Abstract
HIV testing in emergency departments (EDs) remains underutilized. We evaluated a computer tool to facilitate rapid HIV testing in an urban ED. Randomly assigned non-acute adult ED patients to computer tool (‘CARE’) and rapid HIV testing before standard visit (n=258) or to standard visit (n=259) with chart access. Assessed intervention acceptability and compared noted HIV risks. Participants were 56% non-white, 58% male; median age 37 years. In the CARE arm nearly all (251/258) completed the session and received HIV results; 4 declined test consent. HIV risks were reported by 54% of users and there was one confirmed HIV-positive and 2 false-positives (seroprevalence 0.4%, 95% CI 0.01–2.2%). Half (55%) preferred computerized, over face-to-face, counseling for future HIV testing. In standard arm, one HIV test and 2 referrals for testing occurred. Computer-facilitated HIV testing appears acceptable to ED patients. Future research should assess cost-effectiveness compared with staff-delivered approaches.
Keywords: rapid HIV test, computer, emergency departments
INTRODUCTION
Approximately one in five of those estimated to be currently living with HIV in the United States remain unaware of their status (207,600 people; Centers for Disease Control and Prevention [CDC], 2012). Early detection and treatment of HIV disease reduces morbidity and mortality (Deloria-Knoll et al., 2004; Murphy et al., 2001; Palella et al., 2003) as well as secondary transmission risks (Cohen et al., 2011; Fonner, Denison, Kennedy, O'Reilly, & Sweat, 2012; Higgins et al., 1991; Hutchinson, Branson, Kim, & Farnham, 2006). Yet many HIV-positive persons still do not get tested until late in their disease and a third of those who test HIV-positive using traditional antibody testing do not return for their test results (CDC, 2003). This obstructs the opportunity for antiretroviral therapy initiation, which US guidelines now recommend be offered to all upon HIV infection diagnosis, regardless of CD4 cell count (strongest evidence for ART at ≤350 CD4 cell count; Department of Health and Human Services [DHHS], 2011). In an effort to increase HIV seropositive status knowledge – the US Preventive Services Task Force may issue in 2013 a Grade A (strongest level of evidence) recommendation that clinicians screen adolescents and adults ages 15 to 65 years, and younger adolescents and older adults who are at increased risk, for HIV infection (US Preventive Services Task Force [USPSTF], 2012). This follows the Centers for Disease Control and Prevention’s 2006 recommended routine opt-out HIV screening for 13 to 64 year olds in all health care settings including emergency department care settings (EDs) (Branson et al., 2006). This policy means that all people entering EDs should be made aware that they will be tested for HIV unless they do not want a test. A separate written consent for people who accept testing is no longer recommended (Bartlett et al., 2008), nearly all states have now complied with this CDC recommendation (Mooney, 2011) though as of 2011 five states still had laws presenting barriers to CDC-recommended opt-out HIV testing (Neff & Goldschmidt, 2011).
Emergency departments are important locations to increase access to HIV testing (Kecojevic et al., 2011; Kelen, Shahan, & Quinn, 1999; Rothman, Ketlogetswe, Dolan, Wyer, & Kelen, 2003; Walensky et al., 2005) as these may be the only place where many medically underserved persons at risk for HIV seek care (Goggin, Davidson, Cantril, O’Keef, & Douglas, 2000; Hutchinson, Corbie-Smith, Thomas, Mohanan, & del Rio, 2004; Liddicoat et al., 2004; Tao, Branson, Anderson, & Irwin, 2003), there is a higher proportion of HIV-infected adult patients in urban EDs versus the population as a whole (Goggin et al., 2000; Kelen et al., 1999; Walensky et al., 2005), and testing in EDs with high HIV prevalence rates successfully increases awareness of HIV status among attendees (Hutchinson et al., 2006; Kraus, Rothman, Shahan, Quinn, & Kelen, 2006; White, Orujela, Lowery, & Frazee, 2006). Universally offered rapid HIV testing in EDs has shown high levels of patient acceptability, referral to care (Hutchinson et al., 2004; White et al., 2006), and cost effectiveness (Kelen et al., 1999; Rothman et al., 2003) even where HIV prevalence is low (>0.2%) (Paltiel et al., 2006).
However, the need for education, consenting and counseling (in states where required) places a burden on staff time and resources. Due to barriers (Burke et al., 2007) including lack of time, training, funding, and follow up systems (Fincher-Mergi et al., 2002; Murphy, Grusky, Roberts, & Swanson, 2005; Rothman, 2004; Wilson, Mitchell, Bradbury, & Chavez, 1999), and concern about patient volume and throughput, EDs often are unable to provide HIV testing (Ehrenkranz et al., 2008; Gift & Hogben, 2006; Jenkins, Gardner, Thrun, Cohn, & Burman, 2006) even when patients present with HIV risk factors (Fincher-Mergi et al., 2002; Liddicoat et al., 2004; Wilson et al., 1999). A national survey of 223 EDs found that only 3% of providers routinely offered HIV testing to all patients (Hardwicke, Malecha, Lewis, & Grimes, 2008). The National ED HIV Testing Consortium recently published a call for more rigorous evaluation of a range of HIV testing operational models in EDs (Haukoos et al., 2011), echoing earlier calls for research priorities that include computerized aids for HIV testing in EDs (Haukoos, Mehta, Harvey, Calderon, & Rothman, 2009).
Recognizing that these major impediments will require a variety of innovative solutions, we developed an interactive computer tool (Spielberg et al., 2011) to support HIV testing in EDs and other health care settings. Multiple studies in settings including EDs support the effectiveness of computer-delivered health communication tools for HIV prevention (Kiene & Barta, 2006; Lightfoot, Comulada, & Stover, 2007), education (Marsch & Bickel, 2004), HIV testing, consent and counseling (Calderon et al., 2006), as well as for smoking cessation (Noell, Biglan, Hood, & Britz, 1994; Swartz, Noell, Schroeder, & Ary, 2006), diet (Brug, Oenema, & Campbell, 2003; Brug, Steenhuis, van Assema, & de Vries, 1996), diabetes management (Glasgow et al., 1997; Glasgow, Toobert, & Hampson, 1996), and other conditions (Neumann et al., 2006; Rhodes, Lauderdale, He, Howes, & Levinson, 2002). We hypothesized that using an interactive computer tool to facilitate rapid HIV testing would be acceptable among patients and feasible to use in an ED, as documented by HIV testing uptake (i.e., a high percent agreeing to test and receiving test results), behavioral change, and satisfaction measures. Further, we sought to document whether there was potential unmet need with current HIV testing ED practices, as measured by sexual transmission risk behaviors noted in charts and prevalent sexually transmitted infection (STI) status.
