Abstract
Targeting angiogenesis in glioblastoma (GBM) may improve patient outcome by normalizing tumor vasculature and improving delivery of chemotherapeutics and oxygen. Consequently, concomitant administration of small molecule inhibitors of the VEGF pathway will likely have a positive impact on chemoradiation treatment outcome. We conducted a Phase I study of vatalanib, a small molecule inhibitor of VEGFR, PDGFR, and c-kit in patients with newly diagnosed GBM receiving radiation, temozolomide, and an enzyme-inducing anti-epileptic drug in order to determine the MTD of vatalanib in this patient population. We incorporated circulating biomarker and SNP analyses and pharmacokinetic studies. Nineteen patients were enrolled and the MTD was not reached at the time of study termination. Vatalanib was well tolerated with only 2 DLTs (thrombocytopenia and elevated transaminases). Other grade 3/4 toxicities included leukopenia, lymphopenia, neutropenia, and hand-foot syndrome. There were no wound-healing complications. Of the 13 patients evaluable for a radiographic response, 2 had a partial response and 9 had stable disease. Vatalanib significantly increased PlGF and sVEGFR1 in plasma circulation and decreased sVEGFR2 and sTie2. Plasma collagen IV increased significantly by day 50 of treatment. Vatalanib was well tolerated and this study demonstrates the safety of oral small molecule inhibitors in newly diagnosed GBM patients. Blood biomarkers may be useful as pharmacodynamic markers of response to anti-angiogenic therapies.
Keywords: Angiogenesis, Biomarkers, Clinical trial, Glioblastoma, Treatment
Introduction
Despite aggressive treatment with maximal safe surgery, radiation, and chemotherapy, glioblastomas (GBM) remain one of the most challenging solid tumors to treat. These tumors are characterized by an abnormal tumor vasculature that contributes to treatment resistance by promoting tumor hypoxia and elevating interstitial fluid pressure. Vascular endothelial growth factor (VEGF) is one of the principal mediators of this abnormal vasculature and is thus an attractive therapeutic target in GBM patients [1]. Based on glioma mouse models, blocking VEGF is hypothesized to normalize the abnormal tumor vasculature [2, 3]. Normalization improves tumor blood flow and, thus, delivery of chemotherapeutics and oxygen needed for radiation and chemotherapy to be maximally effective. Consequently, combining cytotoxics with anti-angiogenic agents holds promise for improving GBM response to therapy and is currently being evaluated in phase III trials.
Vatalanib (PTK787) is a small molecule tyrosine kinase inhibitor of all 3 VEGF receptors (VEGFR1, -2, and -3), platelet derived growth factor receptor-β (PDGFR-β), and c-kit [4]. The half life of vatalanib is 4–6 h so it should be administered at least twice daily. In a Phase I monotherapy study involving patients with a variety of advanced cancers, the maximal tolerated dose was determined to be 750 mg twice a day and a total dose of ≥1000 mg daily was biologically active [5].
Pre-clinical and clinical studies of vatalanib in glioma demonstrated promising results. Studies involving C6 glioma cell lines that expressed VEGF demonstrated that vatalanib treatment led to tumor regression and increased tumor necrosis [6]. In a Phase I/II trial of recurrent GBM patients treated with single agent vatalanib, 2/47 patients achieved a partial response and 31/47 patients achieved stable disease [7]. The drug was well tolerated with dose limiting toxicities only observed in patients taking ≥1000 mg daily. Vatalanib was also associated with promising extension of time to tumor progression in recurrent GBM when combined with temozolomide or lomustine [8]. Due to concern over the effects of enzyme inducing anti-epileptic drugs (EIAEDs) on vatalanib metabolism, a phase I study of vatalanib in combination with imatinib, a PDGFR inhibitor, and hydroxyurea, a ribonucleoside reductase inhibitor, was conducted in patients with recurrent GBM stratified based on EIAED use [9]. The MTD in this study was 1000 mg daily in both cohorts but EIAEDs decreased vatalanib plasma exposure.
Based on these results in recurrent GBM, we conducted a phase I clinical trial in patients with newly diagnosed GBM who were taking EIAEDs in order to determine the MTD of vatalanib with radiation and temozolomide in this patient population. We incorporated correlative blood biomarker studies to determine the impact of vatalanib on angiogenic signaling pathways as well as pharmacokinetic analyses to determine serum drug levels. Prior studies of anti-angiogenic agents have suggested that several biomarkers (ex. VEGF, PlGF, MMP-10, sVEGFR2, sTie2) change significantly and reversibly after VEGF blockade and can serve as potential pharmacodynamic biomarkers for response with this class of drugs [10–15].
