Abstract
The design of biomaterials for regenerative medicine can require biomolecular cues such as growth factors to induce a desired cell activity. Signal molecules are often incorporated into the biomaterial in either freely-diffusible or covalently-bound forms. However, biomolecular environments in vivo are often complex and dynamic. Notably, glycosaminoglycans (GAGs), linear polysaccharides found in the extracellular matrix, are involved in transient sequestration of growth factors via charge interactions. Biomaterials mimicking this phenomenon may offer the potential to amplify local biomolecular signals, both endogenously produced and exogenously added. GAGs of increasing sulfation (hyaluronic acid, chondroitin sulfate, heparin) were incorporated into a collagen–GAG (CG) scaffold under development for tendon tissue engineering. Manipulating the degree of GAG sulfation significantly impacts sequestration of growth factors from the media. Increasing GAG sulfation improved equine tenocyte metabolic activity in normal serum (10% FBS), low serum (1% FBS), and IGF-1 supplemented media conditions. Notably, previously reported dose-dependent changes in tenocyte bioactivity to soluble IGF-1 within the CG scaffold were replicated by using a single dose of soluble IGF-1 in scaffolds containing increasingly sulfated GAGs. Collectively, these results suggest that CG scaffold GAG content can be systematically manipulated to regulate the sequestration and resultant enhanced bioactivity of growth factor signals on cell behavior within the matrix.
Keywords: Collagen, Scaffold, Tendon, Growth factors, Biomimetic material, Mesenchymal stem cell
1. Introduction
A major focus in the field of tissue engineering is the development of biomaterials able to mimic critical features of the extracellular matrix (ECM), the three-dimensional microenvironment surrounding cells in the tissues and organs of the body. Beyond the use of scaffold mechanical, structural, and compositional signals to impact cell fate, the addition of growth factors into the biomaterial is often a primary way of providing instructive signals within the matrix [1]. Methods for biochemical supplementation include providing factors free in solutions [2–6], covalently tethering factors in random and specific orientations to the materials [7–10], and growth factor release vectors [11–13]. However, growth factor activity within the native ECM is often dictated by non-covalent interactions with ECM biomolecules such as proteins and proteoglycans that mediate transient immobilization and release.
Glycosaminoglycans (GAGs) are linear polysaccharides found in the native ECM and are known to play a critical role in sequestering growth factors within the matrix [14–22]. Along with structural variations in their carbohydrate backbone, GAGs can present varying levels of negative charges depending on their degree of sulfation [14,15], making them attractive for developing growth factor sequestering biomaterials. In addition to the nonspecific, electrostatic growth factor–GAG interactions facilitated by the sulfate groups, it has also been shown that the sulfation code, the positions of the sulfate groups on the carbohydrate backbone, has an impact on growth factor binding [15]. Recently Hudalla et al. immobilized heparin-binding peptides on a self-assembled monolayer to demonstrate sequential binding of first heparin and then heparin-binding growth factors to the substrate in order to enhance human mesenchymal stem cell (hMSC) bioactivity [18]. Similar work has also shown that TGF-β1 can be adsorbed onto biomaterials composed of type I collagen and a sulfated hyaluronan [19]. Considering that charged moieties have been shown to sequester biomolecules [15,18–22], systematic incorporation of differentially-charged GAGs within a biomaterial to selectively impact growth factor sequestration represents a promising avenue for tuning biomolecular signals. The efforts described here are therefore targeted at exploring whether the degree of GAG sulfation of a collagen–GAG scaffold could be modified to impact the scaffold’s capacity to transiently sequester activity-impacting molecules within the scaffold network.
Collagen–GAG(CG) scaffolds have been used for a wide variety of applications for skin, peripheral nerve, and cartilage tissue engineering as well as 3D environments for in vitro studies of cell behavior [2,23–28]. Early development of the CG scaffold platform for skin regeneration included comparison of the effects of the type and weight percent of GAG contained in the scaffold [29], though these studies did not consider biomolecule sequestration. Based on results from in vivo kinetics of wound contraction and quality of regeneration studies, CG scaffolds have traditionally included a 11:1 (wt:wt) collagen:GAG ratio employing chondroitin sulfate [30]. Recent efforts in our lab have described modification of the CG scaffold platform for tendon repair applications. As tendon is composed primarily of type I collagen arranged into aligned fibrils [31–33], we described a directional solidification method to fabricate CG scaffolds with highly anisotropic (aligned) morphology composed of longitudinally-aligned ellipsoidal pores [34]. Notably, scaffold anisotropy was found to improve equine tenocyte alignment as well as long-term maintenance of a pro-tenogenic phenotype [34,35]. Further, incorporation of growth factor signals within the anisotropic scaffold in either freely-soluble or covalently-immobilized forms has been shown to impact tenocyte bioactivity in a dose-dependent manner [3]. In particular, soluble or covalently-bound insulin-like growth factor 1 (IGF-1) was found to enhance tenocyte proliferation but at the expense of tenocyte phenotype [34]. Similarly, soluble growth/differentiation factor 5 (GDF-5) was used to increase expression of tenogenic-specific genes within the CG scaffolds [3].