METHODS
Study Design
We conducted a cross-sectional study in which participants were randomly assigned to one of two arms. The intervention group received the CARE tool session, rapid HIV test (OraQuick Advance Rapid HIV Antibody test, OraSure Technologies, Bethlehem, Pennsylvania) and gonorrhea and chlamydia urine tests (nucleic acid amplification test, GenProbe, San Diego, California) prior to standard ED visit. The other group received only the standard ED visit and chart review to assess risk behaviors and HIV/STI test referrals noted. Sample size for the intervention group was set to have reasonable power at alpha 0.05 to detect by two-sided McNemar’s chi-square test a ≥7% absolute difference in proportions of participants willing to make behavioral changes at the end as compared to the start of their computer session. The research protocol was approved by the University of Washington Human Subjects Division, 06-1550-C 01.
Setting
Participants were recruited from the non-trauma side of an urban, public level-1 trauma center ED serving 80,000 patients per year in the Pacific Northwest. By policy this ED did not at the time of the study provide HIV testing for patients other than those being treated for an occupational blood exposure or for clinical indication. Therefore we were unable to conduct this study as a randomized comparison of computer- versus ED staff-delivered HIV testing. Participants were recruited directly after visiting the triage desk during day and night shifts Monday through Saturday.
Selection of participants
Research Assistants (RAs) approached all ambulatory, non-acute, patients after triage and check in, and provided a scripted study description. All RAs were trained in HIV counseling and testing procedures, and were observed for quality assurance. Eligibility criteria included being age 18 or older, clinically stable, English-speaking, HIV-negative or status unknown, and able to understand the consent process. Participants who refused study enrollment were asked to respond to anonymous questions about HIV testing history and risk, and RAs recorded demographic data. Study arm assignment of consented eligible participants was done on the computer using a standard pseudo-random number generation algorithm. Since RAs had to give verbal HIV test results, they could not be blinded to study arm assignment.
Intervention and procedures
The Computer Assessment and Risk reduction Education (CARE)1 tool provides risk assessment, a rapid HIV test video, HIV test consent, personalized feedback based on user risks, tailored behavioral skill-building videos, and development of a specific HIV risk-reduction plan. The session closes with a printout summarizing user plan and customized referrals. Content is based on Integrative Model of Change (Fishbein et al., 2001; Glanz, Rimer, & Lewis, 2002) and social cognitive theory (Bandura, 2001), and counseling elements shown in randomized trials to reduce STI incidence (Kamb et al., 1998). The intervention was delivered on a computer cart locked to the wall in a busy ED corridor.
After accepting study participation, during the waiting period prior to exam, the study was explained to patients by script in a private area in the clinic. Written informed consent was obtained and eligible participants were randomly assigned to intervention or control.
Intervention participants provided a urine sample for STI testing and an oral swab for the rapid HIV test, then took the computer intervention using headphones, while the RA processed the HIV test. After viewing the rapid test video participants consented on the computer in a two-step process: agreeing that they did not believe they would harm self or others if a test result was preliminary positive and saying ‘Yes’ on the test consent form. After the CARE session RAs reviewed the printout to verify that the participant was safe to test and consented to test. They then delivered the rapid HIV test result verbally in a private exam area. If participants did not complete the consent process the specimen was discarded and the test result not read or reported. All RAs were trained HIV test counselors. Protocol for preliminarily positive rapid test results included drawing a whole blood specimen for Western blot confirmatory testing, linking the participant with the HIV clinic located in the hospital, and provision of other appropriate referrals. Intervention and control participants returned after their ED exam for a verbal exit interview and incentive payment ($20 intervention, $5 control).
Data collection and processing
Intervention group data were entered directly into the CARE tablet computer by participants and were wirelessly transmitted via an encrypted, private signal to a SQL server database. No personally identifying information was collected or stored on the tablet. In-person exit interview data were recorded onto standardized paper forms by RAs. Electronic medical record data abstraction using a standardized form was conducted by an RA experienced in chart abstraction and blinded to group assignment. Abstracted data included demographics, presenting complaint, and whether the presenting symptoms were potentially STI-related (urinary tract infection, pelvic pain, urethral/vaginal discharge, genital rash), documentation of patient HIV risk (injection drug use, unprotected sex) or risk reduction plan (e.g., “use condoms”), HIV status, and whether gonorrhea, chlamydia and HIV testing were recommended or ordered.
Time-motion data were collected by RAs on standardized study forms in which the time for activities were charted including study related duties (approach, informed consent) and HIV/STI testing and computer-related duties (collecting specimens, CARE tool intervention, running rapid tests, delivering results). The RA time spent on these activities was multiplied by an average staff wage of $22/hour, including 12% for fringe benefits. Cost estimates also included the market cost of rapid test kits, confirmatory tests, and CARE tool hardware and set-up costs. Cost for treating cases of gonorrhea and chlamydia were not included because these cases were referred to, and paid for by, the public health department.
Outcome measures
Principle outcome measures included uptake of HIV testing (percent agreeing to test and receiving test results prior to discharge from the ED), participant responses to acceptability questions regarding computer-facilitated rapid HIV testing, and cost of the computer intervention. Measures of potential unmet need included comparison of HIV risks, HIV/STI testing and referral in patients randomized to chart review only (control arm) and to CARE.
Data Analysis
The CARE data and chart abstraction data were entered into SPSS (SPSS Inc, Chicago, IL). Outcome measure data were summarized using descriptive statistics and compared using independent or correlated chi-square tests for categorical measure differences. HIV/STI prevalence confidence bounds were calculated using STATA (StataCorp, College Station, TX).