Patients and methods
Study population and patient eligibility
After receiving approval by the local institutional review board (IRB), we conducted a single center, Phase I study of vatalanib in patients with newly diagnosed GBM who were taking EIAEDs. All patients signed an IRB-approved informed consent form prior to enrollment. Inclusion criteria for patients included pathological diagnosis of GBM; age ≥18 years; Karnofsky Performance Score (KPS) ≥ 60; Mini-Mental Status Examination (MMSE) score ≥15; adequate bone marrow function (absolute neutrophil count ≥1500/mm3; hemoglobin ≥ 8 g/dl; platelet count ≥100,000/mm3); serum creatinine and bilirubin ≤1.5 9 institutional upper normal limit; and liver transaminases ≤3.0 × institutional upper normal limit. Exclusion criteria included concurrent use of anti-coagulants; prolonged mean corrected QT interval or patients with a history of familial prolonged QT syndrome; pregnancy or breast feeding; history of uncontrolled hypertension or other serious medical illnesses including, but not limited to, unstable angina, arrhythmia, symptomatic congestive heart failure, active infection; infection with the human immunodeficiency virus; imaging evidence of intratumoral or intracerebral hemorrhage deemed significant by the treating physician; chronic proton pump inhibitor or H2 antagonist use.
Treatment plan
The goal of this Phase I study was to determine the optimal dose of vatalanib in combination with radiation and temozolomide in patients receiving EIAEDs. Patients were treated in cohorts of 3 at a starting dose of 250 mg once daily in the morning. Subsequent planned dose levels were 250 mg twice daily, 500 mg twice daily, 750 mg twice daily and 1000 mg twice daily. Patients started vatalanib 5 days prior to concomitant radiation and temozolomide and vatalanib was continued daily during chemoradiation. Following chemoradiation, patients completed 28 days of vatalanib monotherapy at the same dose as during chemoradiation (first post-radiation cycle of vatalanib). In conjunction with monthly temozolomide (150–200 mg/m2 for 5 consecutive days), vatalanib was administered at 750 mg twice daily for all patients in 28 day cycles. The original protocol was written to stop temozolomide after 6 post-radiation cycles but was amended to allow for 12 cycles of post-radiation temozolomide. Patients remained on treatment with vatalanib until tumor progression, patient withdrawal, or development of unacceptable toxicity.
Patient monitoring and toxicity assessment
All patients were monitored by physical examination, laboratory tests, and contrast-enhanced MRI scans performed at baseline, 4 weeks after the completion of chemoradiation, and then prior to every odd cycle thereafter. Toxicity was graded according to CTCAEv3.0. Hemato-logic dose limiting toxicity (DLT) included grade 4 neutropenia, ≥grade 3 thrombocytopenia, or treatment delay >7 days due to hematologic toxicity. DLT due to nonhematologic toxicity included serum creatinine ≥2.0 × upper limit of normal, ≥grade 2 hypertension or hematuria, or clinically significant grade 3 or greater adverse events attributed to vatalanib. The maximal tolerated dose was defined as the dose level at which 33% of patients experienced a DLT. Response to treatment was determined by neurological examination and MRI scans using Macdonald criteria [16]. The time period over which DLTs were determined was 7 weeks.
Circulating biomarker evaluations
Peripheral blood was obtained from patients prior to therapy then 8 h, 1 day, 2 days, 9 days, 50 days, and 4 weeks after completion of radiation. Circulating cell populations were isolated by flow cytometry using CD31, CD34, CD45 and CD133 as markers per a previously published protocol [17]. Plasma analysis was conducted for circulating VEGF, PlGF, soluble VEGFR1, bFGF, IL-1β, IL-6, IL-8, TNF-α collagen IV, MMP1, MMP2, MMP3, MMP9, and MMP-10 using multiplex ELISA plates from Meso-Scale Discovery (Gaithersburg, MD) as well as soluble VEGFR2, SDF1a, and sTie2 from R&D System (Minneapolis, MN). Every sample was run in duplicate.
Pharmacokinetic assessments
Blood samples for drug concentration were collected in a subset of patients in heparinized tubes at time 0 (pre-dose), 1 h, 2 h, 3 h, 4 h, 6 h, 8 h, and 24 h on day 14 or later, i.e. once steady state had been reached. Plasma was prepared by centrifugation and frozen at −18°C. Plasma concentrations of vatalanib were determined with high performance liquid chromatography by AAIPharma (Shawnee, Kansas). The assay was valid over the range of 5.0–5,000 ng/ml. Pharmacokinetic parameters were determined from plasma concentration versus time data using standard noncompartmental methods.
