Abstract
Background
To retrospectively evaluate outcomes in patients with cutaneous angiosarcoma of the face/scalp treated curatively with surgery, radiation therapy (RT), or a combination of surgery and RT.
Methods
70 patients with non-metastatic angiosarcoma underwent surgery, RT, or combined-modality therapy. Of these, 20 (29%) were treated with surgery alone, 27 (39%) with RT alone, and 23 (33%) with combined-modality therapy. 44 patients received chemotherapy, either neo-adjuvantly or adjuvantly or both.
Results
Median follow-up was 2.1 years. The overall survival (OS) rate was 43% at 5 years, and disease specific survival was 46% at 5 years. Tumor size > 5 cm and satellitosis were prognostic for inferior OS and DSS. Combined-modality therapy (vs. surgery alone or RT alone) was associated with improved OS, DSS, and local control (LC).
Conclusion
Primary local therapy with combined-modality therapy was associated with improved LC, OS, and DSS for patients with angiosarcoma of the face/scalp.
Keywords: cutaneous, angiosarcoma, scalp, radiation therapy, surgery, local control
INTRODUCTION
Angiosarcomas are rare tumors, comprising only 1% of all soft tissue sarcomas. (1) Cutaneous angiosarcoma is the most common presentation of this malignancy with one-third of angiosarcomas occurring in the skin. Head and neck presentations comprise the most common anatomic sites of cutaneous angiosarcoma. (1) Etiologic factors for cutaneous angiosarcoma of the face and scalp remain poorly understood.(2) However, clinical studies have provided insight into the natural history of this disease and indicate that delays in diagnosis can occur given the relatively benign-appearing clinical presentation of bruise-like lesions on the face or scalp or lesions that initially look like benign hemangiomas.(3-5) Furthermore, delays in diagnosis can arise from involvement of the scalp and under-appreciation of the extent of disease due to hair cover. Skip lesions are also common making the full extent of cutaneous territory involved difficult to appreciate for clinicians not familiar with this disease. Clinical outcomes reflect these aspects of angiosarcoma of the face and scalp presentation and the corresponding difficulty controlling the primary tumor with local recurrence rates ranging from 43% to 100%.(6) (7) (2, 3, 8-10) Overall survival rates are also low, ranging from 10% to 51%.(3, 5-12)
Given the potentially diffuse nature of involvement of the scalp or face by the primary tumor and the difficulty in obtaining negative margins, (3) there is some diversity among reported series in recommended approaches to definitive local management with respect to extent of surgery and whether or not to employ both surgery and radiation versus only one of these local modalities. Most studies assert that surgery combined with radiation therapy is the optimal approach to eradicate the primary tumor.(3, 8-10, 12, 13) Some authors have argued that the high rates of distant metastases and corresponding limited survival horizon in patient with this disease justify a more limited surgical procedure so as not to delay further treatment with wound healing, complex reconstructive procedures, or surgical complications. (4) Some investigators have even suggested that definitive radiation therapy (without surgical resection) with or without chemotherapy may offer sufficient primary local therapy.(5, 6, 14) This suggestion is given particular emphasis for settings where chemotherapy and or radiation therapy has affected a complete response with regression of the primary tumor such that surgical planning may be hindered by lack of visible residual tumor. (14, 15) Studies reporting on outcomes for patients with cutaneous angiosarcoma of the face and scalp are comprised of relatively small series ranging from 13 to 72 patients (median, 20 patients). (2-5, 7-14) The largest series of 72 patients was published in 1986, and included patients with metastatic disease as well as patients whose goal of treatment was palliation only. In the current study, we have undertaken a review of our clinical experience of curative treatment for patients with non-metastatic angiosarcoma of the face and scalp at the University of Texas M.D. Anderson Cancer Center. We report on treatment outcomes, prognostic factors, and local therapy-related complications. One goal of this investigation is to provide data regarding the optimal approach to definitive local therapy for patients with this disease.
PATIENTS AND METHODS
Between 1962 and 2009, 75 patients with non-metastatic cutaneous angiosarcoma of the scalp and face received definitive local treatment at MD Anderson Cancer Center. Of these, 5 patients were lost to follow-up. The current study is comprised of the remaining 70 patients who underwent primary local therapy with surgery, radiation (RT), or a combination of surgery and RT and/or chemotherapy. Review of the data for this investigation commenced after approval was obtained from our institution's investigation review board and a waiver of informed consent was granted due to the retrospective nature of this study. Patients underwent a full history, complete physical examination, routine blood tests, and appropriate imaging before their treatment. A histologic diagnosis of angiosarcoma was confirmed in each case through a review of the slides by a pathologist at MD Anderson Cancer Center at the time of presentation.