RESULTS
Characteristics of study subjects
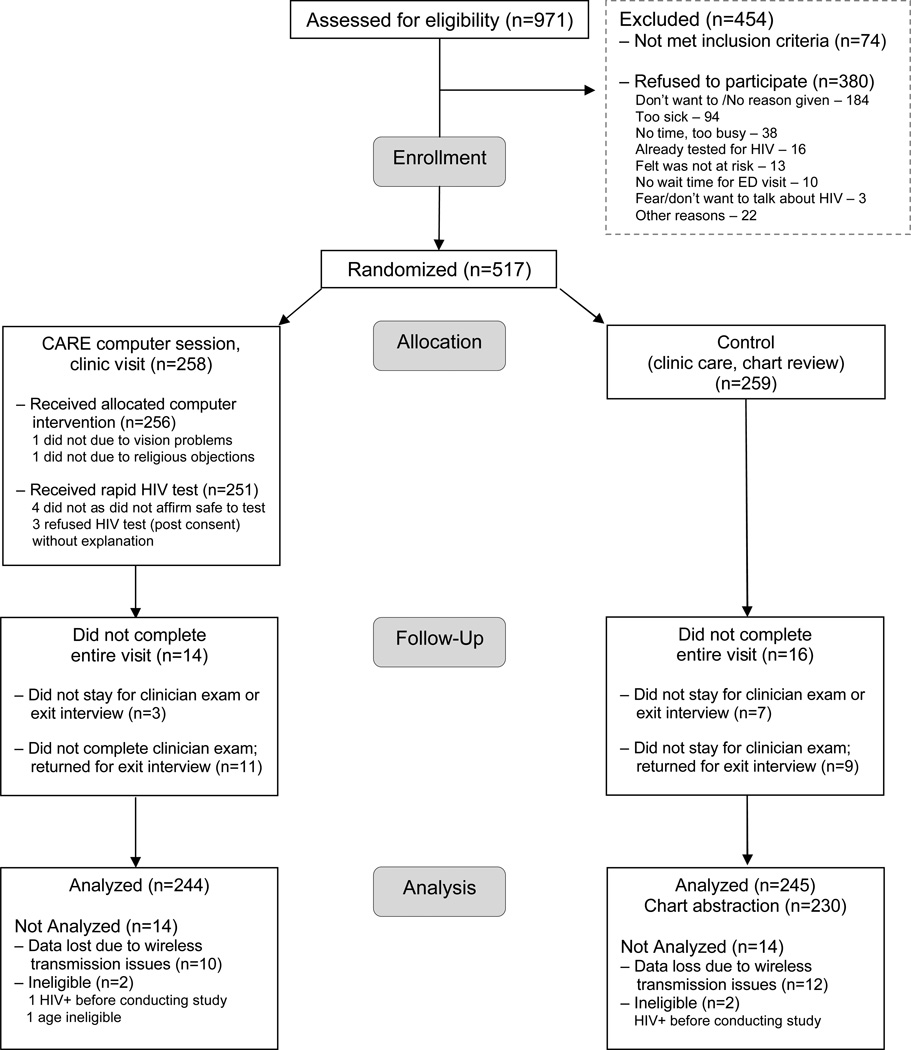
Figure 1 summarizes the study flow. Over half (517/971 or 53%) of those approached accepted study participation, indicating that they were prepared to take a rapid HIV test that day. Among those who did not participate (74/454 or 16%) were not eligible. Half the patients who refused (184/380 or 48%) did not give a reason; among those who gave reasons for refusal (n=196), the most frequently cited were being too sick (94/196 or 48%) or not having time (38/196 or 19%). Relatively few patients refused participation due to having already tested for HIV (16/196 or 8%), not believing they were at risk (13/196 or 7%), or being afraid to consider HIV testing (3/196 or 1%). Study acceptors were more likely than non-acceptors to be non-white (279/517, 53% vs. 154/380, 40%, p<0.001) and to have recent (within last 6 months) sexual HIV transmission risk (145/176, 82% vs. 56/94, 60%, p <0.001), sexual partner with unknown HIV status (105/231, 45% vs.21/102, 21%, p <0.001), and were less likely to have had an HIV test in the last 6 months (59/210, 28% vs. 49/112, 44%, p=0.005).
FIGURE 1.
Computer-Facilitated Rapid HIV Test Study Profile
Participants in the intervention and control groups (Table 1) were similar. Non-whites made up 56% of the study population, females comprised 41% of the sample, and the median age was 37 years. Of those in the CARE intervention group who were sexually active (225/244), over half (132/225) disclosed having unprotected vaginal or anal sex in the prior 2 months, among whom nearly half (63/132) had sex with a partner whose HIV status was positive (1/63) or unknown (62/63).
TABLE 1.
Demographic and risk characteristics*, by randomization arm
| Characteristic | CARE Computer Tool (N = 244†) |
Standard Clinic Visit (Chart data) (N = 245††) |
|---|---|---|
| Gender | N (%) | N (%) |
| Male | 143 (59) | 152 (62) |
| Age (years) | N = 242 | N = 245 |
| < 20 | 7 (3) | 14 (6) |
| 20–29 | 74 (31) | 64 (26) |
| 30–39 | 61 (25) | 58 (24) |
| ≥ 40 | 100 (41) | 109 (44) |
| Mean (SD) | 36.69 (11.022) | 36.86 (11.435) |
| Race/ethnicity | N = 241 | N = 245 |
| African American | 93 (39) | 93 (38) |
| Asian/Pacific Islander | 2 (1) | 6 (2) |
| Latino | 3 (1) | 13 (5) |
| Native American | 6 (2) | 8 (3) |
| White | 106 (44) | 109 (44) |
| Multiple/other | 31 (13) | Not available (N/Av) |
| Unknown | – | 16 (7) |
| ≤ High school education | 166/243 (68) | N/Av |
| Homeless | 52/240 (22) | N/Av |
| Duration since last HIV test | N = 212 | N/Av |
| Past 6 months | 61 (28) | – |
| 6 months to 1 year | 38 (18) | – |
| > 1 year ago | 55 (26) | – |
| Never tested | 58 (27) | – |
| Unprotected sex in past 2 months, any | 132/225 (59) | 3/230 (1)*** |
| Unprotected vaginal or anal sex with risky partner** | 64/227 (28) | N/Av |
| Number of sex partners past 2 months | N = 242 | N/Av |
| 0 | 62 (26) | N/Av |
| 1 | 145 (60) | N/Av |
| ≥ 2 | 35 (14) | N/Av |
| Depression screen positive | 110/241 (46) | N/Av |
| ETOH/chemical dependency screen positive | 117/193 (61) | N/Av |
| Non-injection drug use daily or almost daily | 109/219 (50) | N/Av |
| Drugs used in past 2 months | N = 212 | N/Av |
| Crack/cocaine | 60 (28) | – |
| Methamphetamines | 19 (9) | – |
| Other non-injection drug | 102 (48) | – |
| Heroin | 31 (15) | – |
| Injection drug use (IDU), ever | 62/242 (26) | 5/230 (2)*** |
| IDU daily or almost daily past 2 months | 14/241 (6) | N/Av |
| Needle/works sharing (risky partner**), 2 months | 6/241 (2) | N/Av |
| Unprotected sex or needle sharing (any partner), 2 months | 136/244 (56) | N/Av |
| HIV testing needed (risk within 3 months before or since last test) | 187/204 (92) | N/Av |
Unable to analyze 10 participants due to data loss due to wireless transmission issues; 2 participants excluded from analysis due to ineligibility
Unable to analyze 12 participants due to data loss due to wireless transmission issues; 2 ineligible for study
Sex and needle sharing behaviors in past 2 months
A partner of HIV-positive or unknown HIV status past 2 months
p <0.001
N/Av = data Not Available in charts
Main results
HIV testing uptake and prevalence
Nearly all individuals randomized to the CARE arm consented to and took the rapid HIV test (251/258, 97%) and gave specimens for gonorrhea and chlamydia tests (254/258, 98%). Four participants did not HIV test due to responding affirmatively to CARE tool computer screening questions for potential of harming self or others in the event of a positive result. These patients were counseled to wait to test and to seek support from their health provider or a counselor. Two additional participants did not complete the CARE tool or test for HIV due to: 1) religious objections to sexual questions, 2) vision problems causing difficulty in viewing the computer screen and a third participant refused to test without explanation at the time of HIV test. In the control arm, no HIV tests were conducted and only 2 participants were referred for an HIV test according to chart notes. The chart review of the intervention group revealed that 3 participants were charted as having an HIV test (2 through our study testing and 1 performed for clinical indications by ED clinicians).