This manuscript described the manipulation of the degree of GAG sulfation within the CG scaffold to promote transient, non-covalent sequestration of growth factors for applications in tendon tissue engineering. As prior work has shown dose-dependent tenocyte responses to growth factor within the CG scaffold [3,34], this work investigated whether alterations of GAG content within the scaffold could replicate dose-dependent effects using a single growth factor dose. Notably, it was hypothesized that scaffolds containing a highly sulfated GAG (heparin) would show an increase in transient growth factor sequestration and enhanced bioactivity of cells seeded within these scaffolds relative to less sulfated GAGs such as chondroitin sulfate, the GAG traditionally used in CG scaffolds, or non-sulfated hyaluronic acid. The response of equine tenocytes and human mesenchymal stem cells (hMSCs) to pro-proliferation (IGF-1) and pro-tenocyte phenotype (GDF-5) factors in the culture media as well as metabolically limited culture environments (low serum) was examined to explore the impact of GAG-mediated non-covalent sequestration on cellular bioactivity [3].
2. Materials and methods
2.1. Fabrication of anisotropic CG scaffolds
2.1.1. Preparation of CG suspension
A suspension of collagen and a defined glycosaminoglycan was made by homogenizing type I collagen from bovine Achilles tendon (Sigma–Aldrich, St. Louis, MO) and one of three glycosaminoglycans (GAGs): hyaluronic acid from Streptococcus equi (Sigma–Aldrich #53747, St. Louis, MO), chondroitin sulfate from shark cartilage (Sigma–Aldrich #C4384, St. Louis, MO) or heparin from porcine intestinal mucosa (Sigma–Aldrich #H4784, St. Louis, MO) in 0.05 m acetic acid [23]. A constant collagen concentration (1.5% w/v) and collagen:GAG ratio (11.28:1) was used for all experiments. The suspension was stored at 4 °C and degassed prior to use [36].
2.1.2. Fabrication of CG scaffolds via freeze drying
CG scaffolds were fabricated as previously described [34]. Briefly, the scaffolds were produced via directional solidification using a polytetrafluorethylene (PTFE)-copper mold. The mismatch in thermal conductivity between the mold materials promotes unidirectional heat transfer through the copper bottom when the mold is placed on a precooled freeze-dryer shelf (VirTis, Gardiner, NY). The CG suspension was added to the cylindrical wells of the mold and frozen at −10 °C for 2 h prior to the sublimation of the resulting ice crystals at 0 °C and 200 mTorr. This resulted in a dry, porous scaffold 6 mm in diameter and 20 mm in length with constant pore size along its length [34].
2.1.3. Crosslinking of CG scaffold
Following lyophilization, scaffolds were sterilized and dehydrothermally crosslinked in a vacuum oven (Welch, Niles, IL) at 105 °C under vacuum for 24 h [23]. 5 mm long sections were cut from the scaffold and used for all experiments [34]. Prior to use, these scaffolds were hydrated in 100% ethanol overnight and washed in phosphate-buffered saline (PBS) for 24 h. Scaffolds were subsequently crosslinked using carbodiimide chemistry to make them resistant to tenocyte contraction [30,37]. Scaffolds were immersed in 1-ethyl-3-[3-dimethylaminopropyl] carbodiimide hydrochloride (EDC) and N-hydroxysulfosuccinimide (NHS) at a molar ratio of 5:2:1 EDC:NHS:COOH for 2 h under shaking at room temperature. Following crosslinking, scaffolds were washed with PBS and stored in fresh PBS at 4 °C.
2.2. SEM analysis
Scanning electron microscopy (SEM) was used to visualize the scaffold microstructure. Dry, uncrosslinked sections from the center of the scaffold were used for analysis. Samples were sputter-coated with gold–palladium and imaged with a JEOL JSM-6060LV scanning electron microscope using secondary electron and back-scattered electron detectors under high vacuum.
2.3. Evaluation of CG scaffold microstructure
Microstructural features (pore size, aspect ratio) of the aligned CG scaffold variants were calculated using previously described stereology approaches [34]. Briefly, serial longitudinal and transverse sections were generated from glycolmethacrylate (Polysciences, Warrington, PA) embedded scaffolds using a microtome (Leica Microsystems, Germany) and mounted on slides. Sections were then stained with aniline blue to facilitate the visualization of the scaffold struts on an optical microscope (Leica Microsystems, Germany). Multiple images were captured per section and then analyzed using MATLAB equipped with a linear intercept method which outputs parameters used to calculate pore diameter and aspect ratio [38]. For each GAG variant a minimum of 6 scaffold sections were analyzed (3 longitudinal, 3 transverse) with a minimum of 5 fields of view captured per section.
2.4. Pull down sequestration assay
The degree of growth factor sequestration by CG scaffold variants was determined via a pull down assay. Ten hydrated crosslinked scaffolds were incubated overnight at 37 °C in a single well of an ultra-low attachment 6-well plate (Fisher, Waltham, MA) in 4 mL of a pH 7.4 PBS solution with 500 ng/mL IGF-1 (ProSpec, Israel) and 1% bovine serum albumin (BSA). Scaffolds fabricated from each GAG were tested separately, with wells containing the IGF-1 solution but no scaffolds used as controls. Following incubation, the amount of IGF-1 remaining in solution was measured via an ELISA kit (R&D Systems, Minneapolis, MN). Relative pull down, the amount of IGF-1 trapped within the CG scaffolds, was calculated from the difference in IGF-1 remaining in the media of the experimental versus control wells. Pull down for each CG variant was reported as a percentage of the total IGF-1 concentration in the loading solution.
