Abstract
Diabetes is the most common cause of neuropathy in US and neuropathies are the most common complication of diabetes mellitus affecting up to 50% of patients with type 1 and type 2 diabetes mellitus. Various types of neuropathies can be associated with diabetes mellitus.1 Symptoms usually include numbness, tingling, pain and weakness. Dizziness with postural changes can be seen with autonomic neuropathy. Metabolic, vascular and immune theories have been proposed for the pathogenesis of diabetic neuropathy. Pathologically axonal damage and segmental demyelination can be seen with diabetic neuropathies. Management of diabetic neuropathy should begin at the initial diagnosis of diabetes and mainly requires tight and stable glycemic control. Many medications are available for the treatment of neuropathic pain.
Keywords: Diabetic Neuropathy
Introduction
Diabetes mellitus (DM) has 4 major complications: neuropathy, retinopathy, nephropathy, and vasculopathy. The various neuropathies associated with DM can clinically be divided into symmetric and asymmetric (focal and multifocal) forms (Table 1). In addition, clinicians need to be aware of one muscle disorder, diabetic muscle infarction, that can occur in diabetic patients. A practical approach to the diagnosis and management of diabetic symmetric neuropathies will be reviewed in this chapter.
TABLE 1.
Clinical Classification of Diabetic Neuropathies
| I. | Symmetric Polyneuropathies: | ||
| Relatively fixed deficits: | |||
| Distal sensory polyneuropathy (DSPN) | |||
| Variants: | acute,severe DSPN in early onset diabetes | ||
| pseudosyringomyelic neuropathy | |||
| pseudotabetic neuropathy | |||
| Autonomic neuropathy | |||
| Episodic symptoms: | |||
| Diabetic neuropathic cachexia (DNC) | |||
| Hyperglycemic neuropathy | |||
| Treatment-induced diabetic neuropathy | |||
| II. | Asymmetric/Focal and Multifocal Diabetic Neuropathies: | ||
| Diabetic lumbosacral radiculoplexopathy (DLSRP; Bruns-Garland syndrome; diabetic amyotrophy; proximal diabetic neuropathy) | |||
| Truncal neuropathies (thoracic radiculopathy) | |||
| Cranial neuropathies | |||
| Limb mononeuropathies | |||
EPIDEMIOLOGY
The estimated prevalence of DM in the U.S. in individuals 40–74 years old is 12% if only fasting blood sugar (FBS) criteria are used but 14% if both FBS and glucose tolerance tasting (GTT) criteria are used.2 It is estimated that about half the adults with diabetes in the U.S. are undiagnosed.3 If the entire population is considered, including children, DM has been reported to occur in 1 to 4%.4 In the Rochester, Minnesota population-based study, 1.3% of the population had diabetes mellitus.4 According to the 2011 CDC National Diabetes Fact Sheet, diabetes affects 25.8 million Americans or 8.3% of the US population. That estimate includes 7 million undiagnosed cases. Among U.S. residents aged 65 years and older, 10.9 million, or 26.9%, had diabetes in 2010. In 2005–2008, based on fasting glucose or hemoglobin A1c levels, 35% of U.S. adults aged 20 years or older and 50% of adults aged 65 years or older had “prediabetes”, yielding an estimated 79 million American adults aged 20 years or older with prediabetes. Almost 30% of people with diabetes aged 40 years or older have impaired sensation in the feet.5 Approximately two-thirds of both insulin-dependent DM (IDDM) and non-IDDM (NIDDM) patients had subclinical or clinical evidence of a peripheral neuropathy. Roughly half of the diabetics had a symmetric polyneuropathy, a quarter had carpal tunnel syndrome, about 5% had autonomic neuropathy, and 1% had asymmetric proximal neuropathy. The occurrence of neuropathy correlates with the duration of DM, poor glycemic control and with the presence of retinopathy and nephropathy.4,6–11 In the study by Picart, 7.5% of patients had neuropathy at the time of diagnosis, and after 20 years of DM, 50% had neuropathy.8 Partanen et al showed that after 10 years of follow up the percent of diabetics with neuropathy increased from 8% at baseline to 42%.9
Diabetes Mellitus Diagnostic Criteria
The American Diabetes Association (ADA) issued diagnostic criteria for diabetes mellitus in 19972, with follow-up in 200312 and 201013. The diagnosis is based on one of four abnormalities: hemoglobin A1C (A1C), fasting plasma glucose (FPG), random elevated glucose with symptoms, or abnormal oral glucose tolerance test (OGTT) as follows:
A1C ≥ 6.5%. The test should be performed in a laboratory using a method certified by the national standardization Program (NGSP) and standardized to the Diabetes Control and Complications (DCCT) assay
FPG ≥ 126 mg/dl (7.0 mmol/l) on repeat testing. Fasting is defined as no caloric intake for at least 8 hours
2 hr plasma glucose≥ 200mg/dl (11.1 mmol/l) during an oral GTT. The test should be performed as described by the World Health Organization, using a glucose load containing the equivalent of 75 g anhydrous glucose dissolved in water.
-
In a patient with classic symptoms of hyperglycemia or hyperglycemic crisis, a random glucose ≥ 200mg/dl (11.1mmol/l).
In the absence of unequivocal hyperglycemia, criteria 1–3 should be confirmed by repeat testing. In evaluating a patient with a neuropathy, it is not sufficient to stop with the FBS in excluding DM. While the new criteria allows for the use of HgbA1C as sufficient to diagnose DM, we still usually recommend OGTT in patients who are evaluated for neuropathy in order to consider DM as potential etiology.
Pathogenesis of Diabetic Neuropathy
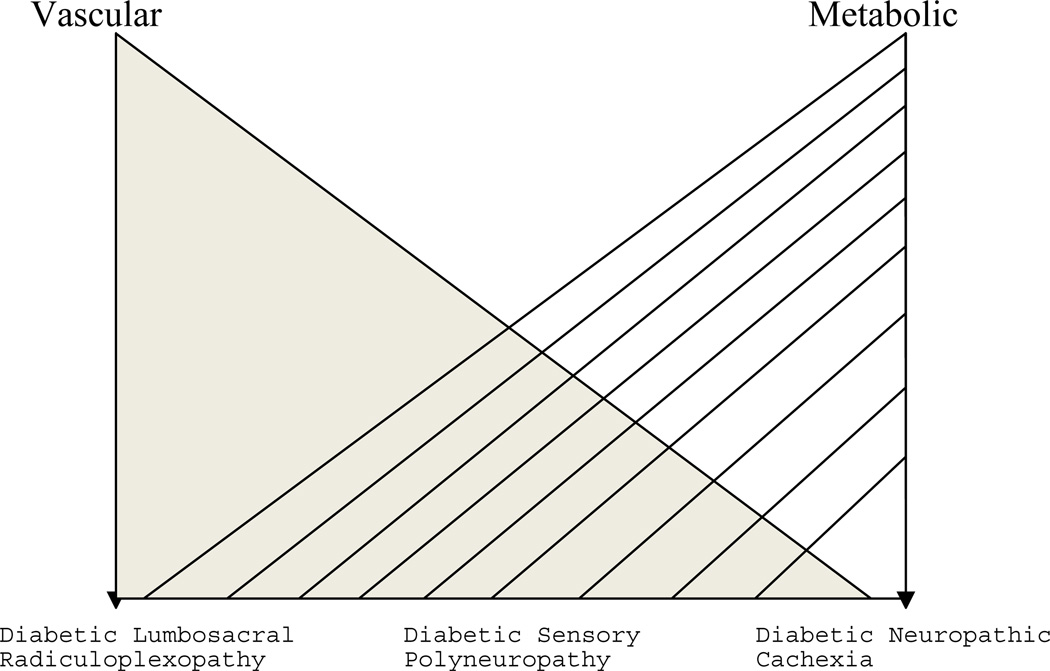
The pathologic basis for diabetic neuropathy remains controversial in spite of massive research efforts. There is evidence that both vascular and metabolic derangements may be responsible for peripheral nerve pathology in diabetes.14–21 An overview of these mechanisms is helpful in approaching the different clinical presentations of diabetic neuropathies and in understanding the various experimental therapeutic trials. A simplified pathophysiologic scheme would primarily attribute the focal and multifocal neuropathies to a vascular basis, and the symmetrical polyneuropathies to a metabolic disorder. However, we believe that there is a spectrum of possible pathophysiologic cause of various diabetic neuropathies and evidence for either vascular or metabolic dysfunction is not restricted to a particular neuropathy (Fig 1).22–26
Figure 1.
Spectrum of possible pathophysiologic causes of various diabetic neuropathies
A. Metabolic pathogenesis
Experimental models of acute, severe hyperglycemia can produce reduction in nerve conduction velocity and axonal shrinkage.27 Glucose and myo-inositol share a structural similarity and hyperglycemia may reduce myo-inositol uptake in diabetic nerve. This secondarily impairs the function of membrane bound sodium/potassium ATPase, which can cause axoglial changes and alterations of conduction velocity.28 However, in nerves from diabetic patients, endoneurial myoinositol was not decreased and in two clinical trials, there was no benefit to patients from myo-inositol supplementation.29–32
Another popular mechanism is an alteration of polyol metabolism. Persistent hyperglycemia activates the enzyme aldose reductase, thereby converting glucose to the polyol, sorbitol, and ultimately to fructose. Sorbitol, a compound with relative impermeability, accumulates in the nerve creating a hypertonic condition and subsequent water accumulation.
