Abstract
Background:
The gene–environment interaction in the pathogenesis of hypertension has not been extensively studied in occupational noise.
Objectives:
The aim of this study was to determine the relationship between noise and hypertension in Egyptian workers, the interaction of angiotensin-converting enzyme (ACE) gene polymorphisms as modifiers, and the possible relationship between noise hearing impairment and hypertension.
Methods:
Study subjects were divided into two groups depending on noise exposure level. The control group (n = 161) was exposed to noise intensity <85 dB and the exposed group (n = 217) was exposed to noise intensity ≧85 dB. A polymerase chain reaction was used to differentiate the various genotypes of ACE insertion/deletion (I/D) and ACE G2350A.
Results:
Noise significantly increased the likelihood of hypertension. Carriers of the genotypes AG, GG, and DD were vulnerable to hypertension on noise exposure. No association between hypertension and hearing impairment or noise-induced hearing loss (NIHL) was found.
Conclusion:
Our results support the association between ACE gene polymorphisms and occurrence of hypertension in noise-exposed workers.
Keywords: ACE I/D gene, ACE G2350A gene, Genetic susceptibility, Gene polymorphism occupational noise, Hypertension
Introduction
Hypertension is an important risk factor for a variety of cardiovascular, renal, and neurological diseases. The average global prevalence of hypertension has declined slightly during the past two decades; meanwhile there is an increasing trend in the middle and low-income countries. Indeed about two-thirds of patients with hypertension are now living in developing countries.1
Elevated blood pressure (BP) is a precursor to excessive morbidity and premature mortality. Although systemic hypertension is a risk factor for disease burden, the risk is uneven, heterogeneous, and unpredictable.2 This clinical scenario, therefore, raises the possibility of genetics in the development of hypertension and/or related complications.
Recent progress in the development of new techniques for locating and identifying genetic variations contributing to common diseases has created new opportunities to advance our understanding of the etiology of many disorders including cardiovascular diseases.3
Accordingly, various genetic hypotheses have been proposed to explain systemic hypertension, but none have been well investigated. Monogenic explanations have not stood the test of evidence. Hence, hypertension may be identified as a polygenic hereditary disease simultaneously influenced by a variety of environmental factors.4 Indeed, genetic factors contribute to 30–50% of causation to essential hypertension.2
The renin–angiotensin system (RAS) contributes to electrolyte homeostasis and regulation of blood pressure. Angiotensin-converting enzyme (ACE) is the key enzyme of the RAS. ACE has two functions: one is to cut the two amino acids of the C-terminal of angiotensin I to generate angiotensin II, an octapeptide that is a potent vasoconstrictor. The other function is to inactivate bradykinin. Imbalance between forces of vasoconstriction over forces of vasodilation elevates vascular tone and leads to systemic elevation of blood pressure. On the basis of this theory, ACE gene polymorphisms associated with high (or inappropriate) levels of ACE can be considered as a genetic model in the development of hypertension and its complications.5
Insertion/deletion (I/D) and G2350A polymorphisms of the ACE gene have been studied with reference to cardiovascular diseases, especially hypertension.6,7 An I/D dimorphism has been shown to co-segregate with tissue and serum ACE function, and D allele is associated with elevated ACE levels. Therefore, the ACE gene can be considered a qualitative trait locus (QTL) modulating ACE levels; ACE I/D dimorphism is a reflection of linkage disequilibrium (LD) with variants located in the ACE gene, implicated in cardiovascular diseases.4
Among the 13 polymorphisms of the ACE gene recently reported, a dimorphism in exon 17, ACE G2350A, has the most significant effect on plasma ACE concentrations. After adjustment for the effect of ACE G2350A dimorphism, the I/D dimorphism was no longer associated with ACE, indicating that it is in LD with ACE G2350A and unlikely to be a functional mutation.8
Despite the fact that significant positive association of ACE gene with hypertension has been reported in several studies, other studies have failed to identify such association.5,9–12
One possible explanation for this is that gene–environment interactions (GEIs) have been largely ignored in the design and analyses of genetic studies. This has hampered the detection of significant genetic effects operating in those exposed to one environment and not another.13
GEIs can be defined as a joint effect of one or more genes with one or more environmental factors that cannot be readily explained by their separate marginal effects.14
Studies of GEI can be useful for investigating biological pathways, discovering genes that act only in particular environments or exposures that are hazardous only to genetically susceptible individuals, setting environmental safety standards, understanding heterogeneity in genetic associations across populations, and predicting individual risk and changes that might result from changes in modifiable risk factors.14
Noise, a psychosocial environmental stressor, disturbs and interferes with many activities including concentration, communication, relaxation, and sleep. High-frequency hearing loss may be associated with occupational noise exposure. A single traumatic exposure to loud sound, such as a gunshot or fireworks, or prolonged and/or repeated exposure to excessive sound over the acceptable daily exposure (85 dBA for 8 hours, a guideline set by the National Institute for Occupational Safety and Health) can cause sensorineural damage to the cochlea. This damage can lead to immediate hearing loss (impulse noise) or chronic progressive noise-induced hearing loss (NIHL).15
Audiometric notch at 3, 4, or 6 kHz with recovery at 8 kHz is a sign of NIHL. Several studies suggest that elevated blood pressure may be associated with exposure to noise as one of the non-auditory effects.16,17 Noise may cause hypertension by activating the hypothalamic–pituitary–adrenal and sympathetic nervous systems and thus causing elevated levels of adrenaline, noradrenaline, and cortisol.18–20 NIHL was used as a biological marker for noise exposure to investigate the risk of hypertension.21
ACE GEIs have been investigated in several studies, including common anthropometrical factors with essential hypertension in an isolated community in the Amazon region in Brazil; outdoor temperature-related blood pressure modulation responses in Korea; and classic cardiovascular risk factors; diabetes mellitus, obesity, smoking, dyslipidemia, and family history in Poland.22–24
GEI in the pathogenesis of hypertension has not been extensively studied in occupational exposure to noise. Genetic polymorphisms in angiotensin converting enzyme and noise exposure are well-established risk factors for the development of hypertension. However, little is known about a potential interaction between these factors. Accordingly, we conducted this study; our first aim was to assess the relationship between occupational noise exposure and hypertension in a group of male Egyptian workers. The second aim was to assess ACE I/D and G2350A gene polymorphisms and their relation with hypertension and the possible gene–environment (noise) interaction. Finally, we aimed to investigate the possible relationship between noise induced hearing impairment and risk of hypertension. This study adds to existing literature by further exploring the possible GEI in the pathogenesis of hypertension.
Methods
Subjects
A total of 378 male workers participated in this case control study, 217 subjects as exposed group and 161 subjects as their referent control. Participants were divided into two groups depending on their exposure to noise intensity at their work sites; the control group was exposed to sound intensity <85 dB and the exposed group exposed to sound intensity ≧85 dB. Exposed workers were production-line workers from two companies in the Helwan industrial area in Southern Cairo, Egypt. One company was a forging company that produces steel forging for feeding multiple industries including: automotive, cement, and sugar. The second company was a major spinning and weaving company. All eligible employees were invited to participate in the study. Eligibility criteria for exposed workers included being exposed to the specific sound level for approximately 8 hours per day for the preceding 5 years and having no other previous work history. A total of 217 workers met the inclusion criteria (113 from the forging company and 104 from the spinning and weaving company). The control group was comprised of 161 individuals employed as security personnel and administrative workers. They were matched to the exposed group by age, sex, educational level, smoking status, and body mass index.
Exclusion criteria for both the exposed and control groups were: any history of alcohol consumption, diabetes, chronic bacterial or viral infection, kidney diseases, and/or the prolonged intake of medications that can affect blood pressure such as corticosteroids or diuretics. To avoid interference from non-occupational exposures, workers were excluded if they had a previous diagnosis of hypertension before their employment in the factories.