SNP analysis
Candidate genes were chosen because of their role in VEGF and VEGF-independent angiogenesis and from published reports of candidate gene or expression studies. Genomic DNA was extracted from peripheral WBC using the QIAamp kit (Qiagen). Multiplex PCR assays are designed using Sequenom SpectroDESIGNER software (version 3.0.0.3) by inputting sequence containing the SNP site and 100 bp of flanking sequence on either side of the SNP. The SNPs are grouped into multiplexes so that the extended product does not overlap in mass with any other oligonucleotide present in the reaction mix, and where no primer–primer, primer–product non-specific interactions will occur. The PCR is carried out in 384-well reaction plates in a volume of 5 ll using 10 ng genomic or WGA DNA. All subsequent steps, up until the reaction is spotted onto the SpectroCHIP, are carried out in the same reaction plate. After PCR, any unincorporated dNTPs from the PCR are removed from the reaction by digestion with Shrimp alkaline phosphatase. The dNTPs should be removed so that they cannot play any role in the extension of the extension oligonucleotide at the SNP site. The extension reaction is carried out in the presence of the extension oligonucleotide and a termination mix containing mass modified dideoxynucleotides which will extend the oligo-nucleotide over the SNP site with one base. Before spotting onto the SpectroCHIP, the reaction was cleaned by incubation with Clean Resin (Sequenom), a cation exchange resin, which removes any salts present. The extension product was then spotted onto a 384 well spectroCHIP before being flown in the MALDI-TOF mass spectrometer. Data was collected in real time using SpectroTYPER Analyzer 4.0.3.18, SpectraAQUIRE 3.3.1.1 and Spectro CALLER 3.3.0.14 (Sequenom).
Statistical analysis
Given the small sample size in this phase I study, there was limited power to assess the correlation of the blood bio-marker studies and survival. As exploratory analyses, we fit Cox regression models with overall survival or progression-free survival as the endpoints and the biomarker measures as continuous predictors. A variable indicating dose cohort was included in the model to adjust for dose. The Kaplan–Meier estimate was used to compute the survival distribution. Differences in blood biomarker levels on each treatment day were compared to baseline (day-1) using the Signed Rank Sum test or ANOVA when dose cohort was included. Bio-markers were log transformed for analyses. Correction for multiple testing was not done because of the predominantly exploratory nature of this analysis.
Results
Patient characteristics
Nineteen patients with newly diagnosed glioblastoma were enrolled in the study from December 2006 to December 2008 (Table 1). Eight of the 19 enrolled patients had only a tumor biopsy prior to study treatment. Seven patients were enrolled at 250 mg daily, 6 patients at 250 mg twice daily, and 6 patients at 500 mg twice daily. One patient was excluded from any analyses after withdrawing consent prior to taking any vatalanib. A separate patient withdrew consent after taking only 1 dose of vatalanib so was only included in the toxicity assessment. An additional 2 patients did not complete combination radiation, temozolomide, and vatalanib because of disease progression (1), and pulmonary embolus (1). The median number of post-chemoradiation cycles of vatalanib was 3 (range 1–14). No patients remain on treatment.
Table 1.
Patient characteristics
| Analyzable patientsa | 18 |
| Age (median) | 58 (29–77) |
| KPS (median) | 90 (70–100) |
| Extent of surgery | GTR (4) |
| STR (6) | |
| Biopsy (8) | |
| MMSE (median) | 28 (17–30) |
| Gender | Men (13) |
| Women (5) | |
| Number of deaths | 12 |
| Median number of post-RT cycles of vatalanib | 3 (1–14) |
| Median follow-up | 14.9 mo |
| mPFS (months)b | 7.2 |
| mOS (months)b | 19.6 |
One patient withdrew consent prior to starting vatalanib
Includes all patients who took >1 dose (17 total)
Safety and tolerability
There were 2 dose limiting toxicities (Table 2). One patient experienced thrombocytopenia during chemoradiation while on 250 mg daily of vatalanib and a second patient experienced elevated transaminases during chemoradiation at 500 mg twice daily of vatalanib. Other Grade 3–4 toxicities possibly, probably, or definitely, related to vatalanib were reported in 5/18 patients (Table 2). These included: elevated transaminases (1), leukopenia (1), neutropenia (1), lymphopenia (1), and hand-foot syndrome (1). Reasons patients were removed from the study are listed in Table 2. Three patients required dose reduction for elevated transaminases (2) and hand-foot syndrome (1).
Table 2.