There was one true HIV positive identified (preliminary positive confirmed by Western blot and HIV-1 RNA), and two false positives (preliminary positive rapid, not confirmed).2 This yielded an HIV seroprevalence of 0.4% (95% CI 0.01 – 2.2%) and a specificity of 99.2% (95% CI 97.2 – 99.9%). One gonococcal and 9 chlamydial infections yielded a combined prevalence of 3.9% (95% CI 1.9 – 7.1). All positive STI test results were referred to the health department STD clinic for follow up, treatment and partner notification which resulted in 8 of 10 participants and 6 of 8 partners receiving treatment for their bacterial infection. Two participants were lost to follow up and these cases were closed by the health department. All preliminary HIV positives were followed up for confirmatory testing and HIV treatment referrals. The one participant who was diagnosed with HIV was referred for, and entered into care, in the hospital-based HIV clinic. Notably, this person had a history of daily injecting drug use and had been seen in the ED twice in the 2 years prior to getting HIV-tested in this study.
Acceptability
Intervention arm participants answered acceptability questions within the tool immediately following the computer session; 86% found the session length “just right,” 97% found the tool either “very easy” (74%) or “easy” (23%) to use, 96% found it provided them “enough privacy” and 91% felt the CARE tool helped in comparison with face-to-face counseling. Verbal exit interviews found that in comparison to human staff-delivered counseling, 83% found the CARE tool more private, 77% more “convenient,” 76% more “comfortable,” 72% “safer,” and 54% found it more “helpful.” Two-thirds (164/244) said it was “very important” for them to change their behavior so they would not acquire HIV or an STI. If tested for HIV in the future, 55% said they would choose a computer over face-to-face counseling, and an additional 6% expressed no difference in preference.
Over half of CARE users (54%) and 46% of control arm participants said they were “’very satisfied’ with their ED visit today.”
Cost
For the study we received free HIV test kits from the distributor but have included 2005 market cost of kits here for analysis. Staff time, CARE hardware, supplies, and study-related costs for conducting computer-facilitated rapid HIV testing (n=251) was US$ 11,047 (Table 2). Programmatic cost (i.e., excluding study-only activity staff time) was US$ 10,017 or about US$ 40 per any HIV test result received and – in this low-prevalence setting – US$ 10,000 for a positive test result received. These results were based on research staff testing one person at a time. When scaled up it would be possible for one staff to test several patients at a time, and hardware costs would amortize over time, bringing overall testing costs down through efficiencies of scale.
Table 2.
Cost data, computer-facilitated rapid HIV testing study
| Category | Measure | Quantity | Subtotal ($USD) |
|
|---|---|---|---|---|
| Personnel Cost | ||||
| (based on $22.40/hourly including benefits @ 12%) | Time | |||
| Program related time | 0.69 hr | 251 (tested for HIV and GC/CT*) | $15.36 × 251 = | 3856.02 |
| (collect rapid & urine test specimen, process rapid test and CARE computer session) | ||||
| Time for HIV test result | 4 minutes | 251 | $1.49 × 251 = | 374.82 |
| (negative and positive) | ||||
| Study related time | 0.183 hr | 251 | 1030.77 | |
| (approach, recruit, consent, study paperwork) | ||||
| Materials | Unit cost - $USD | |||
| Supplies–gloves (0.15) and urine cups (0.10) | 0.25 | 251 | 62.75 | |
| Rapid tests & controls | 12.0 | 251 | 3012.00 | |
| HIV confirmation (Western blot) | 84.0 | 3 | 252.00 | |
| GC and CT tests | 9.0 | 254 | 2259.00 | |
| Locking computer cart | 200 | 1 | 200.00 | |
| CARE tool hardware cost | printer–200 server–600 wireless–500 tablet computer–1700 |
1 of each | 3000.00 | |
| Total Cost | 11,047.36 | |||
| Total Programmatic Cost | ||||
| (excludes study-related staff time) | $10,016.59 | |||
GC = gonorrhea, CT = chlamydia
Evidence of unmet need given current HIV testing practice
As seen in Table 3, HIV risk from sexual contact (unprotected sex in last 2 months) was reported by 59% of the intervention group and noted in 1% of control group charts. Injecting drug use was documented at an order of magnitude higher level in the CARE tool than in control charts. An HIV risk reduction plan was created by nearly all CARE participants (97%, 238/245) while 0.8% (2/230) of control arm charts indicated an HIV risk reduction plan was discussed. Intervention participants were asked about confidence in ability to make changes to reduce HIV risk before and after developing their plan; 78% (190/243) felt “very confident” before their plan and 84% (198/236) felt “very confident” after making a plan (McNemar’s χ2 p = 0.047).
Table 3.
Comparison of HIV risk assessment, testing, and referral needs and services by study arm
| Characteristic | CARE Computer Tool (N = 244) |
Standard Clinic Visit (Chart data) (N = 245) |
|---|---|---|
| Comparison of chart review data | N (%) | N (%) |
| Left before ED provider exam* | 14 | 16 |
| Requested HIV or STI testing from ED provider | 1 | 2 |
| Referred for HIV testing by ED provider | 2 | 2 |
| HIV/STI–related complaint noted in ED chart | 18/241* (8) | 9/230* (4) |
| (UTI, urethral/vaginal discharge, pelvic pain, genital rash) | ||
| Comparison of intervention arm CARE computer data to Standard Care control arm data | ||
| HIV risk noted | CARE | Chart Review |
| Unprotected sex (unprotected sex in last 2 months) | 132/244 (54) | 3/230 (1)**** |
| Injecting drug use–ever | 62/242 (26) | 5/230 (2)**** |
| HIV risk reduction plan made | 238/244 (98) | 2/230 (1)**** |
| Abstinence | 26 (11) | 0**** |
| Have sex w/fewer partners or only one partner | 61 (26) | 0**** |
| Increase condom use (use condoms more or all of the time) | 67 (28) | 2/230 (1)**** |
| Talk to my sex partners about HIV/STIs | 27 (11) | 0**** |
| Safer types of sex (oral, mutual masturbation) | 25 (11) | 0**** |
| Decrease substance use | 32 (13) | 0**** |
| Acceptability Exit Interview–asked in person of both CARE arm and Standard Care control arm | ||
| HIV/STI testing main reason for ED visit | 6/232 (3) | 2/191 (1) |
| Health care provider talked with you about HIV/STI risks today? | 17/237 (7) | 11/215 (5) |
| Health provider offered you HIV testing today? | 6/237 (3) | 5/215 (2) |
| Health provider talked with you about a plan to lower your HIV risk | 7/235 (3) | 5/215 (2) |
| Overall, how satisfied were you with your visit today? | ||
| Not at all satisfied | 8/190 (4) | 9/189 (5) |
| Not very satisfied | 22 (12) | 19 (10)** |
| Somewhat satisfied | 58 (31) | 74 (39)*** |
| Very satisfied | 102 (54) | 87 (46) |
| Comparison of HIV testing performed in CARE arm versus Standard Care control arm | ||
| HIV testing performed | 251/258 | 1**** |
| HIV true positives | 1 | 0 |
| Received confirmatory results | 1 | n/a |
| Followed up for HIV care | 1 | n/a |
| HIV false positives | 2 | n/a |
| Received confirmatory results | 2 | n/a |
| Followed up for primary care | 1 | n/a |
p < 0.10
p < 0.05
p < 0.01
p < 0.001
n/a not applicable
Limitations
Our study had several limitations. We compared patient self-report data to chart notes from clinicians. Participants may be more willing to disclose sensitive risk information on an anonymous computer tool than to a clinician (reporting and social desirability bias). Clinicians may not have charted all HIV/STI risk presented by participants or referrals made for HIV testing. Our findings do reflect the literature that clinicians in many different settings do not consistently discuss HIV risks with patients or screen for HIV/STIs even when patients present with risk factors or are known to be HIV-positive (Marks et al., 2002).