Patient and tumor characteristics
Of the 70 patients, 50 (71%) were male and 20 (29%) were female. Ages ranged from 9 to 85 years (median, 71 years). The distribution of primary lesions was as follows: 39 (56%) on the scalp, 30 (43%) on the face, and 1 (1%) on the ear. Gross tumor size was documented in 60 patients and ranged from 0.2 cm to 12 cm in maximal dimension. The clinically apparent tumor size was ≤ 5 cm in 49 patients (82%) and > 5 cm in 11 patients (18%), with a median size of 3.0 cm. The presence of satellite lesions on the scalp or face was documented in 32 patients (46%), and satellites were absent in 38 patients (54%). Bone invasion occurred in 3 patients (4%). Seven patients (10%) had regional nodal involvement at presentation. Ten patients (14%) presented with disease recurrence after having undergone 1 (8 patients) or more (2 patients) previous attempts at definitive surgery.
Treatment
Because MD Anderson Cancer Center is a referral center, most patients had undergone at least a biopsy of their tumor, and 13 (19%) had had their tumors grossly excised before referral. Definitive local therapy consisted of surgery alone in 20 patients (29%), RT alone in 23 patients (33%), and a combination of surgery and RT in 27 patients (39%). When treatment included both surgery and RT, the RT was delivered post-operatively in all but one case. Of the 57 patients with gross disease at presentation to MD Anderson Cancer Center, 27 (47%) received RT alone to the primary site, while 17 (30%) received surgery alone and 13 (23%) received both surgery and RT. Of the 10 patients who presented to MD Anderson Cancer Center with microscopic disease (positive margin after outside excision or biopsy), 7 were treated with combined surgery and RT, while 3 underwent additional surgery alone. One patient had uncertain margins after outside excision and was treated at MD Anderson Cancer Center with combined surgery and RT. There was no significant association (p=0.88) between tumor size (≤ 5 cm vs. >5 cm) and primary local treatment strategy (surgery vs. RT vs. surgery + RT). Soft tissue reconstruction (e.g., split thickness skin graft) was performed at the primary site in 33 patients and bone reconstruction was performed in 2 patients.
Radiation therapy was delivered with megavoltage (Cobalt-60 or higher) photons or electrons using techniques appropriate to the site of the tumor. The median RT dose for patients treated with RT after surgery was 60 Gy (range, 60.0-70.0 Gy) and the median dose per fraction was 2.0 Gy. The median RT dose for patients treated with RT alone was also 60 Gy (range, 42.0 Gy-75.0 Gy) at a median dose of 2.0 Gy per fraction. Twenty one patients received radiation therapy to the total scalp (16) and 19 patients received radiation therapy to regional lymphatics along with the primary site. For the 27 patients who were treated with RT alone to the primary site, 23 (85%) experienced a clinical complete response, 2 patients (7%) had a partial response, and 2 (7%) experience progressive disease after RT.
Forty-four patients (63%) received chemotherapy as part of up-front management of their disease, either neo-adjuvantly or as an adjuvant to primary local therapy. There was no significant association between the size of the primary lesion (≤ 5 cm vs. > 5 cm, p=0.61) and receipt of chemotherapy. Thirty three-patients (47%) received neo-adjuvant chemotherapy. Neo-adjuvant chemotherapy consisted of gemcitabine and docetaxel in 10 of these patients and paclitaxel alone in 5 patients. Eighteen of these patients received other chemotherapy regimens including: doxorubicin/ifosfamide, CYADIC (cyclosphosphamide, doxorubicin, dacarbazine), interferon, vincristine, doxorubicin/paclitaxel, and various combinations of these drugs. Of these 33 patients, 29 (88%) were documented to have had a response to neo-adjuvant chemotherapy, and 11 (33%) of these had a complete response. Two patients (6%) had progressive disease after receiving neo-adjuvant chemotherapy, and 2 patients experienced neither response nor progression of their disease. Twenty patients (29%) received adjuvant chemotherapy after completion of definitive local therapy. Nine patients (13%) received both neoadjuvant and adjuvant chemotherapy.
Statistical analysis and follow-up
The median follow-up time of patients alive at last contact was 2.1 years (range, 2.4 mos-40 years).