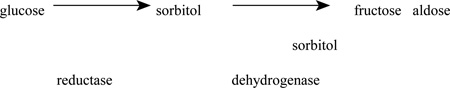
The accumulation of sorbitol and fructose increases the distance between capillaries, producing endoneurial hypoxia and oxidative stress. Aldose reductase inhibitors in animal models decrease sorbitol concentration in the sciatic nerve and restore conduction velocities to normal. In addition, protection from sorbitol elevation in experimental diabetes using aldose reductase inhibitors prevents the loss of myo-inositol from the nerve, which may tie two possible pathogenic mechanisms.33
Alterations of fatty acid metabolism can result from chronic hyperglycemia. In experimental diabetes there is a deficiency of gamma-linolenic acid that could lead to abnormalities in endoneurial blood flow through secondary deficiencies in arachidonic acid and prostaglandins.34 This has led to clinical trials of diets enriched in linolenic acid.35–36
Chronic hyperglycemia increases glycosylation of proteins.37–39 Glycation products can accumulate in tissues producing microvascular disease by direct deposition on endothelial cell membranes or the generation of reactive oxygen species that adds to oxidative stress.40
Aminoguanidine, an inhibitor of advanced glycation, has been used in experimental animal models of diabetes and is currently being studied in humans.41
B. Vascular pathogenesis
It has been postulated that hypoxia or ischemia is involved in diabetic polyneuropathy.16,42–47 The ultrastructural studies of Dyck and colleagues have demonstrated that the increase in basement membrane area and endothelial cell degeneration is associated with severity of polyneuropathy.44–46 On a more macroscopic level, the study of the distribution of fiber loss in diabetic nerves also suggests a vascular pathology.20,48–50 In the study by Dyck et al, the multifocal pattern of fiber loss could still be identified in the sural nerve.50 Johnson et al identified changes in the perineurium and surrounding epineurium that resembled those seen in peripheral nerve vasculitis.20 While these ultrastructural vessel changes could account for these ischemic morphologic features, there is evidence that chronic hyperglycemia may produce hypoxia or frank ischemia.47,51,52
Autopsy material from patients with diabetic cranial third nerve palsy reveals central fascicular injury suggesting ischemia.53–55 Even diabetic patients who never experienced third nerve palsy demonstrate microfasciculation on autopsy when compared to controls.56 An Autopsy study in diabetic asymmetric proximal lower extremity neuropathy by Raff and colleagues57,58 showed ischemic infarcts of the proximal major nerve trunks of the leg and lumbosacral plexus with multiple areas of decreases myelinated fiber density at these levels. Said and colleagues have biopsied the cutaneous nerve of the thigh in patients with this disorder and demonstrated asymmetric fasicular loss in some patients.59,60 In a study of sural nerve biopsies from patients with diabetic lumbosacral radiculoplexopathy, there was multifocal variability in nerve fiber density with non-random fiber loss between and within fascicles.61 In the Mayo Clinic series of Pascoe and colleagues in six biopsied patients with diabetic proximal neuropathy, a multifocal distribution of fiber loss was noted in three sural nerve specimens.62 In a recent Mayo Clinic series changes suggesting ischemia were found in the majority of 33 nerve biopsies with diabetic radiculoplexus neuropathy.63
Local sural nerve blood flow in patients with mild diabetic polyneuropathy was assessed using laser Doppler flowmetry.64. Patients with peripheral nerve vasculitis who did demonstrate abnormal sural nerve blood flow served as "controls". No relationship was found between sural nerve blood flow and the presence or development of distal symmetric neuropathy. One study showed that activation of the complement pathway and formation of the membrane attack complex could injure blood vessels and adversely affect the circulation in the endoneurium.65
C. Immunologic/inflammatory pathogenesis
An immune mediated pathogenesis has recently been advocated in some cases of diabetic neuropathy.66 In a study of proximal asymmetric neuropathy that showed asymmetric nerve fiber loss, an additional feature was lymphocytic epineurial inflammation resembling vasculitis. Krendel and colleagues found similar perivascular inflammation in 7 of 10 patients with asymmetric lumbosacral neuropathy.67 In a Mayo clinic study of diabetic proximal neuropathy, 2/6 sural nerve biopsies showed perivascular mononuclear inflammatory infiltrates.62 Younger and colleagues biopsied 20 patients with diabetic neuropathy - 6 with distal symmetric and 14 with asymmetric neuropathy.68 Seven patients had epineurial vessel inflammation on routine paraffin sections - 2 with distal symmetric and 5 with asymmetric neuropathy. With immunohistochemistry, the number of patients with T-cell microvasculitis increased to 12 (60% of biopsied patients) with the inflammatory cells being predominantly CD8+ T-cells. The presence of tumor necrosis factor, interleukin-6, interleukin-1β and α, and C5b-9 in a number of the specimens further led the authors to suggest an immune-mediated pathogenesis of the neuropathy.
In the Mayo large series of patients with diabetic radiculoplexus neuropathy, perivascular mononuclear cells were found in all 33 biopsied patients, most of whom also showed changes of ischemia.63
Pathology and pathophysiology
Axonal degeneration or segmental demyelination
There has been some debate regarding whether the primary lesion in diabetic neuropathy is the axon or Schwann cell/myelin. Ballin and Thomas identified onion bulbs and teased nerve fiber findings suggesting recurrent demyelination and Vital reported both segmental demyelination and axonal degeneration.69–71 Dyck demonstrated that the changes of axonal degeneration and regeneration were more frequent than those of segmental demyelination and remyelination, and in the setting of multifocal fiber loss, they concluded that axonal degeneration was the primary process.50 Studies by Said of the sural nerves of patients with prominent small fiber sensory loss found pathologic evidence for both axonal degeneration, as well as both primary and secondary segmental demyelination.72,73 Therefore, if a sural nerve biopsy is obtained from a typical distal symmetric diabetic neuropathy patient, one can expect to see a broad spectrum of axonal and myelin pathologic changes. For this reason, the sural nerve biopsy is often not helpful in diabetic neuropathy in attempting to determine if the underlying process is axonal degeneration or demyelination/remyelination.
Similarly, the electrophysiologic studies can show evidence of axonal degeneration and demyelination. An early and characteristic feature of diabetic neuropathy is prolonged distal latencies and F-waves, slow conduction velocity, and reduced amplitude of potentials.7,74–76 Behse and Buchthal believed the conduction velocity slowing was from loss of large fibers.77 However, another reason for slow conduction velocity may be a functional alteration at nodes of Ranvier.16 While frank conduction block and temporal dispersion are usually not found in diabetic neuropathies, the degree of latency and velocity abnormalities can be so severe that socalled "demyelinating" electrophysiologic criteria may be met.78 Krendel found that demyelinating electrophysiologic criteria were met in 6 of 21 patients with proximal diabetic neuropathy.67 In the Mayo clinic series of Pascoe 28 of 42 patients with proximal diabetic neuropathy were classified as axonal and 14 as demyelinating.62 Temporal dispersion was noted in 14 patients, and conduction block in 2 patients. However, invariably the patients with these demyelinating findings also had axonal features as well.
Finding electrophysiologic changes that fulfill demyelinating criteria superimposed on axonal degeneration in a diabetic needs to be interpreted with caution. Chronic inflammatory demyelinating polyneuropathy (CIDP) can develop in diabetic patients79–81, but these cases are not believed to be related to the underlying diabetes. In support of the lack of association, a recent Olmstead county epidemiologic study identified DM in 4% of 23 CIDP cases and in 12% of matched controls.82 Electrophysiologic changes of demyelination in a diabetic with a symmetric distal or an asymmetric proximal neuropathy may not necessarily imply an immune mediated neuropathy that will respond to immunosuppressive therapy. In cases of marked demyelination, correlation with the clinical pattern and temporal profile are essential to distinguish CIDP from distal symmetric peripheral polyneuropathy and avoid unnecessary therapies. Jaradeh presented 15 patients with progressive polyradiculoneuropathy in diabetes with presentation similar to CIDP. Electrophysiology was predominantly axonal, CSF showed increased protein in 14 and oligoconal bands in 5. Sural nerve biopsy performed in 14 patients showed fiber loss, segmental demyelination, inflammatory infiltrates and onion bulbs. All patients had benefit with immunomodulating therapy.83
TYPES OF DIABETIC NEUROPATHIES
Symmetric Neuropathies
A. Symmetric neuropathies with fixed deficits
1. Distal symmetric polyneuropathy (DSPN)
DSPN is the most common form of diabetic neuropathy. Clinically, this is primarily a length-dependent sensory neuropathy, and significant distal weakness is uncommon. However, as with cryptogenic distal sensory neuropathy (CSPN), there is usually electrophysiologic evidence for sub-clinical motor involvement. Indeed, the clinical and electrophysiologic findings in both cryptogenic and diabetic distal sensory and sensorimotor neuropathy are very similar.84 However, since diabetic patients are often monitored closely before they develop symptoms of neuropathy, the earliest signs of neuropathy may be decreased distal vibration, touch, and pin sensation and ankle reflex loss on examination. The first symptoms are usually decreased feeling or tingling in the toes. Dysesthesias, usually burning pain, may develop, although the majority of diabetic patients with a distal sensory neuropathy do not complain of significant discomfort. In a population of 382 insulin-treated diabetic subjects, 41 or 10.7% were found to have painful symptoms.85 In a 2-phase cross-sectional descriptive study of patients with type 2 diabetes (postal survey followed by neurological history and examination) up to 27% of diabetics experienced neuropathic pain or mixed pain resulting in a significant negative effect on quality of life.86 Sensory symptoms can eventually progress up the ankles and knees and to the fingers, hands, and forearms. If sensory loss extends to the elbows, patients can then develop a symmetrical midline truncal-wedge shaped area of sensory loss.87
While there may be atrophy and weakness of the toe extensor and flexor muscles, significant distal ankle weakness is uncommon. If profound distal upper and lower extremity weakness is present in a diabetic patient, an evaluation for other causes of neuropathy is warranted. It should be noted that progression of DSPN is usually quite slow. In the Rochester Diabetic Neuropathy study, none of the 380 diabetics had polyneuropathy that was disabling even when followed for many years.4 An exception to this rule is the unusual cases of severe sensory and autonomic neuropathy that can occur in the first several after the onset of Type 1 diabetes.73 It is not known why some patients develop this unusually severe form of neuropathy in the early stages of the disease and there is no relationship between the neuropathy and hyperglycemia or the initiation of insulin therapy.