2.5. Cell culture
2.5.1. Tenocyte isolation and culture
Tenocytes (tendon cells) were isolated from 2 to 3 year old horses that were euthanized for reasons not related to tendinopathy using previously described methods [39]. Tenocytes were expanded in standard culture flasks in high glucose Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA), 1% Antibiotic–Antimycotic (Invitrogen, Carlsbad, CA), 1% l-glutamine (Invitrogen, Carlsbad, CA), and 50 µg/mL ascorbic acid (Wako, Richmond, VA). The tenocytes were cultured to confluence at 37 °C and 5% CO2 and the media was changed every 3 days. Passage 4 cells were used for all culture experiments.
2.5.2. hMSC culture
Human mesenchymal stem cells (hMSC) from human bone marrow (Lonza, Switzerland) were cultured in standard culture flasks in low glucose Dulbecco’s modified Eagle’s medium supplemented with 10% MSC FBS (Invitrogen, Carlsbad, CA), 1% Antibiotic–Antimycotic (Invitrogen, Carlsbad, CA), 1% l-glutamine (Invitrogen, Carlsbad, CA). Media was changed every 3 days and the cells were cultured to confluence at 37 °C and 5% CO2. Cells were used at passage 6.
2.5.3. Scaffold seeding and culture conditions
Hydrated scaffold sections (6mmdiameter, 5mmthick) were placed in ultra-low attachment 6-well plates (Fisher, Waltham, MA). Confluent tenocytes or hMSCs were trypsinized and resuspended at concentrations of 5 × 105 tenocytes or 7.5 × 104 hMSCs per 20 µL of media. Scaffolds were seeded with either tenocytes or hMSCs using a previously established method [34]. Briefly, 10 µL of the cell suspension was added to each scaffold and then the scaffolds were incubated for 15 min at 37 °C. The scaffolds were flipped over and another 10 µL of cell suspension was added for a total of 5×105 tenocytes or 7.5×104 hMSCs seeded on each scaffold. After 2 h of incubation to facilitate initial cell attachment, additional media was added and scaffolds were incubated at 37 °C and 5% CO2 with the media changed every 3 days for the duration of the experiment. Scaffolds were cultured in tenocyte or hMSC culture media as described above, with or without the addition of serum or soluble growth factors. While standard media contained 10% FBS, some cultures were performed at low serum levels (1%). Experiments including growth factor supplementation (50 ng/mL IGF-1, 500 ng/mL GDF-5) were performed in serum-free media.
2.6. Quantification of cell metabolic activity
The metabolic activity of the cells contained within the CG scaffolds was measured using a non-destructive alamarBlue® assay (Invitrogen, Carlsbad, CA) [34,36] that uses the conversion of resazurin to the fluorescent byproduct resorufin by metabolically active cells. Briefly, scaffolds were removed from culture, rinsed in PBS, then incubated in a 1× alamarBlue® solution at 37 °C for 2 h under shaking [34]. Using a fluorescent spectrophotometer, resorufin fluorescence was measured (excitation: 540 nm, emission: 590 nm) and compared to a standard curve created from a known number of cells (tenocytes, hMSCs). While not all cells are known to attach to the scaffold during seeding [27], metabolic activity at each time point was reported as a percentage of the total number of seeded cells to provide a standard comparison metric.
2.7. RNA isolation and real-time PCR
RNA was isolated from cells (tenocytes or hMSCs) using an RNeasy Plant Mini kit (Qiagen, Valencia, CA). Cell seeded scaffolds were rinsed in PBS, cut longitudinally with a razor, and immersed in the kit’s lysis buffer for 5 min on ice [3]. After lysing, RNA was isolated per the kit’s instructions and total RNA quantified via spectrophotometry. The isolated RNA was reverse transcribed in a Bio-Rad S1000 thermal cycler using a QuantiTect Reverse Transcription kit (Qiagen, Valencia, CA). Real-time PCR reactions were performed in an Applied Biosystems 7900HT Fast Real-Time PCR System (Carlsbad, CA) to measure gene expression levels for collagen type I alpha 2 (COL1A2), scleraxis (SCXB), and tenascin-C (TNC). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a housekeeping gene. All primer sequences used (Table 1) were taken from the literature [40,41], and, excluding the SCXB (human) primer sequences, were synthesized by Integrated DNA Technologies (Coralville, IA). Analysis was completed with Sequence Detection Systems software v2.4 (Applied Biosystems, Carlsbad, CA). All results were expressed as fold changes normalized to the expression levels of cells in CG scaffolds containing hyaluronic acid for the GAG constituent at the first time point unless otherwise noted.
Table 1.
Primer sequences used in RT-PCR.
| Transcript | Sequence | Reference |
|---|---|---|
| COL1A2 (equine) | Forward: 5′-GCACATGCCGTGACTTGAGA-3′ | [40] |
| Reverse: 3′-CATCCATAGTGCATCCTTGATTAGG-5′ | ||
| TNC (equine) | Forward: 5′-GGGCGGCCTGGAAATG-3′ | [40] |
| Reverse: 3′-CAGGCTCTAACTCCTGGATGATG-5′ | ||
| SCXB (equine) | Forward: 5′-TCTGCCTCAGCAACCAGAGA-3′ | [40] |
| Reverse: 3′-TCCGAATCGCCGTCTTTC-5′ | ||
| GAPDH (equine) | Forward: 5′-GCATCGTGGAGGGACTCA-3′ | [40] |
| Reverse: 3′-GCCACATCTTCCCAGAGG-5′ | ||
| SCXB (human) | Qiagen QuantiTect Primer Assay Kit. Commercial product (no sequence available) |
[40] |
| GAPDH (human) | Forward: 5′- CCATGAGAAGTATGACAACAGCC-3′ | [41] |
| Reverse: 5′- CCTTCCACGATACCAAAGTTG-3′ |
2.8. Statistical analysis
One-way analysis of variance (ANOVA) was performed on all datasets followed by Tukey-HSD post-hoc test. A p-value <0.05 was used for significance. All analyses were based on a minimum of n = 3 scaffolds. In figures, error is reported as the standard error of the mean.