Ethical consideration
All subjects were treated according to the Helsinki Declaration of Biomedical Ethics and provided informed consent before participating in the study.25
Methods
Noise exposure level assessment
Obtaining a reliable assessment of noise in occupational settings can be challenging due to several factors, including the temporal and spatial variability of noise levels, the specific acoustic characteristics of the noise sources, the variability of exposure times, and the need to limit the number, and duration of noise measurements due to the inherent costs and production interference, as well as the type of equipment and procedures used in the measurement.26 Accordingly, Fernandez et al. suggested procedures to reduce measurement uncertainty, including the simultaneous use of dosimeters and sound level meters, the correct selection of the measurement period, and workers’ observation during measurements.27
In this study, environmental noise exposure was measured using a sound analyzer (TES-1358; TES Electronic Corp., Taipei, Taiwan) that measures 1-second to 24-hour continuous equivalent sound levels (Leq) in the range of 30–130 dBA as well as time-weighted-average noise levels. This equipment was calibrated with a sound-level calibrator (TES-1356; TES Electronic Corp.) before environmental monitoring. The measurements were collected by industrial hygienists (from the spinning and the forging factories) at 20 locations inside and around each factory using short-term environmental sampling (Task-Based Measurement). For fixed workstation and predictable work patterns, the Task-Based Measurement strategy seems to present lower values, both for uncertainty and for time spent per worker. For all the measurements, the basic measurement quantity considered was the Lp,A,eqT, which is the equivalent continuous A-weighted sound pressure level for a given period of time (T) over which the average is taken, and the corresponding LEX,8h, which represents the noise exposure level normalized to a nominal 8-hour work day.
Locations were selected to be representative of the workers’ tasks. The measurements were performed biweekly for two consecutive weeks. We examined the environmental monitoring records and found that noise levels did not differ significantly over the preceding 5 years in either factory.
In the control areas, measurements were carried out biweekly for two consecutive weeks in 10 different locations representative of the control workplaces.
Questionnaire
A questionnaire was administered during an in-person interview. We investigated confounding variables considered to be possible risk factors for hypertension. The questionnaire consisted of detailed questions regarding self-reported illness, health and well-being, life-style habits such as smoking, alcohol consumption, physical exercise, and diet, medications, occupation, and work environment. Body mass index (BMI) was calculated as body weight (kg)/height (m2).
Blood pressure
Systolic and diastolic blood pressures (SBP and DBP) were measured on interview day, in the middle and at the end of each worker’s shift. A trained nurse took blood pressures using mercury sphygmomanometer. For statistical analysis, the two measurements for SBP and DBP were averaged. According to international guidelines, subjects were considered hypertonic if the mean measurement for SBP was equal to or higher than 140 or DBP was equal to or higher than 90 mmHg.2
Pure tone audiometry
Pure tone air conduction thresholds at 0.5, 1, 2, 3, 4, 6, and 8 kHz were measured using an interacoustics AC40 audiometer with TDH39 headphones. Pure-tone measurements were all performed in a sound-isolated booth. Both ears were tested. All audiometric thresholds were assessed with adequate masking and were expressed in dB hearing loss (HL), according to the standards of diagnostic audiometry. Mean hearing thresholds at 0.5, 1, 2, and 4 kHz were calculated for each subject and the mean level of the better ear was taken as the mean hearing threshold. Presence of a notch at 4 or 6 kHz in the audiogram was an indicator of noise-induced hearing loss. Mean hearing thresholds were divided into mild, moderate, and severe hearing impairment according to established WHO guidelines.28
(Average of 0.5, 1, 2, and 4 kHz in the better ear)
0 (no impairment) ≤25 dB HL
1 (slight) 26–40 dB HL
2 (moderate) 41–60 dB HL
3 (severe) 61–80 dB HL
4 (profound) ≧81 dB HL
Genetic analysis
Blood was collected in 5 ml Na–EDTA tubes and kept frozen at −20°C. DNA was extracted using commercial kit supplied by Thermo-Scientific (http://www.thermoscientificbio.com/fermentas, USA) and stored at −20°C until the time of amplification. The extracted DNA was then amplified using Taq polymerase enzyme and Hybaid thermal cycler (Promega Corporation, Fitchburg, WI, USA). For ACE G2350A gene, the PCR primers used were as follows: F 5′-CTGACGAATGTGATGGCCGC-3′ and R 5′-TTGATGAGTTCCACGTATTTCG-3′. The computerized thermocycler was programmed for the following conditions: initial denaturation at 95°C for 5 minutes, PCR was carried out for 35 cycles, each one comprised of denaturation at 94°C for 30 seconds, annealing at 58°C for 30 seconds, and extension at 72°C for 30 seconds, with a final extension time of 10 minutes at 72°C. The PCR products were digested with 5 U of fast digest BstU1 (supplied by Thermo-Scientific) at 60°C for 2 hours. Digested fragments were separated by electrophoresis on 3.5% agarose gel and identified by ethidium bromide staining using ultraviolet trans-illumination. Allele G2350 was visualized as a 122-bp fragment and allele A2350 as 100-bp and 22-bp fragments and GA genotype appear as 122–100 and 22 bp29 (Fig. 1).
Figure 1.
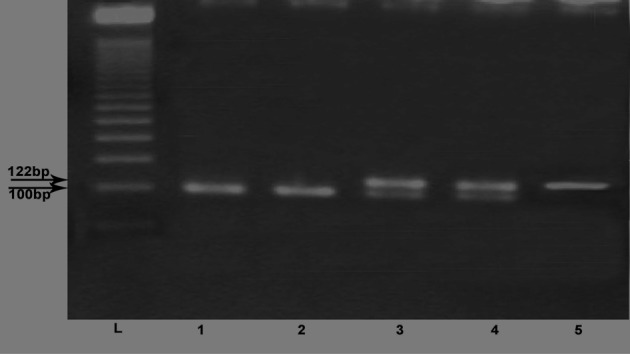
G2350A genotypes. Lane (L): 50-bp ladder; lanes 1 and 2: AA genotype; lanes 3 and 4: GA genotype; and lane 5: GG genotype.
For ACE I/D, the PCR primers used were as follows: sense oligo 5′-CTGGAGACCACTCCCATCCTTTCT-3′ and anti-sense oligo: 5′-GATGTGGCCATCACATTCGTCAGAT-3′. The computerized thermocycler was programmed for the following conditions: initial denaturation at 94°C for 3 minutes. This was followed by 30 cycles of denaturation at 94°C for 1 minute, annealing at 58°C for 1 minute, and extension at 72°C for 1 minute with a final extension at 72°C for 5 minutes. The PCR product was a 190-bp fragment (DD) in the absence of the insertion and a 490-bp fragment (II) in the presence of the insertion. A third fragment (ID) with an intermediate molecular weight (490, 190 bp) was present in PCR from heterozygotes. The products were visualized with ethidium bromide after electrophoresis on 3% agarose gel30 (Fig. 2).
Figure 2.
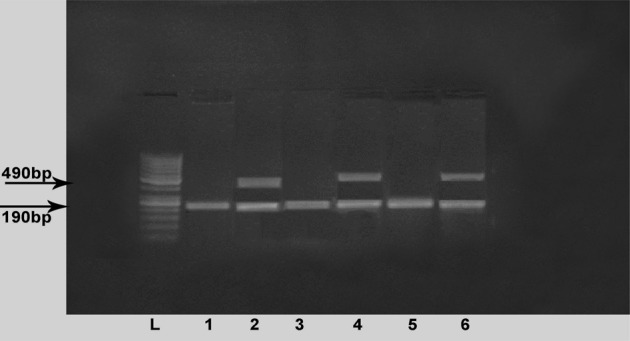
ACE genotypes. Lane (L): 50-bp ladder; lanes 2, 4, and 6: ID genotype; lanes 1, 3, and 5: DD genotype.
Statistical analysis
Data were analyzed using SPSS 15.0 for Windows (SPSS Inc., Chicago, IL, USA; 2006). Demographic data from the two groups were compared using two tailed Student’s t-test and Chi-square test as appropriate. Hardy–Weinberg equilibrium was assessed using the Chi-square test for the total exposed and control groups and for each individual subgroup (hypertensive and normotensive subgroups). The relationships between hypertension and various genotypes in the studied populations were examined using odds ratios (ORs) and 95% confidence intervals (CIs). Effect of noise was assessed in different genotypes carriers through the calculation of ORs considering the control group as the reference. Multivariate analysis of variance was performed to determine if the response variables (systolic and diastolic blood pressures), were altered by the independent variable (exposure to noise). Multivariate analysis of covariance was performed with age and BMI as possible covariates, to show their effects on blood pressure as confounders. However, before the multivariate analysis, we studied the linear correlation (Pearson’s correlation) between age and BMI and the independent variables (SBP and DBP). To consider variables as possible covariates, there must be at least a reasonable correlation between the dependent variables and the possible covariates (age and BMI), with a correlation coefficient between r = 0.3 and 0.9. Moreover, the correlation cannot differ between the independent variables groups. This was measured through an examination of variance covariance matrices via Box’s M test (highly significant outcome, where P<0.001, should be avoided). Pearson correlation analysis was performed to test for linear relations between BP and duration of employment.