Several examination scales have been developed to establish neuropathy.88,89 Most of these focus primarily on large-fiber function. The Utah Early Neuropathy Scale was shown to be a sensitive clinical measure of sensory and small fiber neuropathy.90 England et al recently established the AAN practice parameters for evaluation of distal symmetric polyneuropathy (DSP).91–93 In routine clinical practice, documenting an abnormal sensory, reflex, and occasionally a motor examination is sufficient to diagnose neuropathy in a diabetic patient with appropriate symptoms. The use of monofilaments to assess touch-pressure sensation has become important as well as the Rydell-Seifert semi-quantitative tuning fork.94
Depending on the clinical context, it may be appropriate to perform screening blood tests for other causes of neuropathy (CBC, chem 20, B12 level, VDRL, serum immunofixation). Equipment for detecting sensory loss such as computerized quantitative sensory testing, and both simple and complex grading systems have been developed for assessing diabetic neuropathy, primarily useful in the context of entering patients in research protocols, and they are not needed clinically in most patients.95–96 Skin biopsies have become a tool to diagnose small fiber neuropathy in patients with nomal electrodiagnostic testing.84,88
If severe foot ulcers and neurogenic arthropathies develop, this is often labeled pseudosyringomyelic diabetic neuropathy. There is a selective loss of pain fibers resulting in impaired cutaneous and deep pain and temperature sensation.72,97 Severe proprioceptive loss is uncommon, but occasionally this can occur when there is prominent large fiber involvement. These patients develop sensory ataxia and autonomic manifestations with impotence, bladder atony, and pupillary changes and thus have been called the pseudotabetic form of diabetic neuropathy. However, severe proprioception deficits and ataxia are uncommon in diabetes, and when present should lead to a search for other potentials etiologies (syphilis, B12 deficiency, paraneoplastic or Sjogrens syndrome sensory neuronopathy, CIDP). However, we believe that the pseudosyringomyelic, pseudotabetic, and the early onset neuropathy described by Said and colleagues, are all severe variants of diabetic DSPN and probably not distinct forms of neuropathy.73
Treatment of DSPN
Glucose control: In general, patients with strict control of blood glucose have fewer diabetic neuropathy complications.10,11.98 A number of studies have shown that tight glucose control with aggressive insulin therapy can reduce the risk for development of neuropathy.7,99,100 The DCCT trial convincingly showed that intensive insulin therapy with an insulin pump or three or more daily injections is more effective than conventional therapy in reducing neuropathy.99 Neuropathy occurred in only 5% of intensively treated patients compared to 13% of those conventionally treated. Overall neuropathy was reduced by 64% over 5 years in the intensively treated group. In a follow up 13–14 years after DCCT closeout, the prevalence of neuropathy increased from 9 to 25% in former intensive and from 17 to 35% in former conventional treatment groups (P < 0.001), and the incidence of neuropathy remained lower among former intensive treatment subjects.101 This supports the importance and benefits of early intensive insulin treatment in reducing the risk of neuropathy even more than a decade later.
-
Other experimental approaches: Many different approaches to treat diabetic neuropathy and the other complications of diabetes have been attempted, but in general there has been little success. A number of these experimental approaches were described and referenced above in the section on "metabolic pathogenesis" and aldose reductase inhibitors, diets enriched in linolenic acid, and aminoguanidine to inhibit advanced glycation. Despite numerous trials of various aldose reductase inhibitors, none have been shown to prevent neuropathy or the progression of deficits.11,102–104
In streptozotocin induced diabetic rats, treatment with alpha-lipoic acid improved nerve blood flow and improved conduction velocity. Alpha-lipoic acid (also known as thioctic acid) may act as an anti-oxidant free-radical-scavenger, and it may inhibit nonenzymatic glycation (85,86).105,106 A placebo-controlled trial using intravenous alpha-lipoic acid reduced neuropathic symptoms in diabetic patients.107 In a 5-week randomized controlled trial of oral alpha-lipoic acid 600, 1,200 or 1,800 mg daily, there was a 50% pain reduction as measured by the Total Symptom Score, including stabbing and burning pain, across all doses that was statistically significant as compared to the placebo response (32%, P<0.05).108 A more recent study suggested that four-year treatment with oral alphalipoic acid 600 mg once daily in mild-to-moderate distal symmetric neuropathy did not influence the primary composite end point of Neuropathy Impairment Score of the Lower Limbs and seven neurophysiologic tests.109 Besides being well tolerated, the authors suggested a clinically meaningful improvement and prevention of progression of neuropathic impairment but these results have yet to be duplicated by other investigators. Oral alpha-lipoic acid can be obtained in health-food stores, started at 300 mg daily and increased as high as 600 mg twice a day.
Recently there has been interest in nerve growth factor (NGF) therapy as a treatment of diabetic neuropathy.110 In experimental animal models of diabetes, there is some evidence for decreased NGF expression in various target tissues and NGF treatment in these models prevented the manifestations of neuropathy.111–115 In humans with diabetic neuropathy, NGF levels are reduced in skin biopsy specimens.116 In a phase II placebo controlled trial of subcutaneous NGF in 250 patients with diabetic neuropathy, the only endpoint that reached definite statistical significance was the patient’s overall global symptom assessment that they felt improved. There was a trend toward improvement in quantitative cold detection thresholds and in the small-fiber sensory components of neuropathy impairment score. However, in the larger Phase III trial, there was no benefit in the NGF group on any endpoint.
Symptomatic treatment: If pain is not a part of the diabetic neuropathy patient's complaints, symptomatic treatment is of no value and not necessary. Symptoms of numbness and tingling should not be treated.
The most frequently used oral drugs for the symptomatic treatment of diabetic and non-diabetic painful neuropathy are the tricyclic anti-depressants, carbamazepine, gabapentin, mexiletene and more recently pregabalin and cymbalta.117–125 Each physician has their own preference for first, second, and third line drugs. Our preference and the doses are listed in Table 2. For further information on the treatment refer to the chapter “Treatment of Painful Peripheral Neuropathy in this journal”. A recent Evidence based guidelines were published for treatment of pain in diabetic neuropathy by American Academy of Neurology.93According to this, Pregabalin is established as effective and should be offered for relief of DPN. Venlafaxine, duloxetine, amitriptyline, gabapentin, valproate, opioids (morphine sulfate, tramadol, and oxycodone controlled-release), and capsaicin are probably effective and should be considered for treatment of PDN.123 Other treatments have less robust evidence or the evidence is negative.124 Effective treatments for DPN are available, but many have side effects that limit their usefulness, and few studies have sufficient information on treatment effects on function and quality of life. For further review of neuropathic pain management, the reader is referred to the chapter in this issue titled Treatment of Painful Peripheral Neuropathy.
TABLE 2.
Pharmacologic Therapy for Neuropathic Pain
| ORAL | |||
|---|---|---|---|
| Therapy | Route | Starting Doses |
Maintenance Doses |
| First Line | |||
| Tricyclic anti-depressants | oral | 10–25 mg at bedtime | Increase by 10–25 mg increments to 100–150 mg at |
| Gabapentin (Neurontin) | oral | 300 mg tid | Increase by 300–400 mg increments to 2400-in 3–4 doses |
| Tramadol (Ultram) | oral | 50 mg bid or tid | Increase by 50 mg increments to a maximum of 100 |
| Duloxetine (Cymabalta) | oral | 30 mg a day | Increase by 30 – 60 mg increments up to 120 mg a |
| Pregabalin (Lyrica) | oral | 50 mg tid | Increase to 300 mg/day |
| Second Line | |||
| Venlafaxine XR (Effexor) | oral | 37.5–75 mg once a | Increase by 75 mg increments to 150–225 mg a day |
| Valproate | oral | 250 mg bid to tid | Increase by 250 mg increments up to 1500 mg a day |
| Carbamazepine | oral | 200 mg bid | Increase by 200 mg increments to 200–400 mg three a day; follow drug levels on doses greater than 600 |
| Oxcarbazepine (Trileptal) | oral | 150–300 mg bid | Increase by 300 mg increments to 600–1200 mg two |
| Lamotrigine (Lamictal) | oral | 25 mg once a day or bid | Increase by 25 mg increments weekly to 100 |
| Topiramate (Topamax) | oral | 25–50 mg at bedtime | Increase by 50 mg increments weekly to 200 mg bid |
| Third Line | |||
| Bupropion SR (Welbutrin) | oral | 150 mg a day | After one week, increase to 150 mg bid |
| Tiagabine hydrochloride (Gabitril) | oral | 4 mg a day | Increase to 4 – 12 mg bid |
| Keppra (Levetiracetam) | oral | 250 mg at bedtime | Increase by 250–500 mg increments to 1500 mg two |
| Zonisamide (Zonegran) | oral | 100 mg at bedtime | Increase by 100 mg increments to 400–600 mg at bedtime |
| Mexiletine | oral | 200 mg once a day | Increase by 200 mg increments to a maximum of 200 |
| Phenytoin | oral | 200 mg at bedtime | Increase by 100 mg increments to 300–400 2 doses, following drug levels |
| Newer Drugs | |||
| Savella | oral | 12.5 mg at bedtime × 1 d | 12.5 mg bid × 2 d then 25 mg bid × 4 d the stay on 50 mg bid. May increase up to 100 |
| Vimpat | oral | 50 mg PO bid | In 1week, go to 100 mg bid. May increase up to 2 |
| Topical Agents | |||
| OVER THE COUNTER | |||
| Capsaicin .075% | topical | Apply to affected tid to qid | Continue with starting dose |
| Salicylate 10–15% | topical | Apply to affected tid to qid | Continue with starting dose |
| Menthol 16% / Camphor 3% -+ | topical | Apply to affected tid to qid | Continue with starting dose |
| BY PRESCRIPTION | |||
| Lidocaine 2.5% / Prilocaine 2.5% | topical | Apply to affected tid to qid | Continue with starting dose |
| Lidocaine patch 5% | topical | Apply over adjacent intact skin | Increase up to 3 patches worn for 12 of 24 |
| Doxepin 5% (Zolopan) | topical | Apply to affected bid | Continue with starting dose |
| Diclofenac Sodium (Voltaren Gel 1%) | topical | Apply to affected tid to qid | Continue with starting dose |
| BY PRESCRIPTION - ONLY AT COMPOUNDING PHARMACIES | |||
| Ketoprofen 5% / Amitriptyline 2% / Tetracaine 1% | topical* | Apply to affected bid | Increase up to a qid schedule |
| Ketoprofen 10% / Cyclobenzaprine 1% Lidocaine 5% | topical* | Apply to affected bid | Increase up to a qid schedule |
| Ketamine 5% / Amitriptyline 4% Gabapentin 4% | topical* | Apply to affected bid | Increase up to a tid schedule |
| Carbamazepine 5% Lidocaine 5% | topical* | Apply to affected bid | Increase up to a qid schedule |
| Amitriptyline 2% / Lioresal 2% | topical* | Apply to affected tid to qid | Continue with starting dose |
Key:
- must be compounded by pharmacy (to locate your local compounding pharmacy, call the International Academy of Compounding Pharmacists, 1-800-927-4227)
Topical therapy with capsaicin and lidocaine creams can be tried.126,127 In our experience, a minority of patients respond to these modalities and the creams are difficult to use because they need to be applied several times a day. Lidoderm patches may be effective, but they are expensive and cumbersome to apply to the soles of both feet.128,129 Transcutaneous nerve stimulation is occasionally helpful. Recently, an unusual alternative-medicine approach to painful neuropathies with magnetic inserts has received some attention.130 A blinded-controlled crossover study reported benefit with magnet therapy.131 A larger multicenter study of repetitive and cumulative exposure to low-frequency pulsed electromagnetic fields in 225 subjects with painful diabetic neuropathy did not show any effect on pain reduction.132 Interestingly, it did show in a subgroup of twenty-seven subjects who completed serial biopsies that twenty-nine percent of magnet treated subjects had an increase in distal leg ENFD of at least 0.5 SDs, while none did in the sham group (P=.04).