3. Results
3.1. Scaffold microstructure and pore size analysis
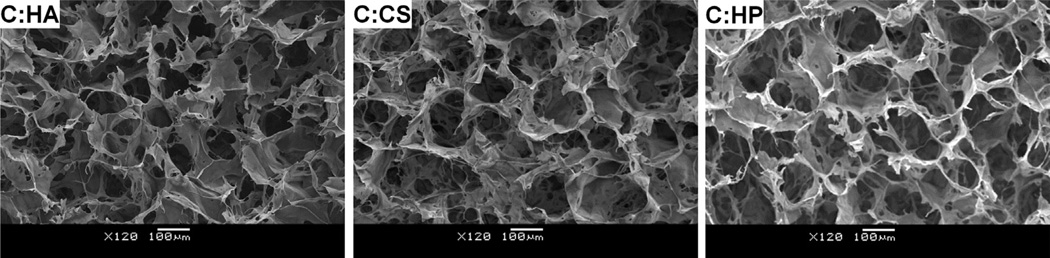
Scaffolds fabricated via freeze drying techniques showed highly porous, sponge-like features when imaged with SEM (Fig. 1). Analysis of scaffold microstructure suggested minimal structural differences between scaffolds. Notably, in the longitudinal direction, mean pore diameters varied between 184 and 199 µm for all three variants, with no statistically significance differences between GAG types (C:HA, C:CS, C:HP) (Table 2). In the transverse plane, mean pore sizes for the scaffold groups varied between 195 and 306 µm with a statistically significant difference (p < 0.05) observed between C:CS and C:HP scaffolds (Table 2). However, increasing (or decreasing) level of GAG sulfation was not correlated with a change in scaffold pore size since the transverse pore sizes of the C:HA scaffolds fall between those of the higher sulfated GAGs.
Fig. 1.
SEM images of CG scaffold pore structure for each GAG variant (left to right): collagen:hyaluronic acid (C:HA), collagen:chondroitin sulfate (C:CS, standard), collagen:heparin (C:HP). Scale bar: 100 µm.
Table 2.
Mean pore size (longitudinal, transverse plane) for the three aligned CG scaffold variants (GAG: Hyaluronic acid, chondroitin sulfate, heparin). Data expressed as mean ± standard deviation, n = 3. (*) Significance (p < 0.05) between groups in the transverse direction.
| Longitudinal pore size | Transverse pore size | ||
|---|---|---|---|
| C:HA | 184 ± 46 µm | 221 ± 27 µm | |
| C:CS | 196 ± 11 µm | 195 ± 27 µm | |
| C:HP | 199 ± 25 µm | 306 ± 31 µm | |
3.2. Growth factor sequestration within CG scaffolds
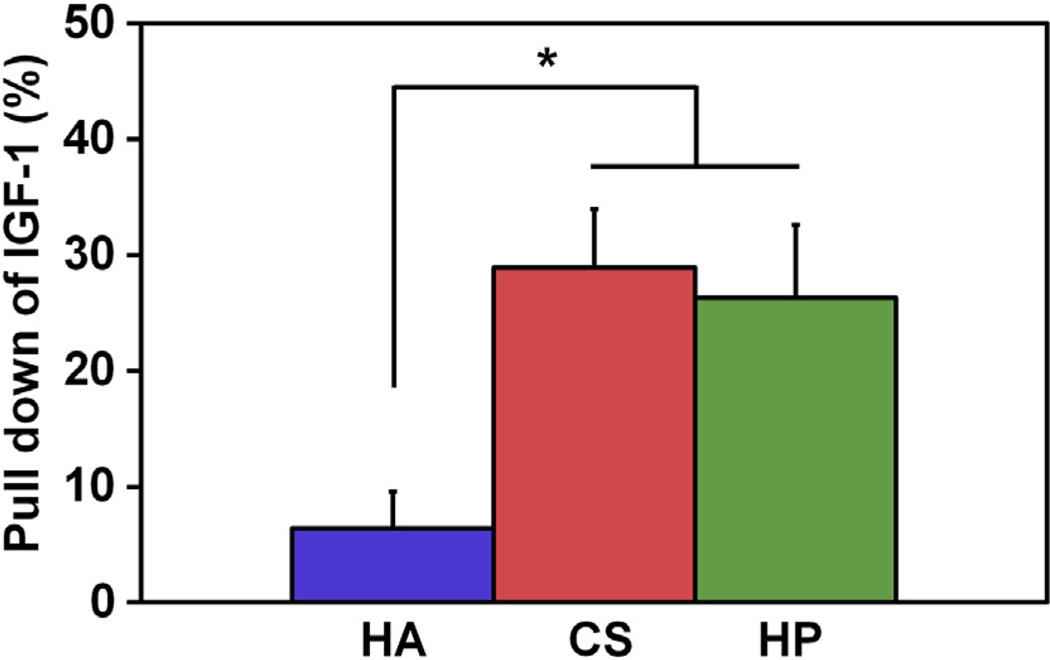
Scaffold GAG content was found to significantly impact sequestration of IGF-1 from the media to the scaffold (Fig. 2). Notably, sulfated C:CS and C:HP scaffolds showed significantly (p < 0.05) increased IGF-1 sequestration compared to non-sulfated C:HA scaffolds. Additionally, no significant increase in IGF-1 pull down was observed for non-sulfated C:HA scaffolds over the control (no scaffolds) (data not shown).