The type of GEI was studied under the conditions of no interaction, synergistic interaction, and antagonistic interaction, for the additive and multiplicative scales, respectively. If the effects of two variables meet the condition of “no interaction on a multiplicative scale,” the data can be said to fit a “multiplicative model,” and if their effects meet the condition of “no interaction on an additive scale,” the data can be said to fit an “additive model.” Disease risk for each of the four combinations of genotype and environmental risk factor is denoted by r11 (for exposed persons with the risk genotype), r10 (for those with the exposure alone), r01 (for those with the genotype alone), and r00 (for those with neither).31 If risks are measured on an additive scale, the effect of an environmental exposure differs among persons with different genotypes (i.e. interaction on an additive scale) when r11−r01≠r10–r00. If risks are measured on a multiplicative scale, the effect of an environmental exposure differs among persons with different genotypes (i.e. interaction on a multiplicative scale) when r11/r01≠r10/r00.
Test for the presence/absence of interaction in an additive model
Expected incidence (I) of A and B = Attributable risk of A alone+attributable risk of B alone+baseline = Incidence of A alone+incidence of B alone–baseline
No interaction: I11–I01 = I10–I00
Synergistic interaction: I11–I01>I10–I00
Antagonistic interaction: I11–I01<I10–I00
Test for the presence/absence of interaction in a multiplicative model
Expected relative risk (RR) for A+B = RR for A only×RR for B only
No interaction: RR11 = RR10×RR01
Synergistic interaction: RR11>RR10×RR01
Antagonistic interaction: RR11<RR10×RR01
Binary logistic regression models using presence or absence of hypertension in the exposed workers as the dependent variable and several factors age, BMI, genotypes, smoking, and NIHL as the independent variables were performed. The relative risk was represented by ORs (or exp (B)) and 95% CIs. A P value less than 0.05 was considered statistically significant.
Results
Demographic data of the study population are presented in Table 1. The exposed and control workers were matched in age, BMI, duration of employment, and smoking history. The prevalence of hypertension was significantly higher in the noise-exposed group (72/217, 33.2%) compared to the control group (27/161, 16.8%) (P<0.001). More than three quarters of the exposed workers had NIHL (168/217, 77.4%). Mean threshold of hearing in the exposed workers at frequencies 0.5, 1, 2, and 4 KHz (in the better ear) was 43.3±16.91, which was significantly higher than in the control group (P<0.001).
Table 1. Characteristics of the study population.
| Exposed (n = 217) | Control (n = 161) | P** | |
| Age (mean±SD) | 44.9±9.58 | 45.3±8.45 | 0.673† |
| Height, cm (mean±SD) | 173.5±5.19 | 174.1±2.53 | 0.18† |
| Weight, kg (mean±SD) | 80.4±3.04 | 80.8±2.82 | 0.22† |
| BMI, kg/m2§ (mean±SD) | 26.8±1.87 | 26.9±1.23 | 0.56† |
| Duration of work (mean±SD) | 22.3±9.86 | 24.4±6.30 | 0.012† |
| Smoking Index* (mean±SD) | 17.6 ±16.31 | 18.2±11.67 | 0.691† |
| Smoking (%) | 84 (38.7%) | 51 (32%) | 0.193†† |
| Systolic blood pressure (mean±SD) | 129.16 ± 15.02 | 120.5±11.89 | 0.001† |
| Diastolic blood pressure (mean±SD) | 83.3 ± 10.78 | 78.7±7.93 | 0.001† |
| Hypertension, % (>140/90) | 72 (33.2%) | 27 (16.8%) | 0.001†† |
| Hearing threshold (dB) (mean±SD) | 43.3±16.91 | 19.7±6.33 | 0.001† |
| NIHL (%)¶ | 168 (77.4%) |
Note: †Two-tailed Student’s t-test.
††Chi-square test.
**Statistical significance at P<0.05.
*Smoking Index = no. of packs per day×years of smoking.
¶NIHL: noise-induced hearing loss.
§BMI: body mass index.
Pearson’s correlation between duration of employment and SBP and DPB in the exposed workers (Figs. 3 and 4) showed a highly significant positive correlation (r = 0.276 and 0.261, respectively, P<0.001).
Figure 3.
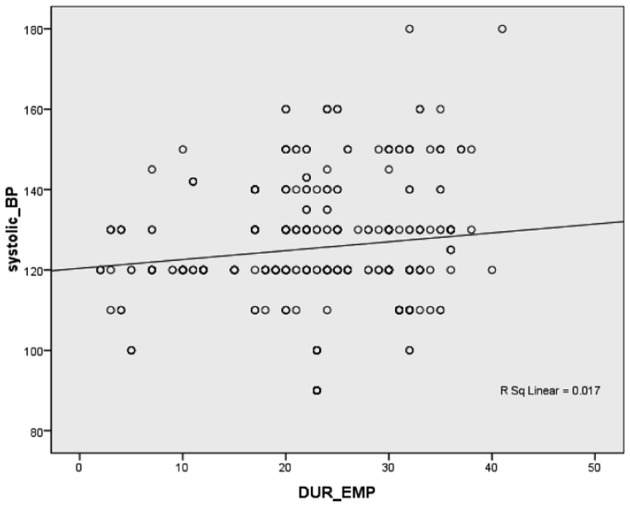
Correlation between duration of employment in the exposed group and systolic blood pressure.
Figure 4.
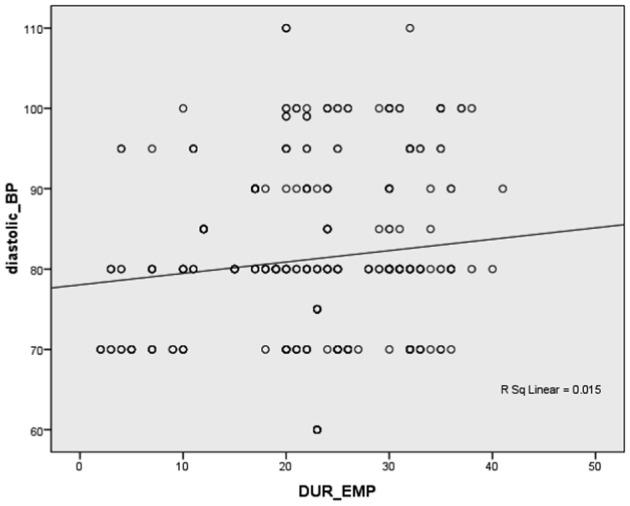
Correlation between duration of employment in the exposed group and diastolic blood pressure.
To explore the confounding effect of age and BMI on blood pressure in the studied groups, multivariate analysis of variance was applied prior to covariate inclusion. A highly significant elevation of blood pressure was reported in the exposed workers compared to their referent controls (lambda = 0.914, F(2,375) = 17.738, P<0.001). This was confirmed across SBP and DBP (P<0.001, F(1,376) = 35.56 and 20.951, respectively). Power to detect the effects was 1.000. Before inclusion in a multivariate analysis of covariance to test the effect of age and BMI as possible covariates, Pearson’s correlation between age and BMI (the possible covariates) with SBP and DBP (the dependent variables) was found to be non-significant. The correlations between age with SBP and DBP were r = 0.021, P = 0.682 and r = 0.005, P = 0.93, respectively. The correlations between BMI with SBP and DBP were r = 0.005, P = 0.919 and r = 0.02, P = 0.642, respectively. Examination of variance covariance matrices via Box’s M test was highly significant (P<0.0001). From the above results and the fact that there was no significant difference between age and BMI between the exposed and control groups, we can conclude that these factors were adjusted for and these covariates can reduce error variance in the outcome study.