Exercise therapy is being investigated as another treatment modality. A small pilot study reported improvements in neuropathic pain and symptoms as well as cutaneous nerve fiber branching on proximal skin biopsy following a 10-week supervised exercise program in 17 patients with diabetic peripheral neuropathy. These findings are particularly promising given the short duration of the intervention, but need to be validated by comparison with a control group in future studies.133
2. Autonomic neuropathy
Autonomic manifestations can affect cardiovascular, genitourinary, or gastrointestinal organ systems so that patients develop orthostasis, tachycardia/tachyarrythmias, gastroparesis, impotence, bladder atony, or impotence. Other autonomic manifestations include profuse nocturnal or postprandial sweating and abnormal pupillary light responses. The nerve fibers that mediate sweating undergo distal damage. One electrophysiologic technique for evaluating these nerve fibers is to test for sympathetic skin responses.123 Diabetic diarrhea and incontinence are rare but can be disabling. Gastrointestinal autonomic dysfunction is assessed with various radiographic techniques, but the easiest is to demonstrate the abnormally slow passage of barium through the gut.134 Impotence is the most common clinical manifestation of autonomic neuropathy, affecting more than 50 percent of men with diabetes.
Treatment
Orthostatic symptoms can be treated with fludrocortisone (0.1 mg bid), the nonsteroidal antiinflammatory agents, ibuprofen and indomethacin, and the oral sympathomimetic agent midodrine (105–107).135–137 Midodrine (ProAmatine) is an alpha-adrenoreceptor agonist that increases blood pressure by producing arterial and venous constriction. The recommended dose is 10 mg 3 times daily. Pharmocotherapy can be tried for delayed gastric emptying (metoclopramide; erythromycin)138,139 and diarrhea (clonidine)140. Impotence can be treated with oral phosphodieterase-5 inhibitors such as sildenafil [Viagra®]141 and less commonly with injectable (phentolamine/papervine) drug therapy or penile prosthesis.142,143
B. Symmetric neuropathies with episodic symptoms
1. Diabetic neuropathic cachexia
Diabetic neuropathic cachexia (DNC) is an uncommon syndrome initially described by Ellenberg in 1973 in which patients develop profound weight loss, a symmetric sensory peripheral neuropathy, and painful dysesthesias over the limbs and trunk, without associated weakness.144–149 Unlike other symmetric neuropathies due to diabetes, diabetic neuropathic cachexia is reversible over a period of weeks to months. Most reported patients have been men, usually in the sixth or seventh decades of life, but there have been two cases described in women. All cases initially show a precipitous weight loss up to 60% of total body weight, leading at times to an incorrect suspicion of an underlying cancer. Patients may experience intense contact hypersensitivity and may also describe intermittent stabbing or shooting pains. The pain tends to be worse at night or during periods of relaxation. The presence of proximal or truncal symmetric dysesthesias associated with profound weight loss should be clinical clues that support the diagnosis of diabetic neuropathic cachexia rather than the more common DSPN of diabetes. Patients may also experience depression, anorexia, and impotence. Sensory impairment associated with diabetic neuropathic cachexia is generally minimal, by contrast with the severity of the patient's complaints of pain, and in some cases, may not be clinically detectable. Some reports describe associated muscle atrophy and "weakness", whereas others have reported normal strength.
Diabetic neuropathic cachexia can occur in both Type 1 and Type 2 diabetic patients. Interestingly, there is a lack of correlation with other microvascular complications of diabetes such as nephropathy or retinopathy. Most cases are associated with poor glucose control. Some of these cases have been associated with malabsorption.150
Treatment of diabetic neuropathic cachexia can be difficult and strict diabetic control is usually necessary. The usual drugs to treat neuropathic pain can be tried, but they are often unsucessful and the temporary use of narcotic is often needed. The prognosis is usually good, and patients typically recover their baseline weight with resolution of the painful sensory symptoms within one year. A residual sensorimotor neuropathy is not uncommon. Recurrent diabetic neuropathic cachexia have also been reported (Figure 2).138
Figure 2.
Patient’s weight over time and the onset of neuropathic pain and initiation of diabetic therapy. +=Initiation of oral hypoglycemic; *=initiation of insulin; ↓=onset of pain
The eventual resolution of diabetic neuropathic cachexia with concomitant weight gain, and the lack of correlation with other microvascular complications of diabetes would suggest a primarily dysmetabolic process (Figure 1). However, the pathophysiologic basis of the disorder remains unknown.
2. Other possible transient symmetric sensory neuropathies
Other cases of transient distal sensory paresthesias and pain have been alleged to be due to hyperglycemia ("hyperglycemic neuropathy") or "insulin neuritis" following the institution of insulin.97,151–152. The insulin neuritis is usually characterized by acute, severe pain, peripheral nerve degeneration, and autonomic dysfunction after intensive glycemic control. This often parallels with worsening retinopathy and resolves in weeks or months153. Clinical features and objective measures of neuropathy can improve in these patients despite a prolonged history of poor glucose control.151 So called hyperglycemic neuropathy may occur at the time of diagnosis or may follow an episode of ketotic coma, and the symptoms rapidly subside once the diabetes is controlled. Llewelyn et reported similar symptoms within 6 weeks of establishing good diabetic control with insulin, and pain persisted for 4 to 5 months.152,125 It is still unclear if these are distinct peripheral neuropathy entities.
KEY POINTS.
Diabetic neuropathy is the most common type of neuropathy
Various types of neuropathies are associated with diabetes mellitus.
Metabolic, Vascular, inflammatory and immune theories have been suggested for pathogenesis
Axonal and demyelination can be seen on electrophysiology and pathology
Treatment is mainly aimed at glycemic control and neuropathic pain management
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Mamatha Pasnoor, Department of Neurology, University of Kansas Medical Center, Kansas City, KS.
Mazen M. Dimachkie, Director, Neuromuscular Section, Department of Neurology, University of Kansas Medical Center, Kansas City, KS.
Patricia Kluding, Department of Physical Therapy and Rehabilitation Science, University of Kansas Medical Center, Kansas City, KS.
Richard J. Barohn, Department of Neurology, University of Kansas Medical Center, Kansas City, KS.