Fig. 2.
IGF-1 sequestration by CG scaffolds with varying degree of GAG sulfation. The degree of pull down is normalized to the no scaffold control. (*) Significance (p < 0.05) between scaffold groups.
3.3. Tenocyte response to altered serum concentrations
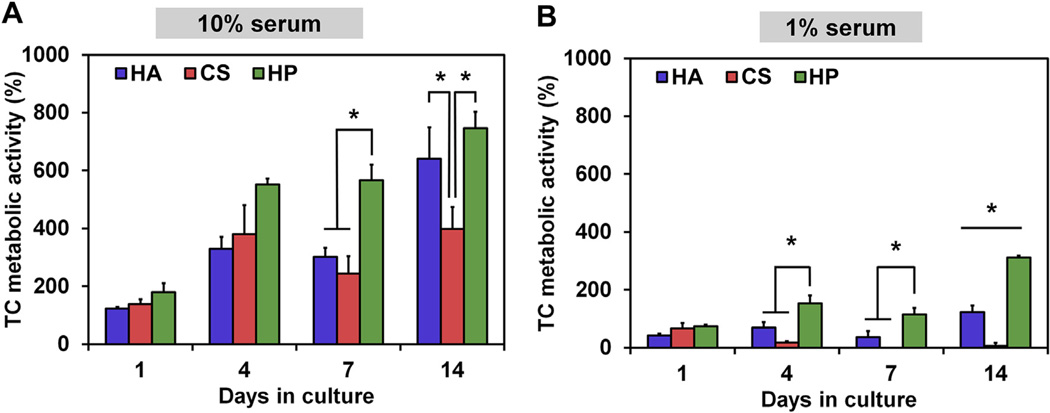
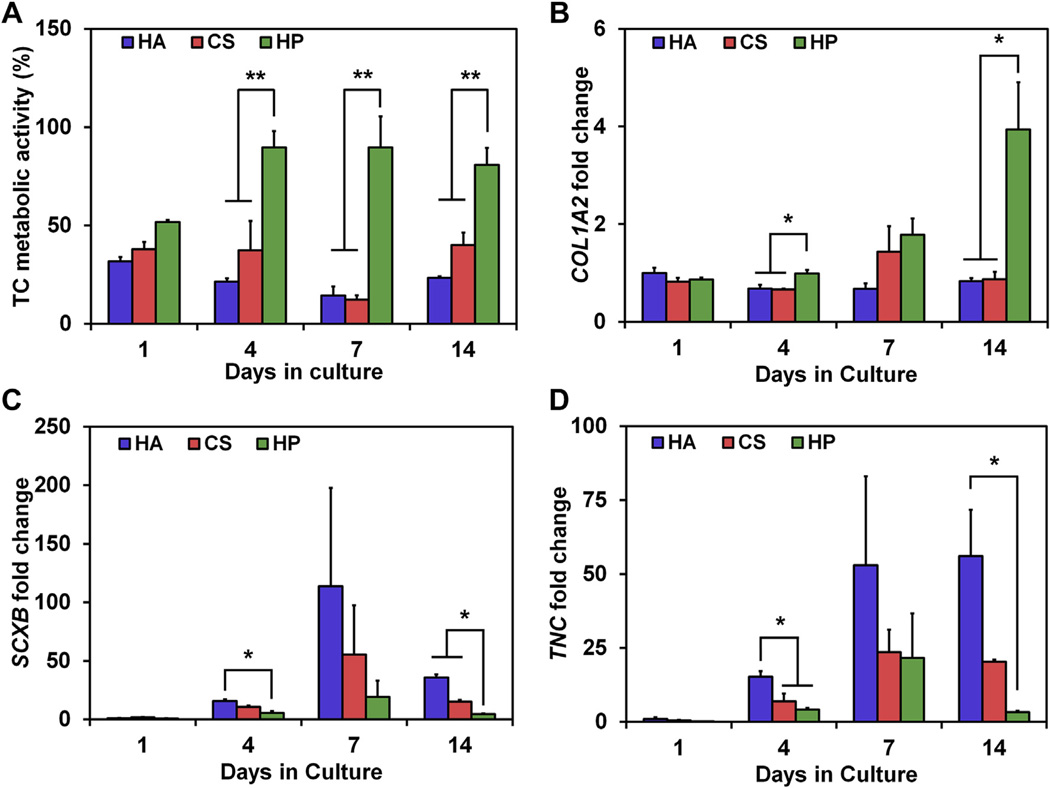
Equine tenocyte metabolic activity was evaluated in each GAG scaffold at days 1, 4, 7 and 14 (Fig. 3). Cells were cultured in media with either normal (10%) or metabolically-limiting (1%) levels of fetal bovine serum (FBS). Tenocytes cultured in 10% media showed GAG-dependent trends in metabolic activity; notably C:HP scaffolds showed elevated metabolic activity over other groups at all experimental timepoints, with a significant (p < 0.05) increase in metabolic activity compared to both C:HA and C:CS scaffolds at day 7 (Fig. 3A). The effect was heightened in low serum conditions, where all groups showed reduced metabolic activity compared to normal serum. However, tenocytes within the highest sulfated C:HP scaffolds showed significantly enhanced metabolic activity over the other CG scaffold variants after the first day in culture. Tenocyte metabolic activity in C:HP scaffold was significantly higher (p < 0.05) at days 4, 7 and 14 compared to all other scaffold groups (C:HA, C:CS; Fig. 3B).