The frequency distribution of the D allele compared to the I allele of the ACE I/D gene was significantly higher in the individual groups and their subgroups (P<0.001) as shown in Table 2. The DD genotype was significantly higher in the control subjects compared to the exposed, while the ID genotype was significantly higher in the exposed workers (P<0.001). Regarding subgroups, the frequency of the DD genotype was significantly higher in all the subgroups; the frequency ranged from 75% in the hypertensive exposed to 93.3% in the normotensive control workers. As for the ACE G2350A genotypes, the frequency of the G allele compared to the A allele was higher in both groups and their subgroups, which was statistically significant in all but the normotensive control subgroup. The homozgotic genotype GG was the predominant in all subgroups, followed by the AA genotype except in the hypertensive subgroup exposed to noise where the frequency of the AG genotype was 27.8%, followed by the AA genotype (16.4%). Genotype distribution among the hypertensive and normotensive groups was further investigated (data not tabulated). Regarding the ACE genotypes, only the AA and ID genotypes were significantly related to blood pressure in the studied workers. Subjects carrying the ID genotype were at increased risk for hypertension (OR = 1.87, 95% CI = 1.03–3.41, P<0.05), while carriers of the AA genotype were at a lower risk (OR = 0.45, 95% CI = 0.26–0.78, P<0.004).
Table 2. Genetic variants of ACE ID and ACE G2350A polymorphism in the exposed and control hypertensive and normotensive subgroups.
| Exposed (n = 217) | Control (n = 161) | |||||
| Genes/alleles | Hypertension (n = 72) | Normotensive (n = 145) | Total (n = 217) | Hypertension (n = 27) | Normotensive (n = 134) | Total (n = 161) |
| ACE I/D | ||||||
| ID | 18 (25%) | 26 (17.9%) | 44 (20.3%)† | 3 (11.1%) | 9 (6.7%) | 12 (7.5%)† |
| DD | 54 (75%) | 119 (82.1%) | 173 (79.7%)† | 24 (88.9%) | 125 (93.3%) | 149 (92.5%)† |
| I‡ | 0.125 | 0.089 | 0.101 | 0.056 | 0.034 | 0.037 |
| D‡ | 0.875 | 0.911 | 0.899 | 0.944 | 0.966 | 0.963 |
| P value | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 | 0.001 |
| ACE G2350A | ||||||
| AA | 12 (16.6%) | 46 (31.7%) | 58 (26.7%)* | 8 (29.6%) | 54 (40.3%) | 62 (38.5%)* |
| AG | 20 (27.8%) | 26 (17.9%) | 46 (21.2%)† | 1 (3.7%) | 14 (10.4%) | 15 (9.3%)† |
| GG | 40 (55.6%) | 73 (50.3%) | 113 (52.1%) | 18 (66.7%) | 66 (49.3%) | 84 (52.2%) |
| A‡ | 0.305 | 0.407 | 0.373 | 0.315 | 0.455 | 0.432 |
| G‡ | 0.695 | 0.593 | 0.627 | 0.685 | 0.545 | 0.568 |
| P value | 0.001 | 0.002 | 0.001 | 0.014 | 0.177 | 0.019 |
Note: *Significance between total (P<0.05).
†Significance between total (P<0.001) (Chi-square test).
‡HWE = Hardy–Weinberg equilibrium.
P value is calculated using Chi-square test in each individual group between alleles frequencies.
We tested the type of statistical interaction between ACE gene polymorphisms and noise on hypertension. When we assessed GEI (additive or multiplicative model), we used the case only design (hypertensive subjects stratified into E+, E− and G+, G−) to produce four groups [E+, G+], [E-, G+], [E+, G−], and [E−,G−].
In an additive model, a synergistic interaction was observed between DD genotype and noise exposure. The observed incidence of hypertension in DD carriers exposed to noise (n = 54) was greater than the expected incidence (n = 39). In the same model, an antagonistic interaction was observed between ID, AA, AG, and GG and noise. The observed incidence of hypertension in these genotypes carriers was: 18, 12, 20, and 40, respectively, which was less than the expected incidence of 33, 49, 27, and 41, respectively.
In a multiplicative model, a synergistic interaction was observed between ID and AG genotype carriers and noise exposure. The observed relative risk for hypertension in ID and AG genotypes carriers exposed to noise was 0.75 and 0.77, respectively, which was greater than the expected relative risks of 0.28 and 0.08, respectively. In the same model, an antagonistic interaction was observed between DD, AA, and GG carriers and noise. The observed relative risk of hypertension in these genotypes carriers was 18, 0.63, and 4.4 respectively, which was less than the expected relative risks of 48.0, 49.0, and 7.1.
The association between ACE genes polymorphisms and hypertension was further investigated (Table 3). Regarding the ACE G2350A gene, carriers of the AG and the GG genotypes were vulnerable to hypertension on noise exposure. AG carriers were 10 times (OR = 10.7, 95% CI = 1.305–88.906) more likely and GG carriers were two times (OR = 2.01, 95% CI = 1.05–3.842) more likely to have hypertension. As for the ACE I/D gene, carriers of the DD genotype were statistically more vulnerable to the development of hypertension related to noise exposure (OR = 2.63, 95% CI = 1.37–4.066). Results without stratified analysis for ACE genotypes indicated that noise significantly increased the likelihood of hypertension (OR = 2.464; 95% CI = 1.493–4.067).
Table 3. Association between ACE genetic polymorphisms with hypertension and noise in the studied subjects.
| Genes | Groups | Hypertensive (99) | Normotensive (279) | OR, 95% CI (HT versus NT‡) | |
| ACE G2350A polymorphism | AA | Control | 8 | 54 | Reference |
| Exposed | 12 | 46 | 1.761 (0.663–4.679) | ||
| GA | Control | 1 | 14 | Reference | |
| Exposed | 20 | 26 | 10.70* (1.305–88.906) | ||
| GG | Control | 18 | 66 | Reference | |
| Exposed | 40 | 73 | 2.01* (1.05–3.842) | ||
| ACE I/D polymorphism | ID | Control | 3 | 9 | Reference |
| Exposed | 18 | 26 | 2.077 (0.423–8.751) | ||
| DD | Control | 24 | 125 | Reference | |
| Exposed | 54 | 119 | 2.363† (1.374–4.066) | ||
| Total | Control | 27 | 134 | Reference | |
| Exposed | 72 | 145 | 2.464† (1.493–4.067) |
Note: *Significance P<0.05.
†Significance P<0.001.
‡HT versus NT: hypertensive versus normotensive.
Hearing impairment and NIHL in the exposed workers was further investigated (Table 4). Hearing impairment ranged from no/mild (106/217, 48.8%), moderate (95/217, 43.8%) to severe (16/217, 7.4%). The severe and moderate hearing impairment groups were compared with the no/mild impairment group. Moreover, workers exposed to higher level of noise (forging company workers) were compared to those exposed to lower levels (spinning and weaving company workers). The results showed that there was no significant difference in mean SBP, DBP, or prevalence of hypertension. Furthermore, we found no significant difference in occurrence of NIHL between the hypertensive and normotensive exposed workers (76.4% and 77.9% respectively, OR: 0.916, 95% CI = 0.468–1.791). Hearing impairment in exposed workers was not associated with hypertension and higher level of noise did not affect blood pressure.
Table 4. Association between hearing impairment and NIHL with hypertension in noise-exposed workers.
| Exposed group (n = 217) | ||||
| Systolic BPMean (SD) | Diastolic BPMean (SD) | Hypertensive (n = 72) | Normotensive (n = 145) | |
| Degree of Hearing impairment* | ||||
| Normal/mild (n = 106) | 128.16 (14.725) | 83.28 (10.319) | 33 (31.1%) | 73 (68.9%) |
| Moderate (95) | 131.18 (14.25)a | 83.45 (11.223)a | 34 (35.8%)c | 61 (64.2%)c |
| Severe (16) | 123.75 (19.958)a | 82.5 (11.690)a | 5 (31.3%)c | 11 (68.7%)c |
| Presence of NIHL (n = 168) | 124.62 (12.71) | 83.44 (12.23) | 55 (76.4%)d | 113 (77.9%) |
| Occupational sector | ||||
| Forging company (113) | 130.52 (15.227) | 84.12 (11.383) | 41 (56.9%)d | 72 (49.7%) |
| Spinning and weaving (104) | 127.66 (14.74)b | 82.41 (10.056)b | 31 (43.1%)d | 73 (50.3%) |
Note: a t-test is used to compare means with the normal/mid hearing impairment group.
bt-test is used to compare means between two companies.
CChi-square test for a significant difference (P<0.05) compared with the normal/mild hearing impairment group.
d Chi-square test for a significant difference (P<0.05) hypertension with normal subjects.
*Degree of hearing impairment: normal and mild (≤40 dB), moderate (41–60 dB), and severe (61–80 dB).