Toxicities associated with vatalanib with chemoradiation in GBM patients
| Dose limiting toxicities (# pts) | Elevated transaminases (1) at 500 mg twice daily Thrombocytopenia (1) at 250 mg daily |
| Other potential grade 3–4 toxicities (# pts) | Leukopenia (1) at 250 mg daily Neutropenia (1) at 250 mg daily Lymphopenia (1) at 250 mg daily Elevated transaminases (1) at 250 mg twice daily Hand-foot syndrome (1) at 1000 mg daily |
| Off study reason | Progressive disease (4) Elevated transaminases (3) Completed protocol (2) Blood clot (2) Thrombocytopenia (1) Fatigue (1) Nausea/vomiting (1) Hand-foot syndrome (1) Asymptomatic ICH (1) Asymptomatic small vessel strokes (1) |
Radiographic response and survival
Fifteen patients completed combination radiation, temozolomide and vatalanib. Two of these 15 patients experienced biopsy proven pseudo-progression so were not evaluable for radiographic response. The best radiographic responses for the 13 evaluable patients were partial response in 2, stable disease in 9, and disease progression in 2 (Table 3; Fig. 1). After a median follow-up of 14.9 months, 13/15 patients have experienced radiographic disease progression and 14/18 patients have died. The median progression-free survival (PFS) for all 18 patients who took 1 or more dose was 7.2 months and median overall survival (OS) was 16.2 months.
Table 3.
Best response to vatalanib with chemoradiation in newly diagnosed GBM
| Best response | Number of patients (15 evaluablea) |
|---|---|
| CR | 0 |
| PR | 2 |
| SD | 10 |
| PD | 3 |
a
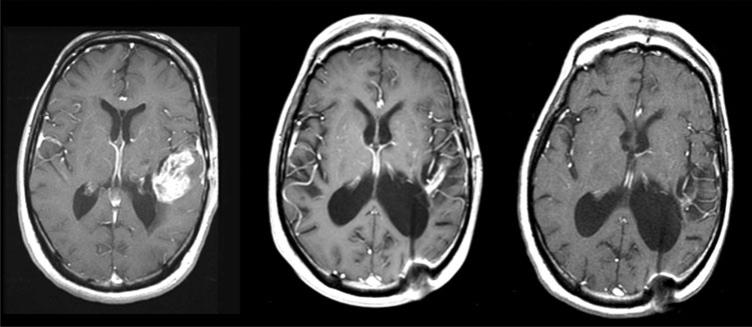
Fig. 1.
Representative MRI scans (T1-tumor enhancement) from a patient who experienced a partial response to vatalanib with chemoradiation. Left baseline scan. Middle 1 month after radiation, temozolomide, and vatalanib. Right after 7 months of post-radiation vatalanib and temozolomide
Biomarker analysis
Seventeen patients were included in the biomarker analysis although the patient who only took 1 dose of vatalanib did not contribute results after the day 1 assessment. Due to scheduling difficulties, one patient could not participate in the biomarker study. Following treatment with vatalanib alone, there was an immediate increase in circulating PlGF and sVEGFR1 which was maintained after combination therapy. Vatalanib with chemoradiation increased plasma collagen IV and CPCs, and decreased plasma sVEGFR2 and sTie2 (Table 4). There was no apparent dose effect on biomarker measurements. Similar to prior reports about early changes in collagen IV, there was a suggestion that circulating collagen IV level after 1 dose of vatalanib was associated with prolonged PFS (HR of 0, 95% CI 0 to 0.884; P = 0.047) [18]. Several other hypothesis-generating correlations with outcome were noted with changes in circulating biomarkers over time such as plasma MMPs, collagen IV, SDF1α and CPCs (Supplemental Table 7) With the small sample size and multiple analyses, these data are preliminary and warrant confirmation in other studies of anti-VEGF agents.
Table 4.