Fig. 3.
Tenocyte metabolic activity as a function of scaffold GAG sulfation as well as media serum level. (A) Tenocyte metabolic activity in scaffolds cultured in media containing normal (10%) FBS concentrations. (B) Tenocyte metabolic activity under reduced (1%) FBS concentrations. (*) Significance (p < 0.05) between groups at a given time point.
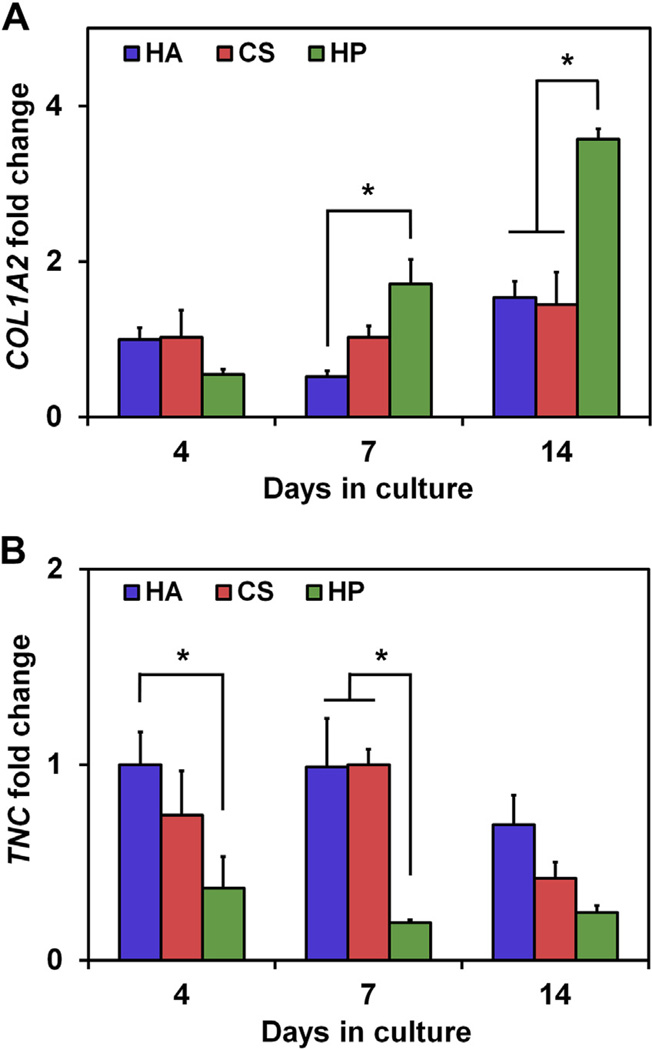
Prior work has linked increases in tenocyte metabolic activity with increased expression levels of the tendon-associated structural matrix gene COL1A2 as well as decreases in the tenocyte phenotype associated gene TNC [3]. Expression levels of COL1A2 and TNC were therefore evaluated for tenocytes cultured in normal (10% FBS)media in the three scaffold variants at days 4, 7, and 14 (Fig. 4). COL1A2 gene expression was significantly upregulated in the most highly-sulfated C:HP scaffolds compared to the C:HA group at day 7 and both lower-sulfated C:HA and C:CS groups at day 14 (p < 0.05) (Fig. 4A). Separately, a trend of downregulation of TNC gene expression was observed with increasing GAG sulfation (Fig. 4B). This effect was significant (p<0.05) in C:HP scaffolds compared to C:HAat day 4 and compared to both scaffold types at day 7 (p < 0.05) (Fig. 4B).
Fig. 4.
Tenocyte gene expression levels for (A) collagen I (COL1A2) and (B) tenascin C (TNC) as a function of scaffold GAG sulfation at days 4, 7, and 14 of culture. Gene expression was normalized to tenocytes cultured in the C:HA scaffold variant at day 4. (*) Significance (p < 0.05) between GAG groups at a given time point.
3.4. Tendon cell metabolic activity and gene expression with IGF-1 supplementation
Prior work has shown dose-dependent effects of IGF-1 supplementation (10, 50, 200 ng/mL) on tenocytes cultured in C:CS scaffolds [3]. These effects were reflected in both measures of growth (metabolic activity and COL1A2 gene expression) and phenotype (gene expression of SCXB and TNC). Here, tenocytes were seeded in the three differentially-sulfated CG scaffold variants, then cultured in serum-free media supplemented with insulin-like growth factor 1 (IGF-1) at a concentration of 50 ng/mL. Previously observed IGF-1 dose-dependent responses for tenocyte metabolic activity as well as gene expression profiles were observed with a single IGF-1 dose across scaffolds of increasing GAG-sulfation (Fig. 5). Tenocytes in the most highly-sulfated C:HP scaffolds showed significantly higher metabolic activity at days 4, 7, and 14 compared to cells seeded in the other GAG variants of our CG scaffolds at the same timepoints (p < 0.01). A trend of increasing COL1A2 expression was observed starting at day 4 in the scaffolds, with significant (p < 0.05) upregulation observed at days 4 and 14 in the most highly-sulfated C:HP scaffolds compared to lesser-sulfated C:HA and C:CS scaffolds (Fig. 5B).
