-silver colloids. Environ Sci Technol. 2010;44(6):2169–75. doi: 10.1021/es9035557. [DOI] [PubMed] [Google Scholar]
- 18.Liu J, Wang Z, Liu FD, Kane AB, Hurt RH. Chemical transformations of nanosilver in biological environments. ACS Nano. 2012;6(11):9887–99. doi: 10.1021/nn303449n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perelshtein I, Applerot G, Perkas N, Guibert G, Mikhailov S, Gedanken A. Sonochemical coating of silver nanoparticles on textile fabrics (nylon, polyester and cotton) and their antibacterial activity. Nanotechnology. 2008;19(24):245705. doi: 10.1088/0957-4484/19/24/245705. [DOI] [PubMed] [Google Scholar]
- 20.Quadros ME, Pierson RT, Tulve NS, Willis R, Rogers K, Thomas TA, et al. Release of silver from nanotechnology-based consumer products for children. Environ Sci Technol. 2013;47(15):8894–901. doi: 10.1021/es4015844. [DOI] [PubMed] [Google Scholar]
- 21.von Goetz N, Lorenz C, Windler L, Nowack B, Heuberger M, Hungerbuhler K. Migration of Ag- and TiO2-(Nano)particles from textiles into artificial sweat under physical stress: experiments and exposure modeling. Environ Sci Technol. 2013;47(17):9979–87. doi: 10.1021/es304329w. [DOI] [PubMed] [Google Scholar]
- 22.Agache P, Candas V. Eccrine sweat glands. In: Agache P, Humbert P, editors. Measuring the skin: non-invasive investigations, physiology, normal constants. Germany: Springer-Verlag; 2004. p. 302–9. [Google Scholar]
- 23.Kulthong K, Srisung S, Boonpavanitchakul K, Kangwansupamonkon W, Maniratanachote R. Determination of silver nanoparticle release from antibacterial fabrics into artificial sweat. Part Fibre Toxicol. 2010;7:1187–8. doi: 10.1186/1743-8977-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lazić V, Šaponjić Z, Vodnik V, Dimitrijević S, Jovanćić P, Nedeljković J, et al. A study of the antibacterial activity and stability of dyed cotton fabrics modified with different forms of silver. J Serb Chem Soc. 2012;77(2):225–34. [Google Scholar]
- 25.Lazić V, Šaponjić Z, Vodnik V, Jovančić P, Nedeljković J, Radetić M. Antibacterial and colorimetric evaluation of cotton fabrics dyed with direct dyes and loaded with Ag nanoparticles. Industria Textilă. 2013;64(2):89–97. [Google Scholar]
- 26.Stefaniak AB, Harvey CJ. Dissolution of materials in artificial skin surface film liquids. Toxicol in Vitro. 2006;20(8):1265–83. doi: 10.1016/j.tiv.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Yan Y, Yang HF, Li JF, Lu XJ, Wang C. Release behavior of nano-silver textiles in simulated perspiration fluids. Text Res J. 2012;82(14):1422–9. [Google Scholar]
- 28.Larese-Filon F, D'Agostin F, Crosera M, Adami G, Renzi N, Bovenzi M, et al. Human skin penetration of silver nanoparticles through intact and damaged skin. Toxicology. 2009;255(1–2):33–7. doi: 10.1016/j.tox.2008.09.025. [DOI] [PubMed] [Google Scholar]
- 29.Tulve NS, Stefaniak AB, Quadros ME, Rogers K, Mwilu S, LeBouf RF, et al. Characterization of silver nanoparticles in consumer products to predict children’s potential exposure. Submitted. doi: 10.1016/j.ijheh.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harvey CJ, LeBouf RF, Stefaniak AB. Formulation and stability of a novel artificial human sweat under conditions of storage and use. Toxicol in Vitro. 2010;24(6):1790–6. doi: 10.1016/j.tiv.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 31.Levard C, Reinsch BC, Michel FM, Oumahi C, Lowry GV, Brown GE. Sulfidation processes of PVP-coated silver nanoparticles in aqueous solution: impact on dissolution rate. Environ Sci Technol. 2011;45(12):5260–6. doi: 10.1021/es2007758. [DOI] [PubMed] [Google Scholar]
- 32.Lorenz C, Windler L, von Goetz N, Lehmann RP, Schuppler M, Hungerbuhler K, et al. Characterization of silver release from commercially available functional (nano)textiles. Chemosphere. 2012;89(7):817–24. doi: 10.1016/j.chemosphere.2012.04.063. [DOI] [PubMed] [Google Scholar]
- 33.Rogers KR, Bradham K, Tolaymat T, Thomas DJ, Hartmann T, Ma LZ, et al. Alterations in physical state of silver nanoparticles exposed to synthetic human stomach fluid. Sci Total Environ. 2012;420:334–9. doi: 10.1016/j.scitotenv.2012.01.044. [DOI] [PubMed] [Google Scholar]