References
- 1.Barohn RJ. Diabetic Neuropathy. Decision Making in Pain Management. (Second Edition) 2006:82–83. [Google Scholar]
- 2.The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care. 1997;20:1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 3.Harris MI, Hadden WC, Knowler WC, Bennett PH. Prevalence of diabetes and impaired glucose tolerance and plasma glucose levels in the U.S. population ages 20–74. Diabetes. 1987;36:523–534. doi: 10.2337/diab.36.4.523. [DOI] [PubMed] [Google Scholar]
- 4.Dyck PH, Kratz KM, Karnes JL, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester diabetic neuropathy study. Neurology. 1993;43:817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. National Fact Sheet. Atlanta, GA: CDC, USHHS; 2011. [Accessed on December 1, 2012]. 2011. http://www.cdc.gov/diabetes/pubs/pdf/ndfs_2011. [Google Scholar]
- 6.Melton LJ, Dyck PJ. Clinical features of the diabetic neuropathies: Epidemiology. In: Dyck PJ, Thomas PK, Asbury AK, Winegrad AI, Porte DJ, editors. Diabetic Neuropathy. Philadelphia: WB Saunders; 1987. pp. 27–35. [Google Scholar]
- 7.Diabetes Control and Complications Trial. Effect of intensive diabetes treatment on nerve conduction in the Diabetes Control and Complications Trial. Ann Neurol. 1995;38:869–880. doi: 10.1002/ana.410380607. [DOI] [PubMed] [Google Scholar]
- 8.Picart J. Diabetes mellitus and its degenerative complications: a prospective study of 4,400 patients observed between 1947 and 1973. Diabetes Care. 1978;1:168–188. and 252–263. [Google Scholar]
- 9.Partanen J, Kiskanen L, Lehtinen J, et al. Natural history of peripheral neuropathy in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:89–94. doi: 10.1056/NEJM199507133330203. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM. Long-term complications of diabetes mellitus. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 11.Clark CM, Lee DA. Prevention and treatment of the complications of diabetes mellitus. N Engl J Med. 1995;332:1210–1217. doi: 10.1056/NEJM199505043321807. [DOI] [PubMed] [Google Scholar]
- 12.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, Kitzmiller J, Knowler WC, Lebovitz H, Lernmark A, Nathan D, Palmer J, Rizza R, Saudek C, Shaw J, Steffes M, Stern M, Tuomilehto J, Zimmet P. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003;26(11):3160. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 13.Diagnosis and classification of diabetes mellitus.American Diabetes Association. Diabetes Care. 2010;33(Suppl 1):S62. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown MJ, Asbury AK. Diabetic neuropathy. Ann Neurol. 1984;15:2–12. doi: 10.1002/ana.410150103. [DOI] [PubMed] [Google Scholar]
- 15.Low PA. Recent advances in the pathogenesis of diabetic neuropathy. Muscle Nerve. 1987;10:121–128. doi: 10.1002/mus.880100204. [DOI] [PubMed] [Google Scholar]
- 16.Dyck PJ, Giannini C. Pathologic alterations in the diabetic neuropathies of humans: a review. J Neuropathol Exp Neurol. 1996;55:1181–1193. doi: 10.1097/00005072-199612000-00001. [DOI] [PubMed] [Google Scholar]
- 17.Sima AA. Pathological definition and evaluation of diabetic neuropathy and clinical correlations. Can J Neurol Sci. 1994;21:S13–S17. doi: 10.1017/s0317167100040695. [DOI] [PubMed] [Google Scholar]
- 18.Stevens MJ, Feldman EL, Greene DA. The aetiology of diabetic neuropathy: the combined roles of metabolic and vascular defects. Diabet Med. 1995;12:566–579. doi: 10.1111/j.1464-5491.1995.tb00544.x. [DOI] [PubMed] [Google Scholar]
- 19.Thomas PK. Diabetic neuropathy: models, mechanism, and mayhem. Can J Neurol Sci. 1992;19:1–7. [PubMed] [Google Scholar]
- 20.Johnson PC, Doll SC, Cromey DW. Pathogenesis of diabetic neuropathy. Ann Neurol. 1986;19:450–457. doi: 10.1002/ana.410190505. [DOI] [PubMed] [Google Scholar]
- 21.Dyck PJ, Thomas PK, Asbury AK, Winegrad AI, Porte D., Jr . Diabetic neuropathy. Philadelphia: WB Saunders; 1987. [Google Scholar]
- 22.Cameron N, Eaton SE, Cotter M, Tesfaye S. Vascular factors and metabolic interactions in the pathogenesis of diabetic neuropathy. Diabetalogia. 2001;44(11):1973–1988. doi: 10.1007/s001250100001. [DOI] [PubMed] [Google Scholar]
- 23.Gries FA, Cameron NE, Low PA, Ziegler D. Textbook of diabetic neuropathy. Stuttgart, Germany: Thieme; 2003. [Google Scholar]
- 24.Boulton AJ, Malik RA, Arezzo JC, Sosenko JM. Diabetic somatic neuropathies. Diabetes Care. 2004;27(6):1458–1486. doi: 10.2337/diacare.27.6.1458. [DOI] [PubMed] [Google Scholar]
- 25.Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120(1):1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vincent AM, Hinder LM, Pop-Busui R, Feldman EL. Hyperlipidemia: a new therapeutic target for diabetic neuropathy. J Peripher Nerv Syst. 2009;14(4):257–267. doi: 10.1111/j.1529-8027.2009.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dyck PJ, Lambert EH, Windebank AJ, et al. Acute hyperosmolar hyperglycemia causes axonal shrinkage and reduced nerve conduction velocity. Exp Neurol. 1981;71:507–514. doi: 10.1016/0014-4886(81)90028-5. [DOI] [PubMed] [Google Scholar]
- 28.Greene DA, Lattimer SA. Impaired rat sciatic nerve sodium-potassium adenosine triphosphatase in acute streptozotocin diabetes and its correction by dietary myo-inositol supplementation. J Clin Invest. 1983;72:1058–1063. doi: 10.1172/JCI111030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dyck PJ, Sherman WR, Hallcher LM, et al. Human diabetic endoneurial sorbitol, fructose, and myo-inositol related to sural nerve morphometry. Ann Neurol. 1980;8:590–596. doi: 10.1002/ana.410080608. [DOI] [PubMed] [Google Scholar]
- 30.Dyck PJ, Zimmerman BR, Vilen TH, et al. Nerve glucose, fructose, sorbitol, myoinositol, and fiber degeneration and regeneration in diabetic neuropathy. N Engl J Med. 1988;319:542–548. doi: 10.1056/NEJM198809013190904. [DOI] [PubMed] [Google Scholar]
- 31.Gregersen G, Bertelsen B, Harbo H. Oral supplementation of myoinositol: effects on peripheral nerve function in human diabetics and on the concentration in plasma, erythrocytes, urine and muscle tissue in human diabetics and normals. Acta Neurol Scand. 1983;67:164–172. doi: 10.1111/j.1600-0404.1983.tb04559.x. [DOI] [PubMed] [Google Scholar]
- 32.Gregersen G, Borsting H, Theil P, Servo C. Myoinositol and function of peripheral nerves in human diabetics: a controlled clinical trial. Acta neurol Scand. 1978;58:241–248. doi: 10.1111/j.1600-0404.1978.tb02884.x. [DOI] [PubMed] [Google Scholar]
- 33.Gillon KRW, Hawthorne JN, Tomlinson DR. Myo-inositol and sorbitol metabolism in relation to peripheral nerve function in experimental diabetes in the rat: the effect of aldose reductase inhibition. Diabetoligia. 1983;25:365–371. doi: 10.1007/BF00253203. [DOI] [PubMed] [Google Scholar]
- 34.Horrobin DF. Essential fatty acids in the management of impaired nerve function in diabetes. Diabetes. 1997;46(Suppl 2):S90–S93. doi: 10.2337/diab.46.2.s90. [DOI] [PubMed] [Google Scholar]
- 35.Jamal GA, Carmichael H. the effect of gamma-linolenic acid on human diabetic polyneuropathy: A double-blind placebo-controlled trial. Diabetic Med. 1990;7:319–323. doi: 10.1111/j.1464-5491.1990.tb01397.x. [DOI] [PubMed] [Google Scholar]
- 36.Keen H, Payan J, Allawi J, et al. Treatment of diabetic neuropathy with gamma-linolenic acid. Diabetes Care. 1993;16:8–15. doi: 10.2337/diacare.16.1.8. [DOI] [PubMed] [Google Scholar]
- 37.Brownlee M. Advanced protein glycosylation in diabetes and aging. Ann Rev Med. 1995;46:223–234. doi: 10.1146/annurev.med.46.1.223. [DOI] [PubMed] [Google Scholar]
- 38.Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318:1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- 39.Vlassara H. Recent progress in advanced glycation end products and diabetic complications. Diabetes. 1997;46:S19–S25. doi: 10.2337/diab.46.2.s19. [DOI] [PubMed] [Google Scholar]
- 40.Feldman EL, Russell JW, Sullivan KA, Golovoy D. New insights into the pathogenesis of diabetic neuropathy. Curr Opin Neurol. 1999;12:553–563. doi: 10.1097/00019052-199910000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Soulis-Liparota T, Cooper M, Papazoglou D, et al. Retardation by aminoguanidine of development of albuminuria, mesangial expansion, and tissue fluorescence in streptozocin-induced diabetic rat. Diabetes. 1991;40:1328–1324. doi: 10.2337/diab.40.10.1328. [DOI] [PubMed] [Google Scholar]
- 42.Williams E, Timperly WR, Ward JD, Duckworth T. Electron microscopic studies of vessels in diabetic peripheral neuropathy. J Clin Pathol. 1980;33:462–470. doi: 10.1136/jcp.33.5.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malik RA, Newrick PG, Sharma AK, et al. Microangiopathy in human diabetic neuropathy: Relationship between capillary abnormalities and the severity of neuropathy. Diabetologia. 1989;32:92–102. doi: 10.1007/BF00505180. [DOI] [PubMed] [Google Scholar]