The Kaplan-Meier method (17) was used to calculate actuarial curves for overall survival (OS), disease-free survival (DFS), disease-specific survival (DSS), local control (LC), distant metastatic recurrence (DM), and development of complications, and the log-rank statistic was used to test for significance. Multivariate regression was performed using Cox proportional hazards model.(18) Differences between proportions for categorical variables were analyzed using the chi-square statistic or Fisher's exact test as appropriate.(19) Local recurrence was defined as any recurrence in the skin above the clavicles. Surgical and RT-related complications were scored as follows: mild (self-limited and requiring no treatment), moderate (requiring conservative medical management), and severe (requiring surgical intervention or hospitalization).
RESULTS
Survival
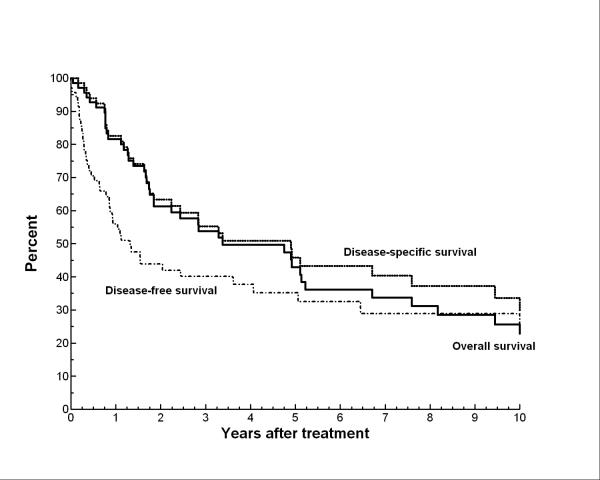
Thirty-seven patients (53%) died of their angiosarcoma. The actuarial OS rates at 5 years and 10 years were 43% and 23%, respectively. Median survival was 41 months. The DSS survival rates were 46% and 30%, respectively at 5 and 10 years. The DFS was 35% and 23% at 5 and 10 years, respectively. The last disease recurrence occurred at 12 years. Figure 1 shows the actuarial curves for OS, DSS, and DFS.
Figure 1.
Overall survival, disease-free survival, and disease-specific survival for the entire study population
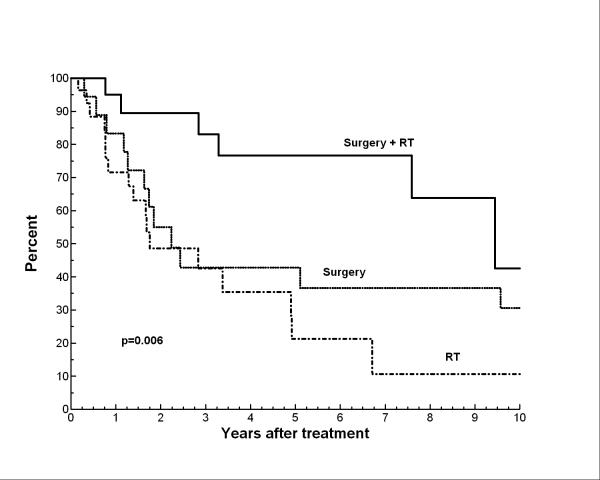
Table 1 shows the univariate analyses for OS as well as DSS. Patients with tumors > 5 cm had significantly worse OS and DSS (p=0.002 and p=0.006, respectively). The presence of satellite lesions around the primary tumor at diagnosis also portended a significantly worse OS and DSS (p=0.02). Scalp location of primary tumor was associated with worse DSS compared to those tumors presenting on the face/ear (p=0.02). Among treatment-related factors, only combined modality local therapy (surgery+ RT vs. surgery alone or RT alone) was significantly associated with improved OS or DSS (Figure 2a and 2b). Neither margin status after surgical resection among those who underwent surgery nor the administration of chemotherapy was associated with any significant differences in OS or DSS.
Table 1.