Fig. 5.
Tenocyte response to IGF-1 supplementation (50 ng/mL in serum-free media) as a function of scaffold GAG content. Tenocyte response is reported as (A) total metabolic activity as well as gene expression levels (normalized to C:HA scaffolds at day 1) for (B) collagen I (COL1A2), (C) scleraxis (SCXB), and (D) tenascin-C (TNC). (*) Significance (p < 0.05) between groups at a given time point. (**) Significance (p < 0.01) between groups at a given time point.
Increasing levels of IGF-1 supplementation has been previously shown to decrease expression of tenocyte phenotypic markers scleraxis (SCXB) and tenascin-C (TNC) [3]. Similarly, expression of SCXB (Fig. 5C) and TNC (Fig. 5D) was significantly downregulated with increasing GAG sulfation. Notably, SCXB expression was significantly lower in the most highly-sulfated C:HP scaffolds than C:HA scaffolds at day 4 and significantly lower than both lesser-sulfated scaffolds variants at day 14 (p < 0.05). Similarly, TNC gene expression was significantly (p < 0.05) downregulated in more-highly sulfated C:CS and C:HP scaffolds compared to non-sulfated C:HA scaffolds at day 4, and significantly (p < 0.05) downregulated in the most highly-sulfated C:HP scaffolds versus non-sulfated C:HA scaffolds at day 14.
3.5. hMSC metabolic activity and gene expression with GDF-5 supplementation
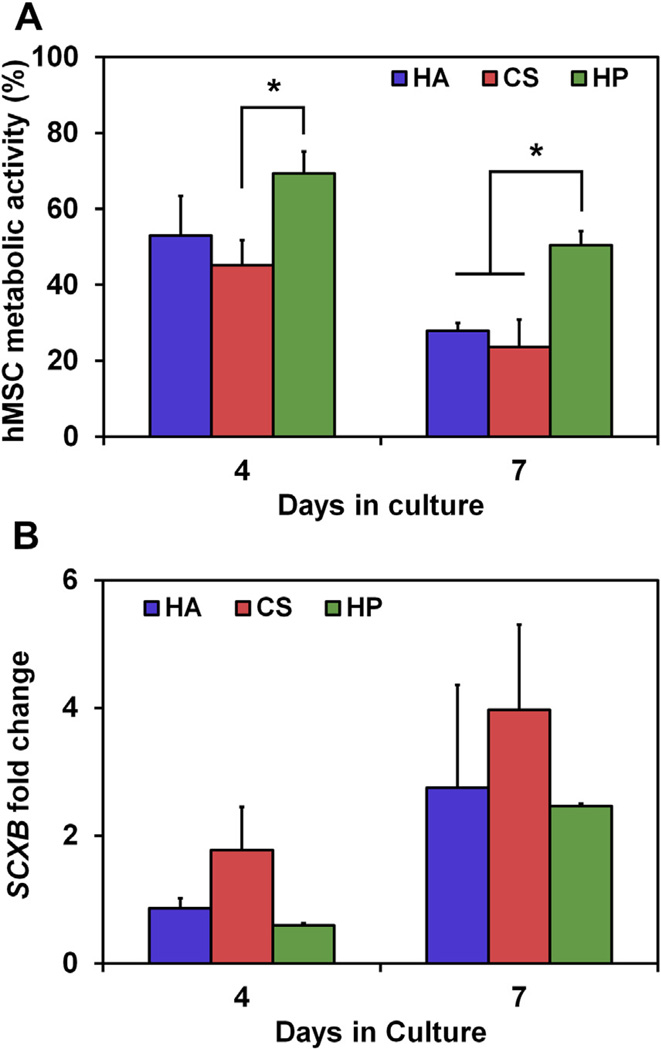
Previous investigations have shown the use of sequestered heparin on chemically-defined culture substrates could impact the bioactivity of human mesenchymal stem cells (hMSCs) through transient sequestration of growth factors [18]. Separately, growth/differentiation factor 5 (GDF-5) has been identified as a pro-tenogenic morphogen [42,43]. Here, the metabolic activity and gene expression levels of the tenogenic gene SCXB were monitored in hMSCs cultured in serum-free media containing 500 ng/mL GDF-5 in the range of CG scaffolds. While hMSC metabolic activity remained low for all variants, likely a consequence of serum free conditions, hMSCs in the most highly-sulfated C:HP scaffolds were significantly (p < 0.05) more metabolically active than those in the C:CS scaffolds at day 4 and significantly (p < 0.05) more metabolically active than either of the lesser-sulfated (C:HA, C:CS) scaffolds at day 7 (Fig. 6A). Further, although the expression of SCXB was globally upregulated at day 7 compared to day 4, there was no significant difference between any of the groups (Fig. 6B).
Fig. 6.