Changes in blood biomarkers after vatalanib with chemoradiation
| Day-1 | 8 h | Day 1 | Day 2 | Day 9 | Day 50 | Day 70 | |
|---|---|---|---|---|---|---|---|
| P1GF | 1.42 (1.38, 1.43) | 1.61 (1.50, 1.67) | 1.60 (1.51, 1.72) | 1.61 (1.50, 1.63) | 1.54 (1.43, 1.66) | 1.57 (1.45, 1.72) | 1.57 (1.40, 1.64) |
| N =17 | N = 16 | N = 17 | N = 15 | N = 15 | N = 14 | N = 10 | |
| NA | P < 0.0001 | P < 0.0001 | P = 0.0003 | P = 0.0009 | P < 0.0001 | P = 0.027 | |
| sVEGFRl | 2.31 (2.23, 2.42) | 2.23 (2.10, 2.32) | 2.29 (2.18, 2.33) | 2.30 (2.14, 2.41) | 2.32 (2.11, 2.38) | 2.23 (2.17, 2.30) | 2.26 (2.18, 2.38) |
| N =17 | N= 16 | N= 17 | N= 15 | N= 15 | N= 14 | N= 11 | |
| NA | P = 0.0017 | P = 0.0032 | P = 0.018 | P= 0.0034 | P= 0.0004 | P = 0.46 | |
| sVEGFR2 | 3.87 (3.81, 3.92) | 3.84 (3.78, 3.90) | 3.85 (3.75, 3.90) | 3.84 (3.77, 3.89) | 3.81 (3.78, 3.89) | 3.76 (3.66, 3.87) | 3.85 (3.78, 3.89) |
| N =17 | N= 14 | N= 15 | N= 14 | N= 13 | N= 12 | N= 9 | |
| NA | P = 0.71 | P = 0.42 | P = 0.54 | P = 0.24 | P= 0.0024 | P = 0.50 | |
| sTie2 | 1.20 (0.99, 1.39) | 1.18 (0.99, 1.33) | 1.16 (0.99, 1.29) | 1.20 (0.96, 1.33) | 1.17 (1.00, 1.35) | 1.15 (0.90, 1.27) | 1.06 (0.95, 1.25) |
| N= 15 | N= 14 | N= 15 | N= 14 | N= 13 | N= 12 | N = 9 | |
| NA | P = 0.76 | P = 1.0 | P = 0.39 | P = 0.17 | P= 0.0068 | P= 0.012 | |
| MMP3 | 1.63 (1.40, 2.0) | 1.71 (1.35, 1.93) | 1.53 (1.39, 1.92) | 1.59 (1.37, 1.83) | 1.55 (1.34, 1.73) | 1.57 (1.25, 1.77) | 1.37 (1.24, 1.66) |
| N= 15 | N= 14 | N= 15 | N= 14 | N= 13 | N= 12 | N= 9 | |
| NA | P = 0.27 | P = 0.11 | P = 0.22 | P= 0.048 | P = 0.18 | P = 0.30 | |
| IL-lβ | 0.16 (0.2, 0.22) | 0.15 (0.01, 0.27) | 0.13 (0.04, 0.23) | 0.12 (0.08, 0.26) | 0.19 (0.06, 0.22) | –0.02 (0.25, 0.30) | 0.04 (0.25, 0.18) |
| N =17 | N= 16 | N =17 | N = 16 | N = 13 | N= 14 | N= 11 | |
| NA | P = 0.67 | P = 0.49 | P = 0.42 | P = 0.81 | P = 0.068 | P= 0.042 | |
| Collagen IV | 0.60 (0.65, 0.57) | 0.62 (0.65, 0.59) | 0.63 (0.66, –0.58) | 0.61 (0.74, 0.56) | 0.62 (0.67, 0.58) | 0.63 (0.69, 0.61) | 0.67 (0.7, 0.64) |
| N =17 | N= 16 | N =17 | N = 16 | N= 14 | N = 14 | N = 11 | |
| NA | P = 0.63 | P = 0.098 | P = 0.43 | P = 0.27 | P = 0.017 | P = 0.0068 | |
| CPCs | 0.92 (1.1, 0.81) | 0.86 (1.07, 0.75) | 0.87 (0.92, 0.74) | 0.9 (1.12, 0.78) | 1.1 (1.22, 0.77) | 1.1 (1.4, 1) | 1.1 (1.3,–0.98) |
| N =17 | N= 16 | N =17 | N= 15 | N= 15 | N = 13 | N= 12 | |
| NA | P = 0.28 | P = 0.19 | P = 0.95 | P = 0.24 | P = 0.0479 | P = 0.054 |
Data are shown as median fold-changes and interquartile ranges and are compared to baseline (Day-1) levels. Bold italic values represent a significant increase in the biomarker level and bold values represent a significant decrease in the biomarker level P values are from Wilcoxon test (without adjustment). A variable representing dose level was not included in the analysis
Pharmacokinetic results
Data from only 10 patients was available for PK analysis since the company contracted to perform the PK analysis inadvertently destroyed the remaining blood samples before processing them. Two patients who received 250 mg daily, 2 who received 250 mg twice daily, and 6 who received 500 mg twice daily were available (Supplemental Table 6). Vatalanib was rapidly metabolized with peak concentration within 1–3 h confirming the need for twice daily dosing. Overall, Cmax appeared to increase with increasing dose.
SNP analysis
Ten patients were available for SNP analysis (Supplemental Table 8). In the context of this small dataset firm conclusions are challenging but we observed that 2 separate SNPs in VEGFR2 were seen in 5 patients with PFS > 200 days and OS > 400.