- 44.Yasuda H, Dyck PJ. Abnormalities of endoneurial microvessels and sural nerve pathology in diabetic neuropathy. Neurology. 1987;37:20–28. doi: 10.1212/wnl.37.1.20. [DOI] [PubMed] [Google Scholar]
- 45.Giannini C, Dyck PJ. Ultrastructural morphometric features of human sural nerve endoneurial microvessels. J Neuropathol Exp Neurol. 1993;52:361–369. doi: 10.1097/00005072-199307000-00003. [DOI] [PubMed] [Google Scholar]
- 46.Giannini C, Dyck PJ. Ultrastructural morphometric abnormalities of sural nerve endoneurial microvessels in diabetes mellitus. Ann Neurol. 1994;36:408–415. doi: 10.1002/ana.410360312. [DOI] [PubMed] [Google Scholar]
- 47.Dyck PJ. Hypoxic neuropathy: Does hypoxia play a role in diabetic neuropathy? The 1988 Robert Wartenberg Lecture. Neurology. 1989;39:111–118. doi: 10.1212/wnl.39.1.111. [DOI] [PubMed] [Google Scholar]
- 48.Sugimura K, Dyck PJ. Multifocal fiber loss in proximal sciatic nerve in symmetric distal diabetic neuropathy. J Neurol Sci. 1982;53:501–509. doi: 10.1016/0022-510x(82)90246-5. [DOI] [PubMed] [Google Scholar]
- 49.Dyck PJ, Karnes JL, O'Brien P, et al. The spatial distribution of fiber loss in diabetic polyneuropathy suggests ischemia. Ann Neurol. 1986;19:440–449. doi: 10.1002/ana.410190504. [DOI] [PubMed] [Google Scholar]
- 50.Dyck PJ, Lais A, Karnes JL, et al. Fiber loss is primary and multifocal in sural nerves in diabetic polyneuropathy. Ann Neurol. 1986;19:425–439. doi: 10.1002/ana.410190503. [DOI] [PubMed] [Google Scholar]
- 51.Low PA, Tuck RR, Dyck PJ, Schmelzer JD, Yao JK. Prevention of some electrophysiologic and biochemical abnormalities with oxygen supplementation in experimental diabetic neuropathy. Proc Natl Acad Sci USA. 1984;81:6894–6898. doi: 10.1073/pnas.81.21.6894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Low PA, Schmelzer JD, Ward KK, Yao JK. Experimental chronic hypoxic neuropathy: Relevance to diabetic neuropathy. Am J Physiol. 1986;251:E94–E99. doi: 10.1152/ajpendo.1986.250.1.E94. [DOI] [PubMed] [Google Scholar]
- 53.Asbury AK, Aldredge H, Herschberg R, Fisher CM. Oculomotor palsy in diabetes mellitus: A clinicopathological study. Brain. 1970;93:555–566. doi: 10.1093/brain/93.3.555. [DOI] [PubMed] [Google Scholar]
- 54.Weber RB, Daroff RB, Mackey EA. Pathology of oculomotor nerve palsy in diabetics. Neurology. 1970;20:835–838. doi: 10.1212/wnl.20.8.835. [DOI] [PubMed] [Google Scholar]
- 55.Dreyfus PM, Hakim S, Adams RD. Diabetic ophthalmoplegia. Arch Neurol Psychiat. 1957;77:337–349. [PubMed] [Google Scholar]
- 56.Smith BE, Dyck PJ. Subclinical histopathological changes in the oculomotor nerve in diabetes mellitus. Ann Neurol. 1992;32:376–385. doi: 10.1002/ana.410320312. [DOI] [PubMed] [Google Scholar]
- 57.Raff M, Asbury AK. Ischemic mononeuropathy and mononeuropathy multiplex in diabetes mellitus. N Engl J Med. 1968;279:17–22. doi: 10.1056/NEJM196807042790104. [DOI] [PubMed] [Google Scholar]
- 58.Raff MC, Sangalang V, Asbury AK. Ishemic mononeuropathy multiplex associated with diabetes mellitus. Arch Neurol. 1968;18:487–499. doi: 10.1001/archneur.1968.00470350045004. [DOI] [PubMed] [Google Scholar]
- 59.Said G, Goulon-Goeau C, Lacroix C, Moulonguet A. Nerve biopsy findings in different patterns of proximal diabetic neuropathy. Ann Neurol. 1994;35:559–569. doi: 10.1002/ana.410350509. [DOI] [PubMed] [Google Scholar]
- 60.Said G, Elgrably F, Lacroix C, et al. Painful proximal diabetic neuropathy: inflammatory nerve lesions and spontaneous favorable outcome. Ann Neurol. 1997;41:762–770. doi: 10.1002/ana.410410612. [DOI] [PubMed] [Google Scholar]
- 61.Barohn RJ, Sahenk Z, Warmolts JR, Mendell JR. The Bruns-Garland syndrome (diabetic amyotrophy): revisited 100 years later. Arch Neurol. 1991;48:1130–1135. doi: 10.1001/archneur.1991.00530230038018. [DOI] [PubMed] [Google Scholar]
- 62.Pascoe MK, Low PA, Windebank AJ, Litchy WJ. Subacute diabetic proximal neuropathy. Mayo Clin Proc. 1997;72:1123–1132. doi: 10.4065/72.12.1123. [DOI] [PubMed] [Google Scholar]
- 63.Dyck PJB, Novell JE, Dyck PJ. Microvasculitis and ischemia in diabetic lumbosacral radiculopexus neuropathy. Neurology. 1999;53:2113–2121. doi: 10.1212/wnl.53.9.2113. [DOI] [PubMed] [Google Scholar]
- 64.Theriault M, Dort J, Sutherland G, Zochodne D. Local human sural nerve blood flow in diabetic and other polyneuropathies. Brain. 1997;120:1131–1138. doi: 10.1093/brain/120.7.1131. [DOI] [PubMed] [Google Scholar]
- 65.Rosoklija GB, Dwork AJ, Younger DS, Karlikaya G, Latov N, Hays AP. Local activation of the complement system in endoneurial microvessels of diabetic neuropathy. Acta Neuropathol. 2000;99(1):55–62. doi: 10.1007/pl00007406. [DOI] [PubMed] [Google Scholar]
- 66.Dyck PJ, Windebank AJ. Diabetic and nondiabetic lumbosacral radiculoplexus neuropathies: new insights into pathophysiology and treatment. Muscle Nerve. 2002;25(4):477–491. doi: 10.1002/mus.10080. [DOI] [PubMed] [Google Scholar]
- 67.Krendel DA, Costigan DA, Hopkins LC. Successful treatment of neuropathies in patients with diabetes mellitus. Arch Neurol. 1995;52:1053–1061. doi: 10.1001/archneur.1995.00540350039015. [DOI] [PubMed] [Google Scholar]
- 68.Younger DS, Rosoklija G, Hays AP, et al. Diabetic peripheral neuropathy: a clinicopathologic and immunohistochemical analysis of sural nerve biopsies. Muscle Nerve. 1996;19:722–727. doi: 10.1002/(SICI)1097-4598(199606)19:6<722::AID-MUS6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 69.Ballin RHM, Thomas PK. Hypertrophic changes in diabetic neuropathy. Acta Neuropathol (Berl) 1968;11:93–102. doi: 10.1007/BF00690213. [DOI] [PubMed] [Google Scholar]
- 70.Vital C, Vallat JM, LeBlanc M, Martin F, Coquet M. Peripheral neuropathies caused by diabetes mellitus. Ultrastructural study of 12 biopsied cases. J Neurol Sci. 1973;18:381–398. doi: 10.1016/0022-510x(73)90133-0. [DOI] [PubMed] [Google Scholar]
- 71.Vital C, LeBlanc M, Vallat JM, Cocquet M, Vallat M, Roques JC. Ultrastructural study of peripheral nerve in 16 diabetics without neuropathy. Comparisons with 16 diabetic neuropathies and 16 non-diabetic neuropathies. Acta Neuropathol (Berl) 1974;30:63–72. doi: 10.1007/BF00685323. [DOI] [PubMed] [Google Scholar]
- 72.Said G, Slama G, Salva J. Progressive centripetal degeneration of axons in small fibre diabetic polyneuropathy. Brain. 1983;106:791–807. doi: 10.1093/brain/106.4.791. [DOI] [PubMed] [Google Scholar]
- 73.Said G, Goulon-Goeau C, Slama G, Tchobroutsky G. Severe early-onset polyneuropathy in insulin-dependent diabetes mellitus. A clinical pathological study. N Engl J Med. 1992;326:1257–1263. doi: 10.1056/NEJM199205073261905. [DOI] [PubMed] [Google Scholar]
- 74.Dyck PJ, Karnes JL, Daube J, O'Brien P, Service FJ. Clinical and neuropathological criteria for the diagnosis and staging of diabetic polyneuropathy. Brain. 1985;108:861–880. doi: 10.1093/brain/108.4.861. [DOI] [PubMed] [Google Scholar]
- 75.Claus D, Mustafa C, Vogel W, Herz M, Neundorfer B. Assessment of diabetic neuropathy: Definition of norm and discrimination of abnormal nerve function. Muscle Nerve. 1993;16:757–768. doi: 10.1002/mus.880160711. [DOI] [PubMed] [Google Scholar]
- 76.Albers JW, Brown MB, Sima AA, Greene DA. Nerve conduction measures in mild diabetic neuropathy in the Early Diabetes Intervention Trial: the effects of age, sex, type of diabetes, disease duration, and anthropometric factors. Tolrestat Study Group for the Early Diabetes Intervention Trial. Neurology. 1996;46:85–91. doi: 10.1212/wnl.46.1.85. [DOI] [PubMed] [Google Scholar]
- 77.Behse F, Buchthal F, Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977;40:1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Research criteria for diagnosis of chronic inflammatory demyelinating polyneuropathy (CIDP) Neurology. 1991;41:617–618. [PubMed] [Google Scholar]
- 79.Cornblath DR, Drach DB, Griffin JW. Demyelinating motor neuropathy in patients with diabetic polyneuropathy. Ann Neurol. 1987;22:126S. (abstract). [Google Scholar]
- 80.Stewart JD, McKelvey R, Durcan L, Carpenter S, Karpati G. Chronic inflammatory demyelinating polyneuropathy (CIDP) in diabetics. J Neurol Sci. 1996;142:59–64. doi: 10.1016/0022-510x(96)00126-8. [DOI] [PubMed] [Google Scholar]
- 81.Uncini A, De Angelis MV, Di Muzio A, et al. Chronic inflammatory demyelinating polyneuropathy in diabetics: motor conductions are important in the differential diagnosis with diabetic polyneuropathy. Clin Neurophys. 1999;110:705–711. doi: 10.1016/s1388-2457(98)00028-5. [DOI] [PubMed] [Google Scholar]