Risk factors for hypertension in noise-exposed workers were studied using the stepwise binary logistic regression analysis model presented in Table 5. In this model, the exposed workers (divided into hypertensive and normotensive) were the dependent variable and age, BMI, smoking, NIHL, and genotypes were the independent variables. Older age and smoking were significantly associated with hypertension occurrence in noise exposure. Workers older than 45 years and smokers were two times more vulnerable for hypertension in noise exposure (exp (B) = 2.035, 95% CI = 3.841–1.078 for age and exp (B) = 2.147, 95% CI = 4.151–1.110 for smoking) (P<0.05).
Table 5. Risk factors for hypertension in noise-exposed workers using binary logistic regression analysis.
| B† | Standard error | Wald†† | Significance | Exp (B)** | 95% confidence interval | ||
| Upper | Lower | ||||||
| NIHL | −0.303 | 0.383 | 0.628 | 0.428 | 0.738 | 1.563 | 0.349 |
| BMI (1) | −0.053 | 0.328 | 0.026 | 0.872 | 0.949 | 1.804 | 0.499 |
| Age (1) | 0.710 | 0.324 | 4.804 | 0.02* | 2.035 | 3.841 | 1.078 |
| Smoking (1) | 0.764 | 0.337 | 5.153 | 0.02* | 2.147 | 4.151 | 1.110 |
| AG (1) | 0.961 | 0.456 | 4.440 | 0.03* | 2.613 | 1.069 | 6.386 |
| GG (1) | 0.709 | 0.396 | 3.209 | 0.07 | 2.031 | 0.935 | 4.410 |
| DD (1) | 0.268 | 0.366 | 0.537 | 0.46 | 1.308 | 0.638 | 2.682 |
Note: Chi-square = 16.023, sig = 0.025.
Overall percentage for prediction = 70%.
−2 log likelihood = 259.760.
The dependent variable in the model was noise-exposed workers coded as hypertensive (1) and normotensive (0). The independent variables were NIHL yes (1) and no (0), smoking yes (1) and no (0), age ≧45 (1) and <45 (0), BMI ≧27 (1) and <27 (0), AG yes (1) and no (0), GG yes (1) and no (0) (AA is the reference genotype), and DD yes (1) and no (0).
*Significance P<0.05.
**Exp (B): exponentiation of the B coefficient.
†B: the coefficient for the constant.
††Wald: Wald Chi-square test.
The environmental assessment results for noise showed that noise levels for the forging company were all greater than 90 dB (mean level: 92.5±2.3 dB) and for the spinning and weaving company were all greater than 87 dB (the mean level: 89±1.7 dB) (P<0.001). The levels for the control sites were all less than 65 dB (mean: 57± 2.1 dB).
Discussion
Noise is a persistent environmental problem and noise exposure is associated with a number of negative health effects including psychosocial effects, such as annoyance, sleep disturbance, disturbance of daily activities and performance, and physical effects, such as hearing loss and cardiovascular diseases including hypertension.32 In this study, we found that the mean levels of SBP, DBP, and the frequency of hypertension were significantly higher in noise-exposed workers. Carriers of the AG and GG genotypes of the ACE G2350A gene and DD genotype of the ACE I/D gene were more vulnerable to hypertension on noise exposure. We found no association between hypertension and hearing impairment or NIHL in noise-exposed workers. The biological mechanisms linking noise to hypertension is thought to be mediated through sympathetic and endocrine stress responses with subsequent acute changes in vascular tension. The hypothesis is that chronic and repetitive noise stimuli modify these otherwise normal responses to a permanent upward resetting of baroreceptors leading to hypertension.33 Indeed, low-frequency noise-induced annoyance may produce hypertension by causing emotional responses that interfere with work performance in jobs requiring selective attention or processing of high load of information.18–20
Several epidemiological studies have shown an association between occupational and environmental noise exposure and hypertension.21,34 van Kempen and co-workers conducted a meta-analysis of 43 epidemiologic studies published between 1970 and 1999 that investigated the relation between occupational and community noise exposure and blood pressure and/or ischemic heart disease. They studied a wide range of effects, from blood pressure changes to myocardial infarction. They found a significant association for both occupational and traffic noise exposure and hypertension, estimating relative risks per 5 dB (A) noise increase of 1.14 (95% CI: 1.01–1.29) and 1.26 (95% CI: 1.14–1.39) respectively.32
Another meta-analysis including articles published between 1950 and 2000, divided 18 658 workers into three groups by noise exposure. The authors found a statistically significant increase in the prevalence of hypertension and ECG abnormalities in high noise-exposed workers.35
In this study, we investigated the risk factors associated with hypertension in exposed workers using binary logistic regression. Smokers and older workers exposed to noise have doubled the risk for hypertension. Although cigarette smoking is a strong risk factor for cardiovascular diseases, its relationship with hypertension remains unclear and indeed abstinence from smoking is not included in current clinical guidelines for the prevention of hypertension.36 Cigarette smoking causes sympathetic activation, oxidative stress, and acute vasopressor effects that are associated with increases in markers of inflammation linked with hypertension.37 A recent population-based study of middle-aged men reported that current cigarette smoking was independently associated with an elevated risk of developing hypertension.38 However, other studies have reported lower BP among smokers and increases in BP after smoking cessation.39,40 As a result, it remains unclear to what extent cigarette smoking is a risk factor for the development of hypertension. As regards the relation of age with hypertension, observational studies have shown a concomitant increase in SBP, DBP, and pulse pressure with increased age until middle adult life. Beyond approximately 60 years, SBP continues to increase, but DBP plateaus or declines gradually, leading to an accelerated rise in pulse pressure.41
It has been estimated that approximately 30% of the inter-individual variability in blood pressure is genetically determined. Special attention has been given to the role of genetic variation in genes implicated in the RAS, particularly the ACE gene. ACE is a key enzyme in the renin–angiotensin–aldosterone and kalikrein–kinin systems, playing a crucial role in blood pressure regulation and electrolyte balance.42 It has been demonstrated that the ACE gene is responsible for 47% of the variance of plasma ACE activity. The ACE gene can contain polymorphisms consisting of either the insertion (I) or deletion (D) of a 287-bp sequence inside intron 16. Notably, the D allele and the DD genotype are associated with elevated levels of ACE and a higher risk of adverse cardiovascular events and end-organ damage. The correlation between the DD genotype and increased propensity for the development of hypertension can be attributed to elevated angiotensin II conversion, suggesting that in these homozygotes, the elevated ACE levels modulate RAS function.43 In addition to association with hypertension, ACE gene polymorphisms independently modulated age related increases in pulse pressure with a significantly higher slope of age–PP and age–systolic blood pressure relationships for DD rather than other genotypes.44
GEI can obscure both environmental effects that may be evident only in genetically susceptible persons and genetic effects that may be evident only in those with appropriate exposure histories. Thus, study of GEI is important for improving accuracy and precision in the assessment of both genetic and environmental influences.14
Understanding the mechanisms that underlie GEIs is a formidable challenge. It is difficult enough to characterize the impact of polymorphisms on gene expression and function under static conditions (i.e. within a constant environment); it is considerably more challenging to elucidate the dynamic intertwining of polymorphic variation and environmental stimuli. This may explain why little has been published about the mechanisms of GEIs, and no major findings have been reported about the relationship between hypertension with noise. Nonetheless, there is consensus that epigenetic mechanisms could explain the interplay between genes and the environment. The dynamic nature of this interplay is a quasi-perfect fit for the plasticity of epigenetic processes, which can be seen as the functional transducers of environmental cues.13 Stable epigenetic alterations can arise during cell development and proliferation, making it possible for the cells of multicellular organisms to be genetically identical but structurally and functionally heterogeneous.45 Modifications in gene expression in response to environmental and/or developmental cues can result from modifications of DNA (e.g. by methylation) or from proteins that intimately associate with DNA (e.g. acetylation, methylation, or phosphorylation of histones).46
Several research groups have already reported the increased likelihood of hypertension due to noise exposure. However, to our knowledge, little research has studied the confounding effects of ACE gene polymorphisms on the association between noise and hypertension. It was observed that different allele carriers of the ACE I/D and ACE G2350A gene polymorphisms have different chances of hypertension on exposure to noise.