We experienced initial data loss due to problems with our initial wireless networking setup (intermittent connection failures that appeared to be caused by other existing wireless networks operating at identical frequencies, as well as suspected interference from ED equipment). Adding additional hardware and redundant network connectivity prevented further data loss. These difficulties experienced and overcome illuminate critical technical issues to be considered when implementing computerized health tools.
Our cost analysis may not accurately reflect the true cost of conducting HIV testing in an ED setting because we had dedicated study staff. On the other hand, staff time was likely over-estimated because RA staff remained with the participant while they conducted their computer session. Finally, our patient population may not be generalizable to other ED populations.
DISCUSSION
As the National ED HIV Testing Consortium has pointed out, effectively scaling up HIV testing in EDs may require various modalities. We found in this randomized trial that a theoretically-based computer tool used in conjunction with staff provision of point-of-care rapid HIV testing was a potentially cost efficient way to support the goal of increasing HIV serostatus knowledge. Computer-facilitated rapid HIV testing in this ED setting led to high testing and results uptake (100% of those in the CARE arm who consented to test got HIV results), and high satisfaction among patients. Additional advantages of such a tool include consistent delivery of evidence-based counseling that has been shown to reduce incident STIs (Kamb et al., 1998; Metcalf et al., 2005) but that is rarely translated into clinical practice (Golden & Manhart, 2005).
This study dispels several concerns about the provision of routine rapid HIV testing in EDs. We found the routine offer of HIV testing to be quite acceptable to the majority of patients visiting EDs, even though they were presenting for other health concerns. Studies evaluating barriers to HIV testing in the past have found that fear of learning their status was the major reason for avoiding HIV testing (Kellerman et al., 2002; Spielberg et al., 2003). When presented in this context, fear was a concern voiced by few patients.
In this study we gave patients the option of keeping their CARE session report confidential or of making an extra printout to share with their provider. Almost none of the patients reported on exit interview that they shared their HIV testing experience with their ED provider. It is not clear how testing rates and visit satisfaction would have been affected if it were routine practice to give providers the information gathered in the CARE session.
Time for the CARE session (mode 19 minutes) allowed patients to complete it after triage and before their clinical visit. This did not interfere with the ED visit and was efficient since it utilized patient (not ED staff) time while waiting for clinical care. Nearly all CARE tool users completed a rapid HIV test, and all who were tested received their preliminary test result prior to leaving the ED. Study staff follow-up time for those testing preliminary HIV-positive was a mean of 49 minutes (SD 35.2 minutes). No participants in the standard of care control arm were HIV-tested. Sexual and parenteral risk for HIV likely was present for a substantial proportion of these individuals (study arm randomization appeared to work, and over half the intervention participants reported unprotected sex, with 1/4 reporting potentially non-HIV-concordant sex). While few indicated that the chief reason for their ED visit was for HIV testing, opportunities for identifying HIV-positives and for reducing risk among HIV-negatives clearly did exist.
Regarding the concern that busy ED providers will not have time for post-test counseling among those with positive test results, we found the burden to be minimal, and that collaboration between our public health department STI clinic and the ED was a successful way to reduce the burden of follow-up on busy ED clinicians (Lyons, Lindsell, Ledyard, Frame, & Trott, 2005).
Operational costs for implementing opt-out HIV testing in EDs has been a consistent concern (Borg, 2007). Several ED providers have voiced concerns that even without separate consent and counseling, the burden of performing the rapid test itself is too much for existing ED staff to absorb. With an interactive computer tool like CARE, routine testing can be provided along with standardized counseling and referrals, at a relatively low cost, without incremental staff resources.
This study also served to highlight the substantial risks and need for referrals that ED patients have. In addition to providing HIV risk reduction counseling, the CARE tool identified that 46% of patients screened positive for depression and 61% screened positive for chemical dependency. The automated referrals to mental health and substance use treatment provided in the CARE tool printout may help patients seek out additional care.
CONCLUSIONS
Establishing computer-facilitated HIV testing in EDs likely will require administrative and clinical leadership, some staff training time, costs for hardware (that amortize over time), and for software localization if necessary. Computer-facilitated HIV testing has been shown to be effective in eliciting sensitive behaviors as well as prompting test offers and administering rapid HIV testing consent and procedures (Cohall et al., 2007; Cohall et al., 2008; Douglas et al., 2005; Merchant et al., 2011; Saifu et al., 2011; Sellors et al., 2002; Sundaram et al., 2009; Welz & Herbst, 2008; Wilbur, Huffman, Lofton, & Finnell, 2011). Such tools would have expanded value if results are integrated into electronic health records. Once these expenditures have been made, computer-facilitated rapid HIV testing has the potential to increase quality of care among underserved populations, while minimizing ongoing operational costs or staffing requirements in these high-burden care settings.
ACKNOWLEDGEMENTS
This study was funded by NIH (University of Washington Center for AIDS Research P30 AI27757, New Investigator Award to Kurth).
We thank the following for their contributions and support: Dave Holt, RN, ARNP and Cheryl Nankervis, RN, Harborview Medical Center; Bill Reidy, PhD now at Columbia University; Nok Chhun at NYU; and the study participants.