- 82.Laughlin RS, Dyck PJ, Melton LJ, et al. Neurology. 2009 Jul 7;73(1):39–45. doi: 10.1212/WNL.0b013e3181aaea47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Jaradeh SS, Prieto TE, Lobeck LJ. Progressive polyradiculoneuropathy in diabetes: correlation of variables and clinical outcome after immunotherapy. J Neurol Neurosurg Psychiatry. 1999 Nov;67(5):607–612. doi: 10.1136/jnnp.67.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pasnoor M, Herbelin L, Johnson M, Ryals J, Dimachkie M, Wright D, Barohn R. Clinical electrophysiologic and skin biopsy findings in cryptogenic sensorimotor polyneuropathy compared to diabetic polyneuropathy and normal controls. Neurology. 2009;72(Suppl 3):A217. [Google Scholar]
- 85.Boulton AJM, Knight G, Drury J, Ward JD. The prevalence of symptomatic diabetic neuropathy in an insulin-treated population. Diabetes Care. 1985;8:125–128. doi: 10.2337/diacare.8.2.125. [DOI] [PubMed] [Google Scholar]
- 86.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006 Jul;29(7):1518–1522. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 87.Waxman SG, Sabin TD. Diabetic truncal polyneuropathy. Arch Neurol. 1981;38:46–47. doi: 10.1001/archneur.1981.00510010072013. [DOI] [PubMed] [Google Scholar]
- 88.Dyck PJ, Davies JL, Litchy WJ, O'Brien PC. Longitudinal assessment of diabetic polyneuropathy using a composite score in the Rochester Diabetic Neuropathy Study cohort. Neurology. 1997;49:229–239. doi: 10.1212/wnl.49.1.229. [DOI] [PubMed] [Google Scholar]
- 89.Feldman E, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 90.Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, Howard J, Smith AG. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. Journal of the Peripheral Nervous System. 2008;13:218–227. doi: 10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- 91.England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of laboratory and genetic testing (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009a;72(2):185–192. doi: 10.1212/01.wnl.0000336370.51010.a1. [DOI] [PubMed] [Google Scholar]
- 92.England JD, Gronseth GS, Franklin G, et al. Practice Parameter: evaluation of distal symmetric polyneuropathy: role of autonomic testing, nerve biopsy, and skin biopsy (an evidence-based review). Report of the American Academy of Neurology, American Association of Neuromuscular and Electrodiagnostic Medicine, and American Academy of Physical Medicine and Rehabilitation. Neurology. 2009b;72(2):177–184. doi: 10.1212/01.wnl.0000336345.70511.0f. [DOI] [PubMed] [Google Scholar]
- 93.Bril V, England J, Franklin GM, Backonja M, Cohen J, Del Toro D, Feldman E, Iverson DJ, Perkins B, Russell JW, Zochodne D. Evidence-based guideline: treatment of painful diabetic neuropathy. Report of the American Academy of Neurology, the American Association of Neuromuscular and Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2011 May 17;76(20):1758–1765. doi: 10.1212/WNL.0b013e3182166ebe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vinik AI, Mehrabyan A. Diabetic neuropathies. Med Clin North Am. 2004;88(4):947–999. doi: 10.1016/j.mcna.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 95.Dyck PJ, Karnes JL, O'Brien PC, Litchy WJ, Low PA, Melton LJD. The Rochester Diabetic Neuropathy Study: Reassessment of tests and criteria for diagnosis and staged severity. Neurology. 1992;42:1164–1170. doi: 10.1212/wnl.42.6.1164. [DOI] [PubMed] [Google Scholar]
- 96.Peripheral Nerve Society. Diabetic polyneuropathy in controlled clinical trials: consensus report of the peripheral nerve society. Ann Neurol. 1995;38:478–482. doi: 10.1002/ana.410380323. [DOI] [PubMed] [Google Scholar]
- 97.Thomas PK, Brown MJ. Diabetic polyneuropathy. In: Dyck PJ, Thomas PK, Asbury AK, Winegrad AI, Porte D, editors. Diabetic Neuropathy. Philadelphia: W.B. Saunders; 1987. pp. 56–65. [Google Scholar]
- 98.Service FJ, Rizza RA, Daube JR, O'Brien PC, Dyck PJ. Near normoglycemia improved nerve conduction and vibration sensation in diabetic neuropathy. Diabetologia. 1985;28:722–727. doi: 10.1007/BF00265018. [DOI] [PubMed] [Google Scholar]
- 99.The Diabetes Control and Complication Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 100.Warmolts JR, Mendell JR, O'Dorisio TM, Cataland S. Comparison of the effects of continuous subcutaneous infusion and split-mixed injection of insulin on nerve function in type I diabetes mellitus. J Neurol Sci. 1987;82:161–169. doi: 10.1016/0022-510x(87)90015-3. [DOI] [PubMed] [Google Scholar]
- 101.Albers JW, Herman WH, Pop-Busui R, Feldman EL, Martin CL, Cleary PA, Waberski BH, Lachin JM. Diabetes Control and Complications Trial /Epidemiology of Diabetes Interventions and Complications Research Group. Effect of prior intensive insulin treatment during the Diabetes Control and Complications Trial (DCCT) on peripheral neuropathy in type 1 diabetes during the Epidemiology of Diabetes Interventions and Complications (EDIC) Study. Diabetes Care. 2010 May 33;(5):1090–1096. doi: 10.2337/dc09-1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lewin IG, O'Brien IAD, Morgan MG, Corall RJM. Clinical and neurophysiological studies with the aldose reductase inhibitor, sorbinil, in symptomatic diabetic neuropathy. Diabetilogia. 1984;26:445–448. doi: 10.1007/BF00262218. [DOI] [PubMed] [Google Scholar]
- 103.Sundkvist G, Armstrong FM, Bradbury JE, et al. Peripheral and autonomic nerve function in 259 diabetic patients with peripheral neuropathy treated with ponalrestat (an aldose reductase inhibitor) or placebo for 18 months: United Kingdom/Scandinavian Ponalrestat Trial. J Diabetes Complications. 1992;6:123–130. doi: 10.1016/1056-8727(92)90023-e. [DOI] [PubMed] [Google Scholar]
- 104.Pfeifer MA, Schumer MP, Gelber DA. Aldose reductase inhibitors: the end of an era or the need for different trial designs? Diabetes. 1997;46(Suppl 2):S82–S89. doi: 10.2337/diab.46.2.s82. [DOI] [PubMed] [Google Scholar]
- 105.Packer L, Roy S, Sen CK. Alpha-lipoic acid: a metabolic antioxidant and potential redox modulator of transcription. Adv Pharmacol. 1997;38:79–101. doi: 10.1016/s1054-3589(08)60980-1. [DOI] [PubMed] [Google Scholar]
- 106.Nagamatsu M, Nickander KK, Schmelzer JD, et al. Lipoic acid improves nerve blood flow, reduces oxidative stress, and improves distal nerve conduction in experimental diabetic neuropathy. Diabetes Care. 1995;18:1160–1167. doi: 10.2337/diacare.18.8.1160. [DOI] [PubMed] [Google Scholar]
- 107.Ziegler D, Hanefeld M, Ruhnau KJ, et al. Treatment of symptomatic diabetic peripheral neuropathy with the anti-oxidant alpha-lipoic acid. A 3-week multicenter randomized controlled trial (ALADIN Study) Diabetologia. 1995;38:1425–1433. doi: 10.1007/BF00400603. [DOI] [PubMed] [Google Scholar]
- 108.Ziegler D, Ametov A, Barinov A, Dyck PJ, Gurieva I, Low PA, Munzel U, Yakhno N, Raz I, Novosadova M, Maus J, Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006 Nov;29(11):2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 109.Ziegler D, Low PA, Litchy WJ, Boulton AJ, Vinik AI, Freeman R, Samigullin R, Tritschler H, Munzel U, Maus J, Schütte K, Dyck PJ. Efficacy and safety of antioxidant treatment with α-lipoic acid over 4 years in diabetic polyneuropathy: the NATHAN 1 trial. Diabetes Care. 2011 Sep;34(9):2054–2060. doi: 10.2337/dc11-0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Apfel SC, Kessler JA, Adornato BT, et al. Recombinant human nerve growth factor in the treatment of diabetic polyneuropathy. Neurology. 1998;51:695–702. doi: 10.1212/wnl.51.3.695. [DOI] [PubMed] [Google Scholar]
- 111.Fernyhough P, Diemel LT, Brewster JW, Tomlinson DR. Altered neurotrophin mRNA levels in peripheral nerve and skeletal muscle of experimentally diabetic rats. J Neurochem. 1995;64:1231–1237. doi: 10.1046/j.1471-4159.1995.64031231.x. [DOI] [PubMed] [Google Scholar]
- 112.Rodriquez-Pena A, Botana M, Gonzalez M, Requejo F. Expression of neurotrophins and their receptors in sciatic nerve of experimentally diabetic rats. Neurosci Lett. 1995;200:37–40. doi: 10.1016/0304-3940(95)12067-e. [DOI] [PubMed] [Google Scholar]
- 113.Fernyhough P, Diemel LT, Trewster WJ, et al. Deficits in sciatic nerve neuropeptide content coincide with a reduction in target tissue nerve growth factor messenger RNA in streptozocin-diabetic rats: effects of insulin treatment. Neuroscience. 1994;62:337–344. doi: 10.1016/0306-4522(94)90368-9. [DOI] [PubMed] [Google Scholar]