We observed an overall higher prevalence of the DD genotype and D allele in the exposed and control normotensive and hypertensive subgroups (P<0.001). Subjects carrying the ID genotype were at a significantly increased risk for hypertension (OR = 1.87, 95% CI = 1.03–3.41, P<0.05, data not tabulated), on the other hand carriers of the DD genotype were highly vulnerable to the development of hypertension on noise exposure (OR = 2.63, 95% CI = 1.37–4.066). Nawaz and Hasnain found that both ID and DD carriers were at increased risk of hypertension with noise exposure [OR = 2.844 (1.32–6.110) and 4.487 (1.549–12.99), respectively].47 Badaruddoza and co-workers investigated the ACE gene polymorphisms and the susceptibility to hypertension among the Bania population in Punjab, India.5 It was observed that the ACE DD genotype was significantly higher in hypertensive subjects, whereas ID genotype was significantly higher in control subjects. Comparative results were found in several other studies.11,48,49 Discordantly, the same polymorphisms did not affect blood pressure in a population living in the Western Black Sea region of Turkey or a Pakistani population.44,50 Such variations of genotype frequencies across communities might be related to genetic drift.
The ACE gene is viewed as a QTL that modulates circulating ACE levels, and the ACE I/D dimorphism is a marker thought to be in LD with functional variants located in the ACE gene.51
The results of a combined segregation-linkage analysis in French families suggested that the I/D dimorphism was in strong LD with an unmeasured functional mutation of the ACE gene.51 This mutation appeared to be frequent and explained the major part of the genetic variance of plasma ACE. A similar study performed in African Caribbean families confirmed the existence of an ACE-linked QTL influencing ACE levels, but also revealed a weaker LD between this QTL and the I/D dimorphism and suggested the existence of a second QTL unlinked to ACE.52
This instigated a search for new polymorphisms in the ACE gene to identify better markers or actual functional variants. Accumulated evidence points to the existence of two QTLs at this chromosomal locus. A genome-scan analysis by the Framingham Heart Study found strong evidence for a QTL on chromosome 17, located close to the ACE gene and linked to blood pressure.53 A polymorphism in exon 17, ACE G2350A, has a significant effect on plasma ACE concentrations as it accounts for 19% of the total variance in ACE plasma levels.29 We found that the prevalence of the G allele and GG genotype were higher in all the subgroups, followed by the AA genotype, except in the hypertensive group exposed to noise. These findings were comparable to the results of studies in a Pakistani population and a Gulf (Emirati) population.7,29,47 The AA genotype carriers were not at increased risk for hypertension on noise exposure; on the other hand, carriers of the AG and GG were significantly vulnerable to hypertension on noise exposure. Nawaz and Hasnain reported similar results.47 In a Malaysian population, the same polymorphism was found to be associated with hypertension, but increased chances of hypertension were linked to the A allele.54
When we tested the model (additive and multiplicative) that may define GEI in this study, there was a positive interaction between DD, ID, and AG genotype carriers and noise in the development of hypertension. The interaction was a synergistic additive interaction for DD and synergistic multiplicative interaction for ID and AG genotype carriers.
However, this leaves questions about which scale should be used to define interaction. Rothman et al. has advocated the use of a fixed reference point to define interaction in epidemiologic studies, arguing that the additive scale of measurement was the only meaningful reference point.55 This argument was based on a conceptual, rather than purely statistical, definition of interaction: co-participation of two factors in a single causal mechanism. Indeed, the choice of either the additive or the multiplicative scale can be appropriate under different conditions. For example, if the disease etiology involves a multistage process, two factors that act at the same stage will generally fit an additive model, whereas those that act at different stages will generally fit a multiplicative model.56 Accordingly, both scales may be applicable to define the interaction between ACE gene and noise exposure on the development of hypertension.
NIHL is a complex disease resulting from the interaction between intrinsic and environmental factors. NIHL is a sensorineural hearing impairment that develops over years of exposure to noise at moderately high levels. About 16% of hearing loss worldwide is attributable to occupational noise exposure. It is predominantly noted in the high-frequency region, with typical notch at 4–6 kHz.57 In this study, noise-exposed workers had a significantly higher mean threshold of hearing compared to their referent control, and more than three quarters had NIHL.
Cells in the human body depend on a proper supply of oxygen and nutrients in order to maintain their functions and such supply depends on the functional and structural integrity of the heart and blood vessels. This integrity may be disturbed by hypertension.57 Moreover, high BP may cause inner ear minute hemorrhages, which may cause progressive or sudden hearing loss. This circulatory system pathology may directly affect hearing in a number of ways. One of the vascular physiopathological mechanisms described is the increase in blood viscosity, which reduces capillary blood flow and ends up reducing oxygen transport, causing tissue hypoxia, and hearing impairment. Moreover, arterial hypertension may cause ionic changes in cell potentials, resulting in hearing loss.58,59
It is possible that hypertension could, through such factors, cause hearing impairment, and consequently, it would be expected that workers exposed to high noise levels and having hypertension would be more prone to hearing impairment and hearing loss.59
The occurrence of hearing impairment in our exposed population was not associated with hypertension. There was no statistically significant difference between hypertensive and normotensive exposed workers regarding their mean hearing threshold and NIHL. Moreover, a statistically significant positive correlation was found between duration of employment and mean hearing threshold (r = 0.234, P<0.01). However, there was no statistically significant correlation between mean hearing threshold and systolic or diastolic blood pressure (r = 0.036, P = 0.602 and r = 0.051, P = 0.455, respectively). Wu et al. found comparable results and the authors concluded that hearing loss is not appropriate as a noise exposure index to measure the relationship between noise exposure and blood pressure.60 Hypertension is an independent risk factor for hearing loss. Few studies have used hearing loss at high frequencies (NIHL) as a biological marker for noise exposure to investigate the risk of hypertension. One field study reported significantly higher means of systolic and diastolic blood pressures in workers with an auditory impairment greater than or equal to 65 dB compared with those with normal hearing.61 Another study reported that high and median hearing loss workers had significantly higher risks of hypertension and slightly greater mean values of resting DBP than low hearing loss workers.21 Future studies with a follow-up design that include participants exposed to different levels of noise may deduce the effectiveness of NIHL as a useful tool for investigating the chronic effects of noise exposure including cardiovascular effects.
To the best of our knowledge, this is the first study investigating gene–noise interaction in the development of hypertension in an Egyptian population. Our results support the role ACE I/D and ACE G2350A polymorphisms in the pathophysiology of hypertension. Moreover, these polymorphisms modulate the risk of hypertension in the presence of noise. On the other hand, we found no association between hypertension and hearing impairment or NIHL in noise-exposed workers. It is important to acknowledge that this study was restricted to industrial settings. The possible distinct workplaces characteristics in non-industrial environments may affect some of the conclusions presented herein. Another limitation was the cross-sectional design of the study, which limits a conclusive causal relationship. We recommend studies with a longitudinal design to allow for better evaluation of this relationship. Also, noise exposure was assessed by fixed measurements with sound level meters. The characterization of personal exposure is a problem especially concerning long-term effects. In general, the reporting of noise-related factors, such as fluctuation of noise levels, frequency, and peak or continuous noise, was incomplete. Moreover, several confounding factors for hypertension including plasma lipid profile levels, and occupational chemical cardiotoxins were not investigated in this research.
This study provides preliminary research for noise–gene interaction in hypertension. Given the complex nature of genetic, environmental, and occupational susceptibility for hypertension, further studies with larger sample sizes and modified designs are needed.
Finally, our results indicate that preventive measures should be considered to reduce occupational noise which contributes to the burden of cardiovascular diseases. To reduce the adverse effects of occupational noise exposure, it is important to evaluate the degree, type, and source of exposure. Optimally, noisy work processes should be isolated and enclosed; and personal protective equipment used. Even with such measures, some settings may witness rates of exposure that exceed guidelines. Consequently, it is clear that continued efforts are needed to train and supervise workers in order to promote worker safety with regard to noise exposure. Policy makers and responsible authorities should impose and enforce stricter regulations to control industrial noise exposure. It is recommended that in addition to audiometry testing, periodic medical examination of noise-exposed workers should include examination of the cardiovascular system and BP monitoring.
Disclaimer Statements
Contributors Nermin Zawilla: idea, field work, writing of the paper; Dr Dalia Shaker: field work, editing; Dr Amaal Adelaal: genetic testing, editing; Dr Wael Aref: clinical, editing.
Funding None.
Conflicts of interest Any of the authors of the above manuscript has any conflict of interest which may arise from being named as an author on the manuscript.