Univariate analysis for overall survival and disease-specific survival
| Characteristic | No. of patients (%) | 5-yr OS | p value | 5-yr DSS | p value |
|---|---|---|---|---|---|
| Entire cohort | 70 | 43% | 46% | ||
| Gender | |||||
| Male | 50 (71) | 36% | 0.85 | 40% | 0.55 |
| Female | 20 (29) | 57% | 61% | ||
| Tumor size | |||||
| ≤ 5 cm | 49 (82)* | 42% | 0.002 | 42% | 0.006 |
| > 5 cm | 11(18) | 12% | 22% | ||
| Site | |||||
| Scalp | 39 (56) | 36% | 0.18 | 34% | 0.03 |
| Face/ear | 31 (44) | 51% | 60% | ||
| Satellites at presentation | |||||
| No | 38 (54) | 58% | 0.02 | 63% | 0.02 |
| Yes | 32 (46) | 20% | 20% | ||
| Nodal disease at diagnosis | |||||
| No | 63 (90) | 43% | 0.89 | 46% | 0.93 |
| Yes | 7 (10) | 38% | 44% | ||
| Local therapy | |||||
| Surgery alone | 20 (29) | 40% | 0.01 | 43% | 0.006 |
| RT alone | 27 (39) | 22% | 22% | ||
| Surgery + RT | 23 (33) | 68% | 76% | ||
| Local therapy | |||||
| Single modality (S or RT) | 32% | 0.01 | 33% | 0.006 | |
| Combined modality (S + RT) | 68% | 76% | |||
| Surgical resection margin** | |||||
| Positive/uncertain | 23 (53) | 60% | 0.80 | 73% | 0.84 |
| Negative | 20 (47) | 47% | 47% | ||
| Neoadjuvant chemotherapy | |||||
| No | 37 (53) | 47% | 0.73 | 53% | 0.47 |
| Yes | 33 (47) | 36% | 36% | ||
| Adjuvant chemotherapy | |||||
| No | 50 (71) | 43% | 0.40 | 49% | 0.52 |
| Yes | 20 (29) | 44% | 42% | ||
| Any chemotherapy (neoadjuvant, adjuvant, or both) | |||||
| No | 26 (37) | 39% | 0.54 | 47% | 0.76 |
| Yes | 44 (63) | 45% | 45% | ||
| Total scalp RT | |||||
| No | 49 (70) | 47% | 0.28 | 51% | 0.11 |
| Yes | 21 (30) | 34% | 34% | ||
cm= centimeter; S= surgery; RT= radiation therapy; OS=overall survival; DSS= disease specific survival;
Tumor size was not available for 10 patients.
Only includes the 43 pts who received surgery (with or without RT) for primary local therapy
Figure 2.
Disease-specific survival by definitive local therapy
On multivariate analysis, local treatment with single modality (surgery or RT alone vs. surgery+ RT; p=0.02) and larger tumor size (as a continuous variable; p=0.008) were significantly predictive of worse OS survival. For DSS, local treatment with a single modality (surgery or RT vs. surgery + RT; p=0.004), larger tumor size (p=0.003), and scalp location of primary tumor (vs. face/ear; p=0.01) were predictive of worse DSS on multivariate analysis.
Patterns of disease recurrence
Of the 70 patients, 44 (63%) experienced disease recurrence. Thirty-seven patients (53%) experienced local recurrence (LR). The actuarial LC rates at 5 and 10 years were 43% and 29%, respectively. The interval to development of LR was < 1 month to 12 years (median, 11 months), and 34 of the 37 LRs (92%) had occurred by 5 years. Eleven patients (16%) experienced nodal relapse, and the actuarial rate of nodal relapse was 21% at 5 years. Univariate analysis of prognostic factors including tumor size, presence of satellites, nodal disease at diagnosis, receipt of chemotherapy (neoajuvant, adjuvant, or any chemo), and definitive local management strategy showed no factor to be significantly predictive of nodal relapse. Twenty-five patients (36%) developed DM. The interval to development of DM was < 1 month to 10.7 years (median, 15 months). Of these 11 had DM to one site (17 to the lung, 3 to bone, 3 to liver, 1 to pleural fluid, 1 to epidural tissues), and 14 had DM to multiple sites (lung, bone, liver, distant skin metastasis, kidney, viscera, and brain). The actuarial rate of DM development was 45% at 5 years and beyond. On univariate analyses (Table 2), patients with satellite lesions around the primary tumor at diagnosis were significantly more likely to develop DM than those without satellitosis (p=0.003). On multivariate analysis that included tumor size (continuous variable), modality(ies) of definitive local therapy, treatment with chemotherapy, and presence of satellite lesions, only the presence of satellite lesions (p=0.009) and tumor size (p=0.03) were significantly predictive for the development of DM.
Table 2.