Footnotes
Conflicts of Interest and Source of Funding:
The authors have no conflicts of interest to disclose
Resources Online, email Jim Larkin [jim@ronline.com], website: http://www.ronline.com/
Quality control checks showed no problems and the company (OraSure Technologies) was notified. They conducted an investigation and found no identifiable lot or other problems.
REFERENCES
- Bandura A. Social cognitive theory: an agentic perspective. Annu Rev Psychol. 2001;52:1–26. doi: 10.1146/annurev.psych.52.1.1. [DOI] [PubMed] [Google Scholar]
- Bartlett JG, Branson BM, Fenton K, Hauschild BC, Miller V, Mayer KH. Opt-out testing for human immunodeficiency virus in the United States: progress and challenges. JAMA. 2008;300(8):945–951. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- Borg KT. To test or not to test? HIV, emergency departments, and the new Centers for Disease Control and Prevention guidelines. Ann Emerg Med. 2007;49(5):573–574. doi: 10.1016/j.annemergmed.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, Clark JE. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. quiz CE11-14. [PubMed] [Google Scholar]
- Brug J, Oenema A, Campbell M. Past, present, and future of computer-tailored nutrition education. Am J Clin Nutr. 2003;77(4 Suppl):1028S–1034S. doi: 10.1093/ajcn/77.4.1028S. [DOI] [PubMed] [Google Scholar]
- Brug J, Steenhuis I, van Assema P, de Vries H. The impact of a computer-tailored nutrition intervention. Prev Med. 1996;25(3):236–242. doi: 10.1006/pmed.1996.0052. [DOI] [PubMed] [Google Scholar]
- Burke RC, Sepkowitz KA, Bernstein KT, Karpati AM, Myers JE, Tsoi BW, Begier EM. Why don't physicians test for HIV? A review of the US literature. AIDS. 2007;21(12):1617–1624. doi: 10.1097/QAD.0b013e32823f91ff. [DOI] [PubMed] [Google Scholar]
- Calderon Y, Haughey M, Bijur PE, Leider J, Moreno-Walton L, Torres S, Bauman LJ. An educational HIV pretest counseling video program for off-hours testing in the emergency department. Ann Emerg Med. 2006;48(1):21–27. doi: 10.1016/j.annemergmed.2006.01.003. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Advancing HIV prevention: new strategies for a changing epidemic-United States, 2003. Morbidity and Mortality Weekly Report. 2003;52(15):329–332. [PubMed] [Google Scholar]
- Centers for Disease Control (CDC) Monitoring selected national HIV prevention and care objectives by using HIV surveillance data-United States and 6 U.S. dependent areas—2010. HIV Surveillance Supplemental Report. 2012;17(No. 3, part A) [Google Scholar]
- Cohall AT, Dini S, Senathirajah Y, Nye A, Neu N, Powell D, Hyden C. Feasibility of using computer-assisted interviewing to enhance HIV test counseling in community settings. Public Health Rep. 2008;123(Suppl 3):70–77. doi: 10.1177/00333549081230S309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohall AT, Senathirajah Y, Dini S, Nye A, Powell D, Powell B. An online audio computer-assisted self-interview for pre-screening prior to rapid HIV testing in a vulnerable population. AMIA Annu Symp Proc. 2007:915. [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Fleming TR. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Holmberg SD, Palella FJ. Factors related to and consequences of adherence to antiretroviral therapy in an ambulatory HIV-infected patient cohort. AIDS Patient Care STDS. 2004;18(12):721–727. doi: 10.1089/apc.2004.18.721. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services (DHHS) Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1- infected adults and adolescents. 2011 Jan 10;:1–166. 2011: Available at http://aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf.
- Douglas GP, Killam WP, Hochgesang MS, Deula RA, Limbe W, Davis MK. Improving completeness, accuracy & timeliness of HIV voluntary counseling & testing client data in Malawi using touchscreen computers. AMIA Annu Symp Proc. 2005:942. [PMC free article] [PubMed] [Google Scholar]
- Ehrenkranz PD, Ahn CJ, Metlay JP, Camargo CA, Jr, Holmes WC, Rothman R. Availability of rapid human immunodeficiency virus testing in academic emergency departments. Acad Emerg Med. 2008;15(2):144–150. doi: 10.1111/j.1553-2712.2008.00028.x. [DOI] [PubMed] [Google Scholar]
- Fincher-Mergi M, Cartone KJ, Mischler J, Pasieka P, Lerner EB, Billittier A. Assessment of emergency department heatlh care professionals’ behaviors regarding HIV testing and referral for patients with STDs. AIDS Patient Care STDS. 2002;16549-53(11):549–553. doi: 10.1089/108729102761041100. J., 4th. [DOI] [PubMed] [Google Scholar]
- Fishbein M, Hennessy M, Kamb M, Bolan GA, Hoxworth T, Iatesta M, Zenilman JM. Using intervention theory to model factors influencing behavior change. Project RESPECT. Eval Health Prof. 2001;24(4):363–384. doi: 10.1177/01632780122034966. [DOI] [PubMed] [Google Scholar]
- Fonner VA, Denison J, Kennedy CE, O'Reilly K, Sweat M. Voluntary counseling and testing (VCT) for changing HIV-related risk behavior in developing countries. Cochrane Database Syst Rev. 2012;9:CD001224. doi: 10.1002/14651858.CD001224.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gift TL, Hogben M. Emergency department sexually transmitted disease and human immunodeficiency virus screening: findings from a national survey. Acad Emerg Med. 2006;13(9):993–996. doi: 10.1197/j.aem.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Glanz K, Rimer BK, Lewis FM, editors. Health Behavior and Health Education: Theory, Research and Practice (3rd ed.) San Francisco: Jossey-Bass; 2002. [Google Scholar]
- Glasgow RE, La Chance PA, Toobert DJ, Brown J, Hampson SE, Riddle MC. Long-term effects and costs of brief behavioural dietary intervention for patients with diabetes delivered from the medical office. Patient Educ Couns. 1997;32(3):175–184. doi: 10.1016/s0738-3991(97)00039-6. [DOI] [PubMed] [Google Scholar]
- Glasgow RE, Toobert DJ, Hampson SE. Effects of a brief office-based intervention to facilitate diabetes dietary self-management. Diabetes Care. 1996;19(8):835–842. doi: 10.2337/diacare.19.8.835. [DOI] [PubMed] [Google Scholar]
- Goggin MA, Davidson AJ, Cantril SV, O’Keefe LK, Douglas JM. The extent of undiagnosed HIV infection among emergency department patients: results of a blinded seroprevalence survey and a pilot HIV testing program. The Journal of Emergency Medicine. 2000;19(1):13–19. doi: 10.1016/s0736-4679(00)00175-x. [DOI] [PubMed] [Google Scholar]