Ethics approval All procedures were in compliance with the declaration of Helsinki and an informed consent was given by all participants. Participants voluntarily joined the study, and an approval from Internal ethics committee (faculty) was obtained. Confidentiality of the data was followed.
References
- 1.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–23. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 2.Mancia G, de Backer G, Dominiczak A, Cifkova R, Fagard R, Germano G, et al. Guidelines for the management of arterial hypertension. The task force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2007;28(12):1462–536. doi: 10.1093/eurheartj/ehm236. [DOI] [PubMed] [Google Scholar]
- 3.Taylor J, Sun Y, Hunt S, Kardia S. Gene–environment interaction for hypertension among African American women across generations. Biol Res Nurs. 2010;12(2):149–55. doi: 10.1177/1099800410371225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siddhart KM, Kapur S, Venkata S. Angiotensin converting enzyme gene polymorphism and hypertension: no ACE yet in the pack of cards. JAPI. 2012;60:9–10. [PubMed] [Google Scholar]
- 5.Badaruddoza AJS, Bhanwer R, Sawhney NK, Randhawa K, Barna B. A study of angiotensin converting enzyme (ACE) gene polymorphism in essential hypertension among a business community in Punjab. Int J Hum Genet. 2009;9(3–4):231–4. [Google Scholar]
- 6.Kim K. Association of angiotensin-converting enzyme insertion/deletion polymorphism with obesity, cardiovascular risk factors and exercise-mediated changes in Korean women. Eur J Appl Physiol. 2009;105:879–87. doi: 10.1007/s00421-008-0973-6. [DOI] [PubMed] [Google Scholar]
- 7.Alvi FM, Hasnain S. ACE I/D and G2350A polymorphisms in Pakistani hypertensive population of Punjab. Clin Exp Hypertens. 2009;31:471–80. doi: 10.1080/10641960902825479. [DOI] [PubMed] [Google Scholar]
- 8.Zhu X, Bouzekri N, Southam L, Cooper RS, Adeyemo A, McKenzie CA, et al. Linkage and association analysis of angiotensin I-converting enzyme (ACE)-gene polymorphisms with ACE concentration and blood pressure. Am J Hum Genet. 2001;68:1139–48. doi: 10.1086/320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashavaid TF, Shalia KK, Nair KG, Dalal JJ. ACE and AT1R gene polymorphisms and hypertension in Indian population. J Clin Lab Anal. 2000;14:230–7. doi: 10.1002/1098-2825(2000)14:5<230::AID-JCLA6>3.0.CO;2-U. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morshed M, Khan H, Akhteruzzaman S. Association between Angiotensin I-converting enzyme gene polymorphism and hypertension in selected individuals of the Bangladeshi population. J Biochem Mol Biol. 2002;35:251–4. doi: 10.5483/bmbrep.2002.35.3.251. [DOI] [PubMed] [Google Scholar]
- 11.Barley J, Blackwood A, Miller M, Markandu ND, Carter ND, Jeffery S. Angiotensin converting enzyme gene I/D polymorphism, blood pressure and the rennin–angiotensin system in Caucasian and Afro-Caribbean peoples. J Hum Hypertens. 1996;10:31–5. [PubMed] [Google Scholar]
- 12.Stassen JA, Wang JG, Ginocchio G, Petrov V, Saavedra AP, Soubrier F, et al. The deletion/insertion polymorphism of the angiotensin converting enzyme gene and cardiovascular-renal risk. J Hypertens. 1997;15:1579–92. doi: 10.1097/00004872-199715120-00059. [DOI] [PubMed] [Google Scholar]
- 13.Ober C, Vercelli D. Gene–environment interactions in human disease: nuisance or opportunity? Trends Genet. 2011;27(3):107–15. doi: 10.1016/j.tig.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas D. Gene–environment-wide association studies: emerging approaches. Nat Rev Genet. 2010;11(4):259–72. doi: 10.1038/nrg2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong AC, Froud KE, Hsieh YS. Noise-induced hearing loss in the 21st century — a research and translational update. World J Otorhinolaryngol. 2013;3(3):58–70. [Google Scholar]
- 16.Chang TY, Lai YA, Hsieh HH, Lai JS, Liu CS. Effects of environmental noise exposure on ambulatory blood pressure in young adults. Environ Res. 2009;109(7):900–5. doi: 10.1016/j.envres.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Stansfeld SA, Matheson MP. Noise pollution: non-auditory effects on health. Br Med Bull. 2003;68:243–57. doi: 10.1093/bmb/ldg033. [DOI] [PubMed] [Google Scholar]
- 18.Spreng M. Central nervous system activation by noise. Noise Health. 2000;2:49–58. [PubMed] [Google Scholar]
- 19.Babisch W. The Noise/Stress concept, risk assessment and research needs. Noise Health. 2002;4:1–11. [PubMed] [Google Scholar]
- 20.Ising H, Kruppa B. Health effects caused by noise: evidence in the literature from the past 25 years. Noise Health. 2004;6:5–13. [PubMed] [Google Scholar]
- 21.Chang TY, Liu CS, Huang KH, Chen R, Lai JS, Bao BY. High-frequency hearing loss, occupational noise exposure and hypertension: a cross-sectional study in male workers. Environ Health. 2011;10:35. doi: 10.1186/1476-069X-10-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freitas SR, Cabello PH, Moura-Neto RS, Dolinsky LC, Bóia MN. Combined analysis of genetic and environmental factors on essential hypertension in a Brazilian rural population in the Amazon region. Arq Bras Cardiol. 2007;88(4):447–51. doi: 10.1590/s0066-782x2007000400014. [DOI] [PubMed] [Google Scholar]
- 23.Hong YC, Kim H, Lim YH, Yoon HJ, Kwon YM, Park M. Identification of RAS genotypes that modulate blood pressure change by outdoor temperature. Hypertens Res. 2013;36(6):540–5. doi: 10.1038/hr.2012.218. [DOI] [PubMed] [Google Scholar]
- 24.Konopka A, Szperl M, Piotrowski W, Roszczynko M, Stępińska J. Influence of renin–angiotensin system gene polymorphisms on the risk of ST-segment-elevation myocardial infarction and association with coronary artery disease risk factors. Mol Diagn Ther. 2011;15(3):167–76. doi: 10.1007/BF03256407. [DOI] [PubMed] [Google Scholar]
- 25.World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects [document on the Internet]. Revised by the 64th WMA General Assembly, Fortaleza, Brazil, October 2013. Ferney-Voltaire: WMA; 2013 [cited 2014 Jan 13]. Available at: http://www.wma.net/e/policy/b3.html [Google Scholar]
- 26.Cagno E, Di Giulio A, Trucco P. Statistical evaluation of occupational noise exposure. Appl Acoust. 2005;66:297–318. [Google Scholar]
- 27.Fernandez MD, Quintana S, Chavarria N, Ballesteros JA. Noise exposure of workers of the construction sector. Appl Acoust. 2009;70:753–60. [Google Scholar]
- 28.World Health Organization. WHO Grades of hearing impairment [document on the internet]. Geneva: World Health Organization; 2013 [cited 2014 Jan 17]. Available from: http://www.who.int/pbd/deafness/hearing_impairment_grades/en/ [Google Scholar]
- 29.Mahmood MS, Saboohi K, Ali SO, Bokhari AM, Saboohi K, Osman A, et al. Association of the angiotensin-converting enzyme (ACE) gene G2350A dimorphism with essential hypertension. J Hum Hypertens. 2003;17:719–23. doi: 10.1038/sj.jhh.1001600. [DOI] [PubMed] [Google Scholar]
- 30.Qun XU, Wang Y, Tong W, Gu M, Wu G. Interaction and relationship between angiotensin converting enzyme gene and environmental factors predisposing to essential hypertension in Mongolian population of China. Biomed Environ Sci. 2004;17:177–86. [PubMed] [Google Scholar]