Discussion
Targeting VEGF-driven abnormal tumor angiogenesis is a promising mechanism to control GBM growth. By normalizing tumor vasculature, anti-VEGF agents are thought to reduce tumor hypoxia and improve delivery of chemotherapeutics [3]. In this study, we sought to determine the appropriate dose of vatalanib in patients with newly diagnosed GBM undergoing treatment with radiation and temozolomide who were also receiving EIAEDs. Most trials with tyrosine kinase inhibitors exclude patients treated with EIAEDs because of concern for drug–drug interactions since both vatalanib and EIAEDs undergo cytochrome P450 metabolism. However, not every patient tolerates non-enzyme inducing drugs or achieves good seizure control with monotherapy so require initiation of a second anti-epileptic. Consequently, it is important to determine the appropriate dose of oral TKIs in patients who take EIAEDs.
In this Phase I trial vatalanib was well tolerated. The maximal tolerated dose in combination with temozolomide and radiation was not reached because the trial was stopped early due to the decision by the study sponsor to terminate development of vatalanib. Combining vatalanib with radiation and temozolomide did not appear to greatly increase toxicity when compared to the reported side effects of vatalanib alone in recurrent GBM [7–9]. With the exception of hand-foot syndrome and elevated transaminases, the majority of the Grade 3/4 toxicities reported are commonly observed with temozolomide. Elevated transaminases are common with EIAEDs—particularly phenytoin which was the drug received by most patients in this study. One patient experienced a clinically asymptomatic intracerebral hemorrhage and 1 elderly patient experienced asymptomatic small vessel strokes. No patient developed wound dehiscence or infection. These toxicity results are similar to a Phase I/II study of vatalanib in combination with chemoradiation conducted in Europe that was restricted to patients not taking EIAEDs [19]. Although there is a strong possibility of selection bias in this single arm study, the PFS and OS are encouraging, especially in light of the fact that 6/15 radiographically evaluable subjects received only a pre-treatment needle biopsy.
There were an inadequate number of patients to achieve sufficient power to fully test blood biomarkers of angio-genesis or SNPs but we observed that collagen IV level on the first day of treatment might be a marker of response and warrants further study—similar to a prior report in recurrent GBM patients treated with cediranib [18]. In addition, results from that same trial reported a similar immediate increase in PlGF and a delayed decrease in sVEGFR2 and sTie2. This increase in PlGF and decrease in sVEGFR2 has been reported in several other trials of anti-angiogenic agents as well but the clinical implications are unclear at this time [10, 11, 13–15]. Nevertheless, given the changes seen in circulating proteins and cell populations with treatment, there remains potential to use them as pharmacodynamic biomarkers of anti-VEGF treatment.
Vatalanib is metabolized by CYP3A4 and EIAEDs induce the CYP3A4 system but with only 6 patients treated at the presumed biologically active dose, it was hard to fully assess the interaction between vatalanib and EIAEDs in our study. Pharmacokinetic data confirmed that twice daily dosing of vatalanib is required in patients taking EIAEDs and temozolomide. Cmax and time to reach maximal concentration in our study were similar to prior reports on vatalanib in combination with EIAEDs confirming that EIAEDs decrease vatalanib plasma exposure [9]. A wide inter-patient variability in PK measurements was found so other patient-specific factors may be playing a role as well.
Limitations of this study include the early discontinuation of the trial prior to achieving the primary endpoint of determining the MTD of vatalanib and lack of MGMT testing performed in patients. Nevertheless, vatalanib was safe and well tolerated up to 500 mg twice daily when combined with radiation and temozolomide in patients with newly diagnosed GBM. Although the study sponsor decided against further development of this particular TKI, the results of this study suggest that targeting VEGFR or other angiogenic receptors with an oral, small molecule TKI is feasible, safe and may improve both progression-free and overall survival in patients with newly diagnosed glioblastoma. Therefore, combining oral anti-VEGF therapy with radiation and temozolomide should be pursued and several studies of other TKI that target the VEGF pathway are underway.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s11060-010-0390-7) contains supplementary material, which is available to authorized users.
Contributor Information
Elizabeth R. Gerstner, Stephen E. & Catherine Pappas Center for Neuro-Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit Street, Yawkey 9E, Boston, MA 02114, USA
April F. Eichler, Stephen E. & Catherine Pappas Center for Neuro-Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit Street, Yawkey 9E, Boston, MA 02114, USA
Scott R. Plotkin, Stephen E. & Catherine Pappas Center for Neuro-Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit Street, Yawkey 9E, Boston, MA 02114, USA
Jan Drappatz, Department of Adult Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA.