(A) Metabolic activity and (B) normalized (to 2D control at day 4) scleraxis gene expression of hMSCs cultured in CG scaffolds with varying GAG content in serum-free media supplemented with 500 ng/mL GDF-5. (*) Significance (p < 0.05) between groups at a given time point.
4. Discussion
This study sought to determine whether the degree of sulfation of the GAG component of a model CG scaffold could impact growth factor sequestration and, further, whether such sequestration was sufficient to alter the bioactivity of both differentiated (equine tenocytes) and non-differentiated (hMSCs) cells. This effort was inspired by GAG-mediated sequestration of factors within the native ECM [1] and prior work showing heparin-binding peptides incorporated within a chemically-defined two-dimensional substrate were able to bind heparins from the cell culture media which in turn were able to transiently sequester growth factors on the culture surface [18,22]. Incorporating charged moieties into a biomaterial able to replicate such sequestration of the ECM offers a route to locally amplify biomolecular signals, to either reduce required factor dose or direct autocatalytic processes. Systematic variation of sequestration capacity using bioinspired GAG design has not before been implemented in 3D collagen-based biomaterial systems.
GAGs with known differences in their degree of sulfation were integrated into a three-dimensional CG scaffold. CG scaffolds have been previously used for a range of in vitro and in vivo applications, including prior work in our lab examining the importance of soluble growth factor supplementation schemes to enhance tenocyte bioactivity for tendon tissue engineering applications [34–36]. In particular, insulin-like growth factor 1 (IGF-1) and growth and differentiation factor 5 (GDF-5) were shown to drive dose-dependent responses in tenocyte metabolic activity and gene expression [3]. Notably, IGF-1 enhanced tenocyte proliferation but at the expense of phenotype [5,44] while GDF-5 could be used to maintain tenogenic phenotype in vitro but at the expense of proliferation [42,43]. Importantly for this application, dose-dependent increases in COL1A2 gene expression and collagen biosynthesis as well as dose-dependent decreases in SCXB and TNC gene expression in equine tenocytes with IGF-1 supplementation have been previously described. Additionally, dose-dependent increases in expression of tenogenic markers SCXB and TNC in equine tenocytes with GDF-5 supplementation were noted [3].
The three CG scaffold variants all displayed microstructural features characteristic of low-density, open-cell foams (Fig. 1). All three scaffolds contained consistent pore structures in the longitudinal plane (Table 2), the direction previously identified as having the primary impact on tenocyte bioactivity [34,35]. Sequestration of growth factors from the media into the scaffold structure was examined across the three CG scaffold variations using a modified pull down assay (Fig. 2). There was a significant increase in the amount of IGF-1 bound to the moderately (CS) and highly (HP) sulfated GAG scaffolds compared to the non-sulfated GAG (HA) scaffold. This supports the claim that the presence of charged glycosaminoglycans within the CG scaffold, even at relatively low concentration (11.28:1 collagen:GAG) can impact the sequestration of growth factors. We acknowledge that the difference in transverse pore size between C:CS and C:HP scaffolds could impact cell activity. Indeed, CG scaffold pore size has previously been observed to impact cell attachment [27], motility [25], and even tenocyte alignment and distribution within the scaffold [34]. However, the differences in pore size between the C:CS and C:HP scaffolds in this study are outside the range of pore sizes (50–150 µm) previously reported to affect cell activity. Further, the difference in pore size in this study was only observed between two scaffold variants and in only a single plane, so does not explain the entirety of the reported data that showed increases and decreases in cell activity across the range of GAG types.
In order to study the effects of GAG-mediated sequestration on tenocyte bioactivity, the metabolic activity of cells cultured in the CG scaffold variants at two concentrations of FBS was examined (Fig. 3). The objective was to determine whether the highly sulfated GAG scaffolds could support enhanced cellular bioactivity even in metabolically limited cultures. Such observations had been previously made for hMSCs cultures on 2D, chemically-defined culture substrates presenting heparin binding peptides [18], but had not been extended to a fully-3D biomaterial composed of native ECM proteins. Since serum contains a large number of growth factors, scaffold bioactivity was evaluated at normal (10%) and restricted (1%) serum levels. At normal serum levels tenocytes proliferated in all scaffold variants and scaffold GAG sulfation impacted tenocyte metabolic activity (Fig. 3A) with the greatest effect observed in metabolically-limited cultures (1% serum) (Fig. 3B). Here, a significant effect of GAG type was seen for all culture conditions, with the most highly-sulfated C:HP scaffolds inducing significant increases in tenocyte metabolic activity at days 4, 7, and 14 in low serum conditions (Fig. 3B). This supports the hypothesis that the highly sulfated heparin GAG scaffolds enhance tenocyte metabolic activity even at reduced metabolic support, which may be critical for future in vivo studies where metabolic support is significantly reduced immediately after implantation.
Further, when examining gene expression profiles for tenocyte growth in the scaffolds under normal metabolic support, GAG sulfation-dependent increases in COL1A2 expression was observed (Fig. 4A) indicating an increase in the signal to produce collagen type I, a primary tendon-associated structural protein. Future investigations will employ longer culture times (4–8 weeks) to quantify differences in collagen production via ELISA or histology. Such studies are not included as part of this work because the current goal was to elucidate whether the initial GAG content of the scaffolds could impact cell bioactivity; longer-term experiments would involve significant scaffold remodeling, making it difficult to assess GAG-