Local control: univariate analysis for all 70 patients
| Characteristic | No. of patients (%) | 5-yr LC | p value | 5-yr DMFS | p value |
|---|---|---|---|---|---|
| Entire cohort | 70 | 43% | 55% | ||
| Sex | |||||
| Male | 50 (71) | 45% | 0.91 | 48% | 0.19 |
| Female | 20 (29) | 40% | 74% | ||
| Tumor size | |||||
| ≤ 5 cm | 49 (82)* | 55% | 0.05 | 53% | 0.34 |
| > 5 cm | 11(18) | 0% | 0% | ||
| Site | |||||
| Scalp | 39 (56) | 46% | .50 | 53% | 0.26 |
| Face/ear | 31 (44) | 31% | 58% | ||
| Satellites at presentation | |||||
| No | 38 (54) | 49% | 0.08 | 67% | 0.003 |
| Yes | 32 (46) | 38% | 35% | ||
| Nodal disease at diagnosis | |||||
| No | 63 (90) | 39% | 0.19 | 56% | 0.78 |
| Yes | 7 (10) | 83% | 48% | ||
| Local therapy | |||||
| Surgery alone | 20 (29) | 25% | 0.0003 | 69% | 0.03 |
| RT alone | 27 (39) | 22% | 24% | ||
| Surgery + RT | 23 (33) | 84% | 67% | ||
| Local therapy | |||||
| Single modality (S or RT) | 24% | 0.0001 | 48% | 0.19 | |
| Combined modality | 84% | 67% | |||
| Surgical resection margin** | |||||
| Positive/uncertain | 23 (53) | 62% | 0.55 | 72% | 0.81 |
| Negative | 20 (47) | 46% | 63% | ||
| Neoadjuvant chemotherapy | |||||
| No | 37 (53) | 41% | 0.99 | 69% | 0.06 |
| Yes | 33 (47) | 44% | 38% | ||
| Adjuvant chemotherapy | |||||
| No | 50 (71) | 34% | 0.04 | 60% | 0.53 |
| Yes | 20 (29) | 63% | 45% | ||
| Any chemotherapy (neoadjuvant, adjuvant, or both) | |||||
| No | 26 (37) | 29% | 0.11 | 72% | 0.16 |
| Yes | 44 (63) | 52% | 45% | ||
| Total scalp RT | |||||
| No | 49 (70) | 44% | 0.56 | n/a | |
| Yes | 21 (30) | 39% | |||
cm= centimeter; RT= radiation therapy; LC=local control rate; DMFS=distant metastasis free survival; CMT=combined modality local therapy (surgery + RT or RT + surgery)
Tumor size was not available for 10 patients.
Only includes the 43 pts who received surgery (with or without RT) for primary local therapy
LR and the primary local therapy
Of the 50 patients who received RT either alone or in combination with surgery, 22 (44%) experienced LR. Fifteen of these (68%) were within the irradiated field, 5 (23%) were at the field edge, and 2 were outside of the RT field.
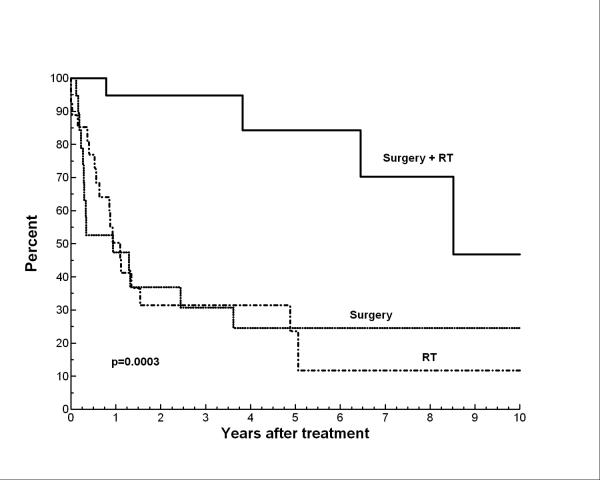
On univariate analyses (Table 2), local control was significantly better if definitive local therapy included both surgery and RT (vs. surgery alone or RT alone) as shown in Figure 3. Similarly, on multivariate regression analyses that included definitive local therapy strategy (surgery or RT vs. surgery + RT), tumor size, presence of satellite lesions, receipt of chemotherapy (any, neoadjuvant, adjuvant) revealed that the only factor significantly predictive of better LC was local therapy with surgery+ RT (p=0.0003). Also, in the multivariate analysis larger tumor size, as a continuous variable, was of borderline predictive significance (p=0.05) for local recurrence.
Figure 3.
Local control by definitive local therapy
Outcomes after recurrence
At 5 and 10 years after recurrence (either LR or DM), OS was 14% and 6%, respecitively. Similarly, DSS was 15% and 6% at those respective time points. There was no significant difference in survival after relapse with respect to whether relapse was LR only or involved DM (18% vs. 8%, respectively at 5 yrs; p=0.44). Thus, isolated LR portended as poor a prognosis as relapse with DM. There was also no significant difference in OS after relapse with respect to approach to local salvage treatment (14% for surgery or RT vs. 17% for surgery + RT at 5 yrs; p=0.91).