Colin L. Doyle, Stephen E. & Catherine Pappas Center for Neuro-Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit Street, Yawkey 9E, Boston, MA 02114, USA
Lei Xu, Steele Laboratory, Department of Radiation Oncology, Massachusetts General Hospital, Boston, MA, USA.
Dan G. Duda, Steele Laboratory, Department of Radiation Oncology, Massachusetts General Hospital, Boston, MA, USA
Patrick Y. Wen, Department of Adult Oncology, Dana-Farber Cancer Institute, Harvard Medical School, Boston, MA, USA
Rakesh K. Jain, Steele Laboratory, Department of Radiation Oncology, Massachusetts General Hospital, Boston, MA, USA
Tracy T. Batchelor, Stephen E. & Catherine Pappas Center for Neuro-Oncology, Massachusetts General Hospital Cancer Center, Harvard Medical School, 55 Fruit Street, Yawkey 9E, Boston, MA 02114, USA
References
- 1.Jain R. Antiangiogenic therapy for cancer: current and emerging concepts. Oncology. 2005;19(4 Suppl 3):7–16. [PubMed] [Google Scholar]
- 2.Winkler F, Kozin SV, Tong RT, Chae SS, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK, Winkler F, Kozin SV, Tong RT, Chae S-S, Booth MF, Garkavtsev I, Xu L, Hicklin DJ, Fukumura D, di Tomaso E, Munn LL, Jain RK. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 3.Jain R. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]
- 4.Wood J, Bold G, Buchdunger E, Cozens R, Ferrari S, Frei J, Hofmann F, Mestan J, Mett H, O'Reilly T, Persohn E, Rosel J, Schnell C, Stover D, Theuer A, Towbin H, Wenger F, Woods-Cook K, Menrad A, Siemeister G, Schirner M, Thierauch K, Schneider M, Drevs J, Martiny-Baron G, Totzke F. PTK787/ZK 222584, a novel and potent inhibitor of vascular endothelial growth factor receptor tyrosine kinases, impairs vascular endothelial growth factor-induced responses and tumor growth after oral administration. Cancer Res. 2000;60(8):2178–2189. [PubMed] [Google Scholar]
- 5.Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, Masson E, Puccio-Pick M, Laurent D, Steward WP. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23(18):4162–4171. doi: 10.1200/JCO.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 6.Goldbrunner R, Bendszus M, Wood J, Kiderlen M, Sasaki M, Tonn J. PTK787/ZK222584, an inhibitor of vascular endothelial growth factor receptor tyrosine kinases, decreases glioma growth and vascularization. Neurosurgery. 2004;55(2):426–432. doi: 10.1227/01.neu.0000129551.64651.74. [DOI] [PubMed] [Google Scholar]
- 7.Conrad C, Friedman H, Reardon D, Provenzale J, Jackson E, Serajuddin H, Laurent D, Chen B, Yung WKA. A phase I/ II trial of single-agent PTK 787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in patients with recurrent glioblastoma multiforme (GBM). ASCO Annual Meeting Proceedings: Abstr 1512. 2004 [Google Scholar]
- 8.Reardon D, Friedman H, Yung WKA, Brada M, Conrad C, Provenzale J, Jackson E, Serajuddin H, Chen B, Laurent D. A phase I/II trial of PTK787/ZK 222584 (PTK/ZK), a novel, oral angiogenesis inhibitor, in combination with either temozolomide or lomustine for patients with recurrent glioblastoma multiforme (GBM). ASCO Annual Meeting Proceedings: Abstr 1513. 2004 [Google Scholar]
- 9.Reardon DA, Egorin MJ, Desjardins A, Vredenburgh JJ, Beumer JH, Lagattuta TF, Gururangan S, Herndon JE II, Salvado AJ, Friedman HS. Phase I pharmacokinetic study of the vascular endothelial growth factor receptor tyrosine kinase inhibitor vatalanib (PTK787) plus imatinib and hydroxyurea for malignant glioma. Cancer. 2009;115(10):2188–2198. doi: 10.1002/cncr.24213. doi:10.1002/cncr.24213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burstein HJ, Elias AD, Rugo HS, Cobleigh MA, Wolff AC, Eisenberg PD, Lehman M, Adams BJ, Bello CL, DePrimo SE, Baum CM, Miller KD. Phase II study of sunitinib malate, an oral multitargeted tyrosine kinase inhibitor, in patients with metastatic breast cancer previously treated with an anthracycline and a taxane. J Clin Oncol. 2008;26(11):1810–1816. doi: 10.1200/JCO.2007.14.5375. doi:JCO.2007.14. 5375[pii]10.1200/JCO.2007.14.5375. [DOI] [PubMed] [Google Scholar]