- 114.Apfel SC, Arezzo JC, Brownlee M, et al. Nerve growth factor administration protects against experimental diabetic sensory neuropathy. Brain Res. 1994;634:7–12. doi: 10.1016/0006-8993(94)90252-6. [DOI] [PubMed] [Google Scholar]
- 115.Hellweg R, Vavich G, Hartung HD, et al. Axonal transport of endogenous nerve growth factor (NGF) and NGF receptor in experimental diabetic neuropathy. Exp Neurol. 1994;130:24–30. doi: 10.1006/exnr.1994.1181. [DOI] [PubMed] [Google Scholar]
- 116.Anand P, Terenghi G, Warner G, Kopelman P, et al. The role of endogenous nerve growth factor in human diabetic neuropathy. Nature Med. 1996;2:703–707. doi: 10.1038/nm0696-703. [DOI] [PubMed] [Google Scholar]
- 117.Max MB, Lynch SA, Muir J, Shoaf SE, Smoller B, Dubner R. Effects of desipramine, amitriptyline, and fluoxetine on pain in diabetic neuropathy. N Engl J Med. 1992;326:1250–1256. doi: 10.1056/NEJM199205073261904. [DOI] [PubMed] [Google Scholar]
- 118.Kvinesdal B, Molin J, Froland A, Gram L. Imipramine treatment of painful diabetic neuropathy. JAMA. 1984;251:1727–1730. [PubMed] [Google Scholar]
- 119.Mendel CM, Klein RF, Chappell DA, Dere W, et al. A trial of amitriptyline and fluphenazine in the treatment of painful diabetic neuropathy. JAMA. 1986;255:637–639. [PubMed] [Google Scholar]
- 120.Dejgard A, Petersen P, Kastrup J. Mexiletine for treatment of chronic painful diabetic neuropathy. Lancet. 1988;1:9–11. doi: 10.1016/s0140-6736(88)90999-3. [DOI] [PubMed] [Google Scholar]
- 121.Backonja M, Beydoun A, Edwards KR, et al. Gabapentin for the symptomatic treatment of painful neuropathy in patients with diabetes mellitus: a randomized controlled trial. JAMA. 1998;280:1831–1836. doi: 10.1001/jama.280.21.1831. [DOI] [PubMed] [Google Scholar]
- 122.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–638. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 123.Harati Y, Gooch C, Swenson M, et al. Double-blind randomized trial of tramadol for the treatment of the pain of diabetic neuropathy. Neurology. 1998;50:1842–1846. doi: 10.1212/wnl.50.6.1842. [DOI] [PubMed] [Google Scholar]
- 124.Nelson KA, Park KM, Robinovitz E, et al. High-dose oral dextromethorphan versus placebo in painful diabetic neuropathy and postherpetic neuralgia. Neurology. 1997;48:1212–1218. doi: 10.1212/wnl.48.5.1212. [DOI] [PubMed] [Google Scholar]
- 125.Lesser H, Sharma U, LaMoreaux L, Poole RM. Pregabalin relieves symptoms of painful diabetic neuropathy: a randomized controlled trial. Neurology. 2004;63:2104–2110. doi: 10.1212/01.wnl.0000145767.36287.a1. [DOI] [PubMed] [Google Scholar]
- 126.Ross DR, Varipara RJ. Treatment of painful diabetic neuropathy with topical capsaicin. N Engl J Med. 1989;321:474–475. [PubMed] [Google Scholar]
- 127.The Capsaicin Study Group. Treatment of painful diabetic neuropathy with topical capsaicin: a multi-center, double-blind, vehicle controlled study. Arch Int Med. 1991;151:2225–2229. doi: 10.1001/archinte.151.11.2225. [DOI] [PubMed] [Google Scholar]
- 128.Rowbotham MC, Davies PS, Verkempnick, et al. Lidocaine patch: a double-blind controlled study of a new treatment method for postherpetic neuralgia. Pain. 1996;65(1):39–44. doi: 10.1016/0304-3959(95)00146-8. [DOI] [PubMed] [Google Scholar]
- 129.Galer BS, Rowbotham MC, Pevander J, et al. Topical lidocaine patch relieves postherpetic neuraliga more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain. 1999;80(3):533–538. doi: 10.1016/S0304-3959(98)00244-9. [DOI] [PubMed] [Google Scholar]
- 130.Weintraub MI, Wolfe GI, Barohn RA, Cole SP, Parry GJ, Hayat G, Cohen JA, Page JC, Bromberg MB, Schwartz SL. Magnetic Research Group. Static magnetic field therapy for symptomatic diabetic neuropathy: a randomized, double-blind, placebo-controlled trial. Arch Phys Med Rehabil. 2003 May;84(5):736–746. doi: 10.1016/s0003-9993(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 131.Weintraub MI. Magnetic bio-stimulation in painful diabetic peripheral neuropathy: a novel intervention - A randomized, double-placebo crossover study. Am J Pain Management. 1999;9:8–17. [Google Scholar]
- 132.Weintraub MI, Herrmann DN, Smith AG, Backonja MM, Cole SP. Pulsed electromagnetic fields to reduce diabetic neuropathic pain and stimulate neuronal repair: a randomized controlled trial. Arch Phys Med Rehabil. 2009 Jul;90(7):1102–1109. doi: 10.1016/j.apmr.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 133.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, Sharma NK, Wright DE. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complications. 2012 Sep-Oct;26(5):424–429. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Malcolm A, Camilleri M. Treatment of diabetic gastroparesis and diarrhea. In: Dyck PJ, Thomas PK, editors. Diabetic neuropathy. 2nd ed. Philadelphia: WB Saunders; 1999. pp. 517–529. [Google Scholar]
- 135.Robertson D, Davis TL. Recent advances in the treatment of orthostatic hypotension. Neurology. 1995;45(suppl 5):S26–S32. [PubMed] [Google Scholar]
- 136.Jankovic J, Gilden JL, Hiner BC, et al. Neurogenic orthostatic hypotension: a doubleblind placebo-controlled study with midodrine. Am J Med. 1993;95:34–48. doi: 10.1016/0002-9343(93)90230-m. [DOI] [PubMed] [Google Scholar]
- 137.Low PA, Gilden JL, Freeman R, et al. Efficacy of midodrine vs placebo in neurogenic orthostatic hypotension. A randomized, double-blind multicenter study. JAMA. 1997;227:1046–1051. [PubMed] [Google Scholar]
- 138.Snape WJ, Jr, Battle WM, Schwartz SS, Braunstein SN, et al. Metoclopramide to treat gastroparesis due to diabetes mellitus: a double-blind, controlled trial. Ann Intern Med. 1982;96:444–446. doi: 10.7326/0003-4819-96-4-444. [DOI] [PubMed] [Google Scholar]
- 139.Janssens J, Peeters TL, Vantrappen G, et al. Improvement of gastric emptying in diabetic gastroparesis by erythromycin: preliminary studies. N Engl J Med. 1990;332:1028–1031. doi: 10.1056/NEJM199004123221502. [DOI] [PubMed] [Google Scholar]
- 140.Fedorak RN, Field M, Chang EB. Treatment of diabetic diarrhea with clonidine. Ann Intern Med. 1985;102:197–199. doi: 10.7326/0003-4819-102-2-197. [DOI] [PubMed] [Google Scholar]
- 141.Goldstein I, Lue TF, Padma-Nathan H, et al. Oral sildenafil in the treatment of erectile dysfunction. N Engl J Med. 1998;338:1397–1404. doi: 10.1056/NEJM199805143382001. [DOI] [PubMed] [Google Scholar]
- 142.Gasser TC, Roach RM, Larsen EH, et al. Intracavernous self-injection with phentolamine and papaverine for the treatment of impotence. J Urol. 1987;137:678–680. doi: 10.1016/s0022-5347(17)44172-3. [DOI] [PubMed] [Google Scholar]
- 143.Scott FB, Fishman IJ, Light JK. An inflatable penile prosthesis for treatment of diabetic impotence. Ann Intern Med. 1980;92:340–342. doi: 10.7326/0003-4819-92-2-340. [DOI] [PubMed] [Google Scholar]
- 144.Ellenberg M. Diabetic neuropathic cachexia. Diabetes. 1974;23:418–423. doi: 10.2337/diab.23.5.418. [DOI] [PubMed] [Google Scholar]
- 145.Jackson CE, Barohn RJ. Diabetic neuropathic cachexia: report of a recurrent case. J Neurol Neurosurg Psychiatry. 1998;64:785–787. doi: 10.1136/jnnp.64.6.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Archer AG, Watkins PJ, Thomas PK, Sharma AK, Payan J. The natural history of acute painful neuropathy in diabetes mellitus. J Neurol Neurosurg Psychiat. 1983;46:491–499. doi: 10.1136/jnnp.46.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Gade GN, Hofeldt FD, Treece GL. Diabetic neuropathic cachexia: beneficial response to combination therapy with amitriptyline and fluphenazine. JAMA. 1980;243:1160–1161. doi: 10.1001/jama.243.11.1160. [DOI] [PubMed] [Google Scholar]
- 148.Blau RH. Diabetic neuropathic cachexia: report of a woman with this syndrome and review of the literature. Arch Intern Med. 1983;143:2011–2012. doi: 10.1001/archinte.143.10.2011. [DOI] [PubMed] [Google Scholar]
- 149.Wright DL, Shah JH. Diabetic neuropathic cachexia and hypothyroidism in a woman. Mo Med. 1987;84:143–145. [PubMed] [Google Scholar]
- 150.D'Costa DF, Price DE, Burden AC. Diabetic Neuropathic Cachexia Associated with Malabsorption. Diabetic Medicine. 1992;9:203–205. doi: 10.1111/j.1464-5491.1992.tb01758.x. [DOI] [PubMed] [Google Scholar]
- 151.Caravati CM. Insulin neuritis: a case report. Va Med Mon. 1933;59:745–746. [Google Scholar]
- 152.Llewelyn JG, Thomas PK, Fonseca V, et al. Acute painful diabetic neuropathy precipitated by strict glycaemic control. Acta Neuropathol (Berl) 1986;72:157–163. doi: 10.1007/BF00685978. [DOI] [PubMed] [Google Scholar]
- 153.Gibbons CH, Freeman R. Treatment-induced diabetic neuropathy: a reversible painful autonomic neuropathy. Ann Neurol. 2010;67(4):534–541. doi: 10.1002/ana.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]