- Golden MR, Manhart LE. Innovative approaches to the prevention and control of bacterial sexually transmitted infections. Infect Dis Clin North Am. 2005;19(2):513–540. doi: 10.1016/j.idc.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Hardwicke R, Malecha A, Lewis ST, Grimes RM. HIV testing in emergency departments: a recommendation with missed opportunities. J Assoc Nurses AIDS Care. 2008;19(3):211–218. doi: 10.1016/j.jana.2008.03.005. [DOI] [PubMed] [Google Scholar]
- Haukoos JS, Mehta SD, Harvey L, Calderon Y, Rothman RE. Research priorities for human immunodeficiency virus and sexually transmitted infections surveillance, screening, and intervention in emergency departments: consensus-based recommendations. Acad Emerg Med. 2009;16(11):1096–1102. doi: 10.1111/j.1553-2712.2009.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haukoos JS, White DA, Lyons MS, Hopkins E, Calderon Y, Kalish B, Rothman RE. Operational methods of HIV testing in emergency departments: a systematic review. Ann Emerg Med. 2011;58(1 Suppl 1):S96–S103. doi: 10.1016/j.annemergmed.2011.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins DL, Galavotti C, O'Reilly KR, Schnell DJ, Moore M, Rugg DL, Johnson R. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991;266(17):2419–2429. [PubMed] [Google Scholar]
- Hutchinson AB, Branson BM, Kim A, Farnham PG. A meta-analysis of the effectiveness of alternative HIV counseling and testing methods to increase knowledge of HIV status. AIDS. 2006;20(12):1597–1604. doi: 10.1097/01.aids.0000238405.93249.16. [DOI] [PubMed] [Google Scholar]
- Hutchinson AB, Corbie-Smith G, Thomas SB, Mohanan S, del Rio C. Understanding the patient’s perspective on rapid and routine HIV Testing in an inner-city urgent care center. AIDS Education and Prevention. 2004;16(2):101–114. doi: 10.1521/aeap.16.2.101.29394. [DOI] [PubMed] [Google Scholar]
- Jenkins TC, Gardner EM, Thrun MW, Cohn DL, Burman WJ. Risk-based human immunodeficiency virus (HIV) testing fails to detect the majority of HIV-infected persons in medical care Settings. Sex Transm Dis. 2006;33(5):329–333. doi: 10.1097/01.olq.0000194617.91454.3f. [DOI] [PubMed] [Google Scholar]
- Kamb ML, Fishbein M, Douglas JM, Jr, Rhodes F, Rogers J, Bolan G, Peterman TA. Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. Project RESPECT Study Group. JAMA. 1998;280(13):1161–1167. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- Kecojevic A, Lindsell CJ, Lyons MS, Holtgrave D, Torres G, Heffelfinger J, Rothman RE. Public health and clinical impact of increasing emergency department-based HIV testing: perspectives from the 2007 conference of the National Emergency Department HIV Testing Consortium. Ann Emerg Med. 2011;58(1 Suppl 1):S151–S159. e151. doi: 10.1016/j.annemergmed.2011.03.040. [DOI] [PubMed] [Google Scholar]
- Kelen GD, Shahan JB, Quinn TC. Emergency department-based HIV screening and counseling: experience with rapid and standard serologic testing. Ann Emerg Med. 1999;33(2):147–155. doi: 10.1016/s0196-0644(99)70387-2. [DOI] [PubMed] [Google Scholar]
- Kellerman SE, Lehman JS, Lansky A, Stevens MR, Hecht FM, Bindman AB, Wortley PM. HIV testing within at-risk populations in the United States and the reasons for seeking or avoiding HIV testing. J Acquir Immune Defic Syndr. 2002;31(2):202–210. doi: 10.1097/00126334-200210010-00011. [DOI] [PubMed] [Google Scholar]
- Kiene SM, Barta WD. A brief individualized computer-delivered sexual risk reduction intervention increases HIV/AIDS preventive behavior. Journal of Adolescent Health. 2006;39(3):404–410. doi: 10.1016/j.jadohealth.2005.12.029. [DOI] [PubMed] [Google Scholar]
- Kraus C, Rothman RE, Shahan JB, Quinn TC, Kelen GD. Trends in human immunodeficiency infection in the emergency department: a 16-year review. Acad Emerg Med. 2006;13(5) Suppl. 1:S22. [Google Scholar]
- Liddicoat RV, Horton NJ, Urban R, Maier E, Christiansen D, Samet JH. Assessing missed opportunities for HIV testing in medical settings. Journal of General Internal Medicine. 2004;19(4):349–356. doi: 10.1111/j.1525-1497.2004.21251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lightfoot M, Comulada WS, Stover G. Computerized HIV preventive intervention for adolescents: indications of efficacy. Am J Public Health. 2007;97(6):1027–1030. doi: 10.2105/AJPH.2005.072652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MS, Lindsell CJ, Ledyard HK, Frame PT, Trott AT. Health department collaboration with emergency departments as a model for public health programs among at-risk populations. Public Health Rep. 2005;120(3):259–265. doi: 10.1177/003335490512000307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks G, Richardson JL, Crepaz N, Stoyanoff S, Milam J, Kemper C, McCutchan A. Are HIV care providers talking with patients about safer sex and disclosure?: A multi-clinic assessment. AIDS. 2002;16(14):1953–1957. doi: 10.1097/00002030-200209270-00013. [DOI] [PubMed] [Google Scholar]
- Marsch LA, Bickel WK. Efficacy of computer-based HIV/AIDS education for injection drug users. Am J Health Behav. 2004;28(4):316–327. doi: 10.5993/ajhb.28.4.3. [DOI] [PubMed] [Google Scholar]
- Merchant RC, Clark MA, Langan TJ, 4th, Mayer KH, Seage GR, 3rd, DeGruttola VG. Can computer-based feedback improve emergency department patient uptake of rapid HIV screening? Ann Emerg Med. 2011;58(1 Suppl 1):S114–S119. e111–e112. doi: 10.1016/j.annemergmed.2011.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalf CA, Douglas JM, Jr, Malotte CK, Cross H, Dillon BA, Paul SM, Peterman TA. Relative efficacy of prevention counseling with rapid and standard HIV testing: a randomized, controlled trial (RESPECT-2) Sex Transm Dis. 2005;32(2):130–138. doi: 10.1097/01.olq.0000151421.97004.c0. [DOI] [PubMed] [Google Scholar]
- Mooney BL. HIV-testing consent requirements still vary by state. Medical Economics: smarter business, better patient care. 2011 Nov 3; 2011. Available at http://medicaleconomics.modernmedicine.com/news/hiv-testing-consent-requirements-still-vary-state. Accessed on January 22, 2013. [Google Scholar]