- 11.Drevs J MM, Mross K, et al. Phase I clinical evaluation of AZD2171, a highly potent VEGF receptor tyrosine kinase inhibitor, in patients with advanced tumors. J Clin Oncol 23: Abstract 3002. 2005 [Google Scholar]
- 12.Drevs J, Zirrgiebel U, Schmidt-Gersbach CI, Mross K, Medinger M, Lee L, Pinheiro J, Wood J, Thomas AL, Unger C, Henry A, Steward WP, Laurent D, Lebwohl D, Dugan M, Marme D. Soluble markers for the assessment of biological activity with PTK787/ZK 222584 (PTK/ZK), a vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitor in patients with advanced colorectal cancer from two phase I trials. Ann Oncol. 2005;16(4):558–565. doi: 10.1093/annonc/mdi118. doi:mdi118[pii]10.1093/annonc/mdi118. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Michaelson MD, Redman BG, Hudes GR, Wilding G, Figlin RA, Ginsberg MS, Kim ST, Baum CM, DePrimo SE, Li JZ, Bello CL, Theuer CP, George DJ, Rini BI. Activity of SU11248, a multitargeted inhibitor of vascular endothelial growth factor receptor and platelet-derived growth factor receptor, in patients with metastatic renal cell carcinoma. J Clin Oncol. 2006;24(1):16–24. doi: 10.1200/JCO.2005.02.2574. doi:JCO.2005.02.2574[pii]10.1200/JCO.2005.02.2574. [DOI] [PubMed] [Google Scholar]
- 14.Norden-Zfoni A, Desai J, Manola J, Beaudry P, Force J, Maki R, Folkman J, Bello C, Baum C, DePrimo SE, Shalinsky DR, Demetri GD, Heymach JV. Blood-based biomarkers of SU11248 activity and clinical outcome in patients with metastatic imatinib-resistant gastrointestinal stromal tumor. Clin Cancer Res. 2007;13(9):2643–2650. doi: 10.1158/1078-0432.CCR-06-0919. doi:13/9/2643[pii]10.1158/1078-0432.CCR-06-0919. [DOI] [PubMed] [Google Scholar]
- 15.Zhu AX, Sahani DV, Duda DG, di Tomaso E, Ancukiewicz M, Catalano OA, Sindhwani V, Blaszkowsky LS, Yoon SS, Lahdenranta J, Bhargava P, Meyerhardt J, Clark JW, Kwak EL, Hezel AF, Miksad R, Abrams TA, Enzinger PC, Fuchs CS, Ryan DP, Jain RK. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study. J Clin Oncol. 2009;27(18):3027–3035. doi: 10.1200/JCO.2008.20.9908. doi:JCO.2008.20. 9908[pii]10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macdonald DR, Cascino TL, Schold SC, Jr, Cairncross JG. Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol. 1990;8(7):1277–1280. doi: 10.1200/JCO.1990.8.7.1277. [DOI] [PubMed] [Google Scholar]
- 17.Duda DG, Cohen KS, Scadden DT, Jain RK, Duda DG, Cohen KS, Scadden DT, Jain RK. A protocol for phenotypic detection and enumeration of circulating endothelial cells and circulating progenitor cells in human blood. Nat Protoc. 2007;2(4):805–810. doi: 10.1038/nprot.2007.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sorensen AG, Batchelor TT, Zhang WT, Chen PJ, Yeo P, Wang M, Jennings D, Wen PY, Lahdenranta J, Ancukiewicz M, di Tomaso E, Duda DG, Jain RK. A “Vascular normalization index” As potential mechanistic biomarker to predict survival after a single dose of cediranib in recurrent glioblastoma patients. Cancer Res. 2009;69(13):5296–5300. doi: 10.1158/0008-5472.CAN-09-0814. doi:0008-5472.CAN-09-0814[pii] 10.1158/0008-5472.CAN-09-0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brandes AA, Stupp R, Hau P, Lacombe D, Gorlia T, Tosoni A, Mirimanoff RO, Kros JM, van den Bent MJ. Eortc study 26041–22041: Phase I/II study on concomitant and adjuvant temozolomide (TMZ) and radiotherapy (RT) with PTK787/ZK222584 (PTK/ZK) in newly diagnosed glioblastoma. Eur J Cancer. 2009;46(2):348–354. doi: 10.1016/j.ejca.2009.10.029. doi:S0959-8049(09)00816-8[pii]10.1016/ j.ejca.2009.10.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.