- 31.Ottman R. Gene–environment interaction: definitions and study designs. Prev Med. 1996;25(6):764–70. doi: 10.1006/pmed.1996.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.van Kempen EE, Kruize H, Boshuizen HC, Ameling CB, Staatsen BA, de Hollander AE. The association between noise exposure and blood pressure and ischemic heart disease: a meta-analysis. Environ Health Perspect. 2002;110:307–17. doi: 10.1289/ehp.02110307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Babisch W. Health aspects of extra-aural noise research. Noise Health. 2004;6(22):69–81. [PubMed] [Google Scholar]
- 34.Chang TY, Jain RM, Wang CS, Chan CC. Effects of occupational noise exposure on blood pressure. J Occup Environ Med. 2003;45:1289–96. doi: 10.1097/01.jom.0000100003.59731.3d. [DOI] [PubMed] [Google Scholar]
- 35.Tomei G, Fioravanti M, Cerratti D, Sancini A, Tomao E, Rosati MV, et al. Occupational exposure to noise and the cardiovascular system: a meta-analysis. Sci Total Environ. 2010;408:681–9. doi: 10.1016/j.scitotenv.2009.10.071. [DOI] [PubMed] [Google Scholar]
- 36.Bowman T, Gaziano M, Buring J, Sesso H. A prospective study of cigarette smoking and risk of incident hypertension in women. J Am Coll Cardiol. 2007;50(21):2085–92. doi: 10.1016/j.jacc.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 37.Narnkiewicz K, Kjeldsen SE, Hedner T. Is smoking a causative factor of hypertension? Blood Press. 2005;14:69–71. doi: 10.1080/08037050510034202. [DOI] [PubMed] [Google Scholar]
- 38.Niskanen L, Laaksonen DE, Nyyssonen K, Punnonen K, Veli-Pekk V, Fuentes R, et al. Inflammation, abdominal obesity, and smoking as predictors of hypertension. Hypertension. 2004;44:859–65. doi: 10.1161/01.HYP.0000146691.51307.84. [DOI] [PubMed] [Google Scholar]
- 39.Green MS, Jucha E, LZ Y. Blood Pressure in smokers and non-smokers: epidemiologic findings. Am Heart J. 1986;111:932–40. doi: 10.1016/0002-8703(86)90645-9. [DOI] [PubMed] [Google Scholar]
- 40.Lee D, Ha M, Kim J, Jacobs DR. Effects of smoking cessation on changes in blood pressure and incidence of hypertension. Hypertension. 2001;37:194–8. doi: 10.1161/01.hyp.37.2.194. [DOI] [PubMed] [Google Scholar]
- 41.Whelton PK, Jiang He, Klag MJ. Blood pressure in westernized populations. In: Swales JD, editors. Textbook of hypertension. London: Blackwell Scientific Publications; 1994. p. 11–21. [Google Scholar]
- 42.Martínez-Rodríguez N, Posadas-Romero C, Cardoso G, Pérez-Rodríguez JM, Pérez-Hernández N, Vallejo M, et al. Association of angiotensin II type 1-receptor gene polymorphisms with the risk of developing hypertension in Mexican individuals. J Renin Angiotensin Aldosterone Syst. 2012;13(1):133–140. doi: 10.1177/1470320311419175. [DOI] [PubMed] [Google Scholar]
- 43.Ajala A, Almeida S, Rangel M, Palomino Z, Strufaldi M, Puccini RF, et al. Association of ACE gene Insertion/Deletion polymorphism with birth weight, blood pressure levels, and ACE activity in healthy children. Am J Hypertens. 2012;25(7):827–32. doi: 10.1038/ajh.2012.50. [DOI] [PubMed] [Google Scholar]
- 44.Saeed M, Khan AN, Siddiqui S, Saboohi K, Ali SO, Frossard PM. Association of ACE gene haplotype with essential hypertension. J Hum Hypertens. 2004;18:913–4. doi: 10.1038/sj.jhh.1001757. [DOI] [PubMed] [Google Scholar]
- 45.Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33:245–54. doi: 10.1038/ng1089. [DOI] [PubMed] [Google Scholar]
- 46.Jones PA, Takai D. The role of DNA methylation in mammalian epigenetics. Science. 2001;293:1068–70. doi: 10.1126/science.1063852. [DOI] [PubMed] [Google Scholar]
- 47.Nawaz S, Hasnain S. Effect of ACE polymorphisms on the association between noise and hypertension in a Pakistani population. J Renin Angiotensin Aldosterone Syst. 2011;12(4):516–20. doi: 10.1177/1470320310395799. [DOI] [PubMed] [Google Scholar]
- 48.Higaki J, Baba S, Katsuya T, Sato N, Ishikawa K, Mannami T, et al. Deletion allele of angiotensin-converting enzyme gene increases risk of essential hypertension in Japanese men. Circulation. 2000;101:2060–5. doi: 10.1161/01.cir.101.17.2060. [DOI] [PubMed] [Google Scholar]
- 49.Li Y. Angiotensin-converting enzyme gene insertion/deletion polymorphism and essential hypertension in the Chinese population: a meta-analysis including 21,058 participants. Intern Med J. 2012;42(4):439–44. doi: 10.1111/j.1445-5994.2011.02584.x. [DOI] [PubMed] [Google Scholar]
- 50.Tascilar N, Dursun A, Ankarali H, Mungan G, Ekem S, Baris S. Angiotensin-converting enzyme insertion/deletion polymorphism has no effect on the risk of atherosclerotic stroke or hypertension. J Neurol Sci. 2009;285:137–41. doi: 10.1016/j.jns.2009.06.016. [DOI] [PubMed] [Google Scholar]
- 51.Tiret L, Rigat B, Visvikis S, Breda C, Corvol P, Cambien F, et al. Evidence, from combined segregation and linkage analysis, that a variant of the angiotensin I-converting enzyme (ACE) gene controls plasma ACE levels. Am J Hum Genet. 1992;51(1):197–205. [PMC free article] [PubMed] [Google Scholar]
- 52.Villard E, Tiret L, Visvikis S, Rakotovao R, Cambien F, Soubrier F. Identification of new polymorphisms of the angiotensin I-converting enzyme (ACE) gene, and study of their relationship to plasma ACE levels by two-QTL segregation linkage analysis. Am J Hum Genet. 1996;58:1268–78. [PMC free article] [PubMed] [Google Scholar]
- 53.Levy D, DeStefano AL, Larson MG, O’Donnell CJ, Lifton RP, Gavras H, et al. Evidence for a gene influencing blood pressure on chromosome 17. Genome scan linkage results for longitudinal blood pressure phenotypes in subjects from the Framingham heart study. Hypertension. 2000;36:477–83. doi: 10.1161/01.hyp.36.4.477. [DOI] [PubMed] [Google Scholar]
- 54.Vasudevan R, Ismail P, Stanslas J, Shamsudin N. Association of G2350A polymorphism of angiotensin converting enzyme gene with essential hypertension and type 2 diabetes mellitus in Malaysian subjects. J Biol Sci. 2008;8:1045–50. [Google Scholar]
- 55.Rothman KJ, Greenland S, Walker AM. Concepts of interaction. Am J Epidemiol. 1980;112:467–70. doi: 10.1093/oxfordjournals.aje.a113015. [DOI] [PubMed] [Google Scholar]
- 56.Siemiatycki J, Thomas DC. Biological models and statistical interactions: an example from multistage carcinogenesis. Int J Epidemiol. 1981;10:383–7. doi: 10.1093/ije/10.4.383. [DOI] [PubMed] [Google Scholar]
- 57.Pawlaczyk-Luszczynska M, Dudarewicz A, Zaborowski K, Zamojska M, Sliwinska-Kowalska M. Noise induced hearing loss: research in central, eastern and south-eastern Europe and newly independent states. Noise Health. 2013;15:55–66. doi: 10.4103/1463-1741.107157. [DOI] [PubMed] [Google Scholar]
- 58.Nagahar K, Fisch U, Yagi N. Perilymph oxygenation in sudden and progressive sensorineural hearing loss. Acta Otolaryngol. 1983;96:57–6. doi: 10.3109/00016488309132875. [DOI] [PubMed] [Google Scholar]
- 59.Carrasco VN, Prazma J, Faber JE. Cochlear microcirculation effect of adrenergic agonists on arteriole diameter. Arch Otolaryngol Head Neck Surg. 1990;116:411–7. doi: 10.1001/archotol.1990.01870040033009. [DOI] [PubMed] [Google Scholar]
- 60.Wu TN, Chou FS, Chang PY. A study of noise-induced hearing loss and blood pressure in steel mill workers. Int Arch Occup Environ Health. 1987;59(6):529–36. doi: 10.1007/BF00377915. [DOI] [PubMed] [Google Scholar]
- 61.Tarter SK, Robins TG. Chronic noise exposure, high-frequency hearing loss, and hypertension among automotive assembly workers. J Occup Med. 1990;32:685–9. [PubMed] [Google Scholar]