Complications from treatment
Of the 34 patients who underwent surgery at MD Anderson Cancer Center, 5 (15%) experienced a surgical complication, and all were characterized as either moderate (1 patient with a pseudomonas infection of the surgical site) or severe (3 patients with skin flap failure requiring surgical repair; and 1 patient with acquired ectropion requiring multiple reconstructive procedures). The actuarial rate of surgical complications was 16% at 5 years. The median time to surgical complication was 4 months (range, 0-7.6 months).
Of the 50 patients who were treated with RT (with or without surgery) for local management, 15 (30%) experienced an RT-induced complications. Of these 4 were considered mild complications (2 cases of xerostomia, 2 cases of non-functionally significant fibrosis), 6 were considered moderate (4 cases of clinically significant dryeye, 2 cases of chronic ulceration/cellulitis of scalp requiring long-term IV antibiotics), and 5 were considered severe (2 case of tissue necrosis requiring debridement, 2 cases of severe ocular complications requiring surgical intervention, 1 case of severe fibrosis of eyelids requiring surgical manipulation). Actuarial rate of RT-induced complications was 47% at 5 years. The median time to development of RT-induced complications was 5 months (range, 0.5 months to 51 months). Total scalp RT was not significantly associated with higher rates of RT-induced complications than more limited fields (58% vs. 38% at 5 years, respectively; p=0.45). Treatment with combined modality local therapy (surgery + RT) was not associated with a higher rates of RT-induced complications than RT alone (47% vs. 45% at 5 years, respectively; p=0.91).
DISCUSSION
The results of this analysis of the largest series of patients treated curatively for non-metastatic cutaneous angiosarcoma of the face and scalp demonstrate that combined modality local therapy that includes both surgical resection and radiation therapy improves LC, DSS, and OS for patients with angiosarcoma of the face and scalp compared to those who undergo surgery alone or radiation therapy alone for their primary local therapy. Overall, almost two-thirds of patients experienced local recurrence of their disease while only 36% developed DM; and isolated local recurrence portended as poor a prognosis as did the development of DM. This highlights the critical role definitive local management plays in controlling this disease.
We identified certain tumor characteristics that were associated with poor prognosis, namely: tumor size > 5 cm, the presence of satellite cutaneous lesions, and scalp location of the primary tumor (vs. face). Other studies have reported upon prognostic factors in this disease. Tumor size has consistently been shown to be a predictor of outcome in this disease with larger tumor size being associated with inferior local control and/or overall survival by multiple investigators. (5, 10, 12) (3, 6, 7) Pawlik and colleagues were the only other investigators to analyze significance of satellitosis and reported that the presence of multiple or satellite lesions was significantly predictive of shorter time to recurrence. Lastly, we also found the scalp location of the primary tumor was associated with worse disease-specific survival. Ward and colleagues (10) reported a similar finding in their study revealing that scalp location was associated with poorer local control and overall survival.
Our findings regarding the importance of combined modality local therapy employing both surgery and radiation therapy are consistent with those of other investigators. Pawlik and colleagues,(3) in their series of 29 patients with angiosarcoma of the scalp, demonstrated that the addition of RT to surgical excision significantly improved both local control and overall survival. Mark and colleagues (8) also found, in a cohort that included 28 patients with angiosarcoma of the face and scalp, that surgery plus RT was associated with better local control and disease-free survival compared to patients treated with surgery alone or RT alone to the primary site of disease. However, other investigators have reported data suggesting that RT alone may be sufficient for local therapy. Specifically, two series from Japan, one by Sasaki and colleagues(6) and one by Ohguri and colleagues, (14) did not show a prognostic significance to surgery plus RT compared to RT alone in series that included 24 patients and 20 patients, respectively with AFS.