- 34.Stefaniak AB, Harvey CJ, Wertz PW. Formulation and stability of a novel artificial sebum under conditions of storage and use. Int J Cosmet Sci. 2010;32:347–355. doi: 10.1111/j.1468-2494.2010.00561.x. [DOI] [PubMed] [Google Scholar]
- 35.Cobb D. Migration of flame retardant chemicals in mattress barriers. Washington, D.C: U.S. Consumer Product Safety Commission; 2005. [Google Scholar]
- 36.Rijk R. 1998. Determination of release of diisononyl phthalate in saliva simulant. Zeist, The Netherlands: TNO Nutrition and Food Research Institute. [Google Scholar]
- 37.Kelly KL, Coronado E, Zhao LL, Schatz GC. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B. 2003;107(3):668–77. [Google Scholar]
- 38.Zook JM, Long SE, Cleveland D, Geronimo CLA, MacCuspie RI. Measuring silver nanoparticle dissolution in complex biological and environmental matrices using UV-visible absorbance. Anal Bioanal Chem. 2011;401(6):1993–2002. doi: 10.1007/s00216-011-5266-y. [DOI] [PubMed] [Google Scholar]
- 39.Thomas T, Brundage P.Quantitative assessment of potential health effects from the use of fire retardant (FR) chemicals in mattresses Washington, DC: U.S. Consumer Product Safety Commission; 2006 [Google Scholar]
- 40.Ghanem R, Delmani FA. Kinetics of thermal and photolytic degradation of decabromodiphenyl ether (BDE 209) in backcoated textile samples. J Anal Appl Pyrolysis. 2012;98:79–85. [Google Scholar]
- 41.Shelton F, Barnett R, Meyer E. Full-body pressure interface testing as a method for performance evaluation of clinical support surfaces. Appl Ergon. 1998;29(6):491–7. doi: 10.1016/s0003-6870(97)00069-0. [DOI] [PubMed] [Google Scholar]
- 42.Glover RD, Miller JM, Hutchison JE. Generation of metal nanoparticles from silver and copper objects: nanoparticle dynamics on surfaces and potential sources of nanoparticles in the environment. ACS Nano. 2011;5(11):8950–7. doi: 10.1021/nn2031319. [DOI] [PubMed] [Google Scholar]
- 43.Chao JB, Liu JF, Yu SJ, Feng YD, Tan ZQ, Liu R, et al. Speciation analysis of silver nanoparticles and silver ions in antibacterial products and environmental waters via cloud point extraction-based separation. Anal Chem. 2011;83(17):6875–82. doi: 10.1021/ac201086a. [DOI] [PubMed] [Google Scholar]
- 44.Shaff JE, Schultz BA, Craft EJ, Clark RT, Kochian LV. GEOCHEM-EZ: a chemical speciation program with greater power and flexibility. Plant Soil. 2010;330(1–2):207–14. [Google Scholar]
- 45.Riddick T. Control of colloid stability through zeta potential: with a closing chapter on its relationship to cardiovascular disease. Livingston Publishing Company. 1968 [Google Scholar]
- 46.Skoglund S, Lowe TA, Hedberg J, Blomberg E, Odnevall Wallinder I, Wold S, et al. Effect of laundry surfactants on surface charge and colloid stability of silver nanoparticles (Ag NPs). Langmuir. 2013;29(28):8882–91. doi: 10.1021/la4012873. [DOI] [PubMed] [Google Scholar]
- 47.Kent RD, Vikesland PJ. Controlled evaluation of silver nanoparticle dissolution using atomic force microscopy. Environ Sci Technol. 2012;46(13):6977–84. doi: 10.1021/es203475a. [DOI] [PubMed] [Google Scholar]
- 48.Lenz GR, Martell AE. Metal chelates of some sulfur-containing amino acids. Biochemistry. 1964;3:745–50. doi: 10.1021/bi00894a001. [DOI] [PubMed] [Google Scholar]
- 49.Luther GW, Rickard DT. Metal sulfide cluster complexes and their biogeochemical importance in the environment. J Nanopart Res. 2005;7(6):389–407. [Google Scholar]
- 50.Baroli B, Ennas MG, Loffredo F, Isola M, Pinna R, Lopez-Quintela MA. Penetration of metallic nanoparticles in human full-thickness skin. J Invest Dermatol. 2007;127(7):1701–12. doi: 10.1038/sj.jid.5700733. [DOI] [PubMed] [Google Scholar]
- 51.Campbell CS, Contreras-Rojas LR, Delgado-Charro MB, Guy RH. Objective assessment of nanoparticle disposition in mammalian skin after topical exposure. J Control Release. 2012;162(1):201–7. doi: 10.1016/j.jconrel.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 52.Leavens TL, Monteiro-Riviere NA, Inman AO, Brooks JD, Oldenburg SJ, Riviere JE. In vitro biodistribution of silver nanoparticles in isolated perfused porcine skin flaps. J Appl Toxicol. 2012;32(11):913–9. doi: 10.1002/jat.2750. [DOI] [PMC free article] [PubMed] [Google Scholar]