- Murphy EL, Collier AC, Kalish LA, Assmann SF, Para MF, Flanigan TP, Nemo GJ. Highly active antiretroviral therapy decreases mortality and morbidity in patients with advanced HIV disease. Ann Intern Med. 2001;135(1):17–26. doi: 10.7326/0003-4819-135-1-200107030-00005. [DOI] [PubMed] [Google Scholar]
- Murphy K, Grusky O, Roberts KJ, Swanson AN. HIV testing in an urgent-care clinic. Sex Health. 2005;2(4):245–250. doi: 10.1071/sh05026. [DOI] [PubMed] [Google Scholar]
- Neff S, Goldschmidt R. Centers for Disease Control and Prevention 2006 human immunodeficiency virus testing recommendations and state testing laws. JAMA. 2011;305(17):1767–1768. doi: 10.1001/jama.2011.564. [DOI] [PubMed] [Google Scholar]
- Neumann T, Neuner B, Weiss-Gerlach E, Tonnesen H, Gentilello LM, Wernecke KD, Spies CD. The effect of computerized tailored brief advice on at-risk drinking in subcritically injured trauma patients. J Trauma. 2006;61(4):805–814. doi: 10.1097/01.ta.0000196399.29893.52. [DOI] [PubMed] [Google Scholar]
- Noell J, Biglan A, Hood D, Britz B. An interactive videodisc-based smoking cessation program: prototype development and pilot test. Computers in Human Behavior. 1994;10:347–358. [Google Scholar]
- Palella FJ, Jr, Deloria-Knoll M, Chmiel JS, Moorman AC, Wood KC, Greenberg AE, Holmberg SD. Survival benefit of initiating antiretroviral therapy in HIVinfected persons in different CD4+ cell strata. Ann Intern Med. 2003;138(8):620–626. doi: 10.7326/0003-4819-138-8-200304150-00007. [DOI] [PubMed] [Google Scholar]
- Paltiel AD, Walensky RP, Schackman BR, Seage GR, 3rd, Mercincavage LM, Weinstein MC, Freedberg KA. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]
- Rhodes KV, Lauderdale DS, He T, Howes DS, Levinson W. "Between me and the computer": increased detection of intimate partner violence using a computer questionnaire. Ann Emerg Med. 2002;40(5):476–484. doi: 10.1067/mem.2002.127181. [DOI] [PubMed] [Google Scholar]
- Rothman RE. Current Centers for Disease Control and Prevention guidelines for HIV counseling, testing, and referral: critical role of and a call to action for emergency physicians. Ann Emerg Med. 2004;44(1):31–42. doi: 10.1016/j.annemergmed.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Rothman RE, Ketlogetswe KS, Dolan T, Wyer PC, Kelen GD. Preventive care in the emergency department: should emergency departments conduct routine HIV screening? a systematic review. Acad Emerg Med. 2003;10(3):278–285. doi: 10.1111/j.1553-2712.2003.tb02004.x. [DOI] [PubMed] [Google Scholar]
- Saifu HN, Shamouelian A, Davis LG, Santana-Rios E, Goetz MB, Asch SM, Sun BC. Impact of a kiosk educational module on HIV screening rates and patient knowledge. J Telemed Telecare. 2011;17(8):446–450. doi: 10.1258/jtt.2011.110415. [DOI] [PubMed] [Google Scholar]
- Sellors JW, Hayward R, Swanson G, Ali A, Haynes RB, Bourque R, Howard M. Comparison of deferral rates using a computerized versus written blood donor questionnaire: a randomized, cross-over study [ISRCTN84429599] BMC Public Health. 2002;2:14. doi: 10.1186/1471-2458-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberg F, Branson BM, Goldbaum GM, Lockhart D, Kurth A, Celum CL, Wood RW. Overcoming barriers to HIV testing: preferences for new strategies among clients of a needle exchange, a sexually transmitted disease clinic, and sex venues for men who have sex with men. J Acquir Immune Defic Syndr. 2003;32(3):318–327. doi: 10.1097/00126334-200303010-00012. [DOI] [PubMed] [Google Scholar]
- Spielberg F, Kurth AE, Severynen A, Hsieh YH, Moring-Parris D, Mackenzie S, Rothman R. Computer-facilitated rapid HIV testing in emergency care settings: provider and patient usability and acceptability. AIDS Educ Prev. 2011;23(3):206–221. doi: 10.1521/aeap.2011.23.3.206. [DOI] [PubMed] [Google Scholar]
- Sundaram V, Lazzeroni LC, Douglass LR, Sanders GD, Tempio P, Owens DK. A randomized trial of computer-based reminders and audit and feedback to improve HIV screening in a primary care setting. Int J STD AIDS. 2009;20(8):527–533. doi: 10.1258/ijsa.2008.008423. [DOI] [PubMed] [Google Scholar]
- Swartz LH, Noell JW, Schroeder SW, Ary DV. A randomised control study of a fully automated internet based smoking cessation programme. Tob Control. 2006;15(1):7–12. doi: 10.1136/tc.2003.006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao G, Branson BM, Anderson LA, Irwin KL. Do physicians provide counseling with HIV and STD testing at physician offices or hospital outpatient departments? AIDS. 2003;17(8):1243–1247. doi: 10.1097/00002030-200305230-00017. [DOI] [PubMed] [Google Scholar]
- US Preventive Services Task Force (USPSTF) Screening for HIV: U.S. Preventive Services Task Force Recommendation Statement DRAFT. 2012 Available at http://www.uspreventiveservicestaskforce.org/uspstf13/hiv/hivdraftrec.htm. [Google Scholar]
- Walensky RP, Losina E, Malatesta L, Barton GE, O'Connor CA, Skolnik PR, Freedberg KA. Effective HIV case identification through routine HIV screening at urgent care centers in Massachusetts. Am J Public Health. 2005;95(1):71–73. doi: 10.2105/AJPH.2003.031310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welz T, Herbst K. Anonymous HIV testing with participant-controlled access to results using handheld computers: a new model of HIV testing used in a household survey in rural South Africa. Sex Transm Dis. 2008;35(4):372–376. doi: 10.1097/OLQ.0b013e31815fd3fc. [DOI] [PubMed] [Google Scholar]
- White DAE, Orujela DJ, Lowery D, Frazee B. Preliminary report of feasibility and yield of a rapid human immunodeficiency virus screening program in an urban emergency department using indigenous staff for testing and counseling. Acad Emerg Med. 2006;13(5 Suppl. 1):S23. [Google Scholar]
- Wilbur L, Huffman G, Lofton S, Finnell JT. The use of a computer reminder system in an emergency department universal HIV screening program. Ann Emerg Med. 2011;58(1 Suppl 1):S71–S73. e71. doi: 10.1016/j.annemergmed.2011.03.028. [DOI] [PubMed] [Google Scholar]
- Wilson SR, Mitchell C, Bradbury DR, Chavez J. Testing for HIV: current practices in the academic ED. Am J Emerg Med. 1999;17(4):354–356. doi: 10.1016/s0735-6757(99)90085-2. [DOI] [PubMed] [Google Scholar]