While one study by Morgan and colleagues, (7) which included 44 patients with angiosarcoma of the face and scalp, did show that positive surgical margin was associated with poorer overall survival, in our investigation, surgical margin status did not appear to significantly influence disease outcomes. The lack of prognostic significance of surgical margin in angiosarcoma of the face and scalp has also been reported by other investigators as well. (3, 10, 12) In fact, Pawlik and colleagues reported that margins were “chased” by intraoperative frozen section only to find that final permanent pathologic sections actually revealed the margins were ultimately positive. Their investigation lead these authors to report that positive margins were virtually impossible to obtain in angiosarcoma of the face and scalp, especially for patients with scalp presentations and that consequently, wide-field post-operative RT should be used routinely for these patients. These findings along with the fact that angiosarcoma of the face and scalp presents in a diffuse and multifocal nature lends credence to an assertion by Buschmann and associates (4) that the surgical and reconstructive goal should not be to obtain an R0 resection but rather be one of a time-saving debulking procedure with low potential for complications that might delay further therapy. Thus, while surgery is a critical component of definitive local therapy, it does not appear that a radical wide excision with high potential for complications, delayed wound healing, or 2-step reconstructive surgical procedure is necessary or even desirable in the setting of combined modality local therapy which includes RT. In fact, our current approach is gross tumor resection (typically after neoadjuvant chemotherapy) without an attempt at negative margins and with a goal of robust yet simple reconstruction (such as primary closure or local flap rather than skin graft) in order to facilitate tolerance of subsequent post-operative radiotherapy.
Our study did not show an improvement in outcomes with the administration of chemotherapy. The benefit or lack thereof of adjuvant or neoadjuvant chemotherapy is not reliably ascertained from a retrospective, non-randomized case series. The only other series to specifically report on the prognostic influence of chemotherapy also failed to show a significant improvement in disease-free survival with the administration of chemotherapy.(8) The applicability of these data is limited by the heterogenous nature of chemotherapeutic agents administered to the patients in both relatively small cohorts. Furthermore, recent literature suggests that taxane-based chemotherapy offers clinical benefit to patients with angiosarcoma.(20) Indeed some patients have experienced complete response with administration of paclitaxel-based regimens.(15, 21) Currently, we typically employ such neoadjuvant taxane-based chemotherapy in most cases of cutaneous angiosarcoma of the face and scalp. Other investigators have reported promising results with recombinant interleukin-2 immunotherapy-based regimens.
There are obvious limitations to our study, specifically with respect to the limited number of patients and retrospective nature of the data collection. The heterogeneous management approaches among our patients likely reflects selection bias with respect to modalities employed and their sequencing. Specifically, patients with tumors that were more difficult to extirpate or have a worse prognosis at the time of presentation may have been more likely to receive neoadjuvant chemotherapy or referred for radiation therapy alone. Furthermore, these treatments were delivered over several treatment eras with their respective different technologies and surgical or radiation therapy techniques.
In conclusion, we found that combined modality local therapy that includes both surgery and radiation therapy improved LC, DSS, and OS for patients presenting with non-metastatic angiosarcoma of the face and scalp. In our institution, patients are offered neoadjuvant taxane-based chemotherapy. Once maximal response is obtained or chemotherapeutic tolerance has been reached, patients are referred for surgical resection of any residual primary tumor. Postoperative radiation therapy is routinely recommended. Whenever possible, it is preferable that the radiation oncologist and surgeon see the patient in consultation at disease presentation so as to appreciate the full extent of disease prior to any response to chemotherapy. Diagrams or medical photographs may also be helpful at initial consult to aid in local therapy planning after chemotherapy. In the setting of a scalp primary, the total scalp is not specifically targeted (16) unless it is known to be involved at disease presentation. The primary tumor and all satellite lesions with a 3-5cm margin are encompassed by a single or multiple matched appositional electron fields. Other techniques such as intensity-modulated radiation therapy or matched photon/electron technique may be necessary depending on the extent and location of disease involvement. Tissue equivalent bolus material is applied to the skin surface to ensure adequate dose to the entire thickness of the dermal tissues and to minimize dose to underlying brain tissue. For primary lesions occurring on the face, lead mask skin collimation and/or tungsten eye shields may be needed for shielding of the eyes. Custom wax bolus or other similar device may be necessary to provide adequate dose build up to the skin surface. The radiation dose is 60 Gy in 30 fractions. Local recurrence is highly-lethal in this disease, and the concern for complications must be weighed against the need to control the primary tumor. Disease control outcomes and survival remain poor for patients with this disease. While laboratory research is needed to identify effective systemic agents for cutaneous angiosarcoma of the face and scalp, we encourage the development of multi-institutional trials to test such therapies, particularly in the neoadjuvant setting.
Footnotes
There are no conflicts of interest among any of the authors with respect to the data reported here.
REFERENCES
- 1.Weiss SW, Goldblum JR. Enzinger and Weiss's Soft Tissue Tumors. Mosby Elsevier; Philadelphia: 2008. Malignant Vascular Tumors. pp. 703–732. [Google Scholar]
- 2.Maddox JC, Evans HL. Angiosarcoma of skin and soft tissue: a study of forty-four cases. Cancer. 1981;48(8):1907–21. doi: 10.1002/1097-0142(19811015)48:8<1907::aid-cncr2820480832>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 3.Pawlik TM, Paulino AF, McGinn CJ, et al. Cutaneous angiosarcoma of the scalp: a multidisciplinary approach. Cancer. 2003;98(8):1716–26. doi: 10.1002/cncr.11667. [DOI] [PubMed] [Google Scholar]
- 4.Buschmann A, Lehnhardt M, Toman N, Preiler P, Salakdeh MS, Muehlberger T. Surgical treatment of angiosarcoma of the scalp: less is more. Ann Plast Surg. 2008;61(4):399–403. doi: 10.1097/SAP.0b013e31816b31f8. [DOI] [PubMed] [Google Scholar]
- 5.Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer. 1987;59(5):1046–57. doi: 10.1002/1097-0142(19870301)59:5<1046::aid-cncr2820590533>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 6.Sasaki R, Soejima T, Kishi K, et al. Angiosarcoma treated with radiotherapy: impact of tumor type and size on outcome. Int J Radiat Oncol Biol Phys. 2002;52(4):1032–40. doi: 10.1016/s0360-3016(01)02753-5. [DOI] [PubMed] [Google Scholar]
- 7.Morgan MB, Swann M, Somach S, Eng W, Smoller B. Cutaneous angiosarcoma: a case series with prognostic correlation. J Am Acad Dermatol. 2004;50(6):867–74. doi: 10.1016/j.jaad.2003.10.671. [DOI] [PubMed] [Google Scholar]
- 8.Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF. Angiosarcoma. A report of 67 patients and a review of the literature. Cancer. 1996;77(11):2400–6. doi: 10.1002/(SICI)1097-0142(19960601)77:11<2400::AID-CNCR32>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 9.Kohler HF, Neves RI, Brechtbuhl ER, Mattos Granja NV, Ikeda MK, Kowalski LP. Cutaneous angiosarcoma of the head and neck: report of 23 cases from a single institution. Otolaryngol Head Neck Surg. 2008;139(4):519–24. doi: 10.1016/j.otohns.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 10.Ward JR, Feigenberg SJ, Mendenhall NP, Marcus RB, Jr., Mendenhall WM. Radiation therapy for angiosarcoma. Head Neck. 2003;25(10):873–8. doi: 10.1002/hed.10276. [DOI] [PubMed] [Google Scholar]
- 11.Espat NJ, Lewis JJ, Woodruff JM, et al. Confirmed angiosarcoma: prognostic factors and outcome in 50 prospectively followed patients. Sarcoma. 2000;4(4):173–7. doi: 10.1155/2000/575781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lydiatt WM, Shaha AR, Shah JP. Angiosarcoma of the head and neck. Am J Surg. 1994;168(5):451–4. doi: 10.1016/s0002-9610(05)80097-2. [DOI] [PubMed] [Google Scholar]
- 13.Hodgkinson DJ, Soule EH, Woods JE. Cutaneous angiosarcoma of the head and neck. Cancer. 1979;44(3):1106–13. doi: 10.1002/1097-0142(197909)44:3<1106::aid-cncr2820440345>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 14.Ohguri T, Imada H, Nomoto S, et al. Angiosarcoma of the scalp treated with curative radiotherapy plus recombinant interleukin-2 immunotherapy. Int J Radiat Oncol Biol Phys. 2005;61(5):1446–53. doi: 10.1016/j.ijrobp.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 15.Fata F, O'Reilly E, Ilson D, et al. Paclitaxel in the treatment of patients with angiosarcoma of the scalp or face. Cancer. 1999;86(10):2034–7. [PubMed] [Google Scholar]
- 16.Morrison WH, Byers RM, Garden AS, Evans HL, Ang KK, Peters LJ. Cutaneous angiosarcoma of the head and neck. A therapeutic dilemma. Cancer. 1995;76(2):319–27. doi: 10.1002/1097-0142(19950715)76:2<319::aid-cncr2820760224>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 17.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 18.Cox D. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 19.Altman DG. Practical statistics for medical research. Chapman and Hall; London, England: 1991. [Google Scholar]
- 20.Penel N, Lansiaux A, Adenis A. Angiosarcomas and taxanes. Curr Treat Options Oncol. 2007;8(6):428–34. doi: 10.1007/s11864-007-0042-0. [DOI] [PubMed] [Google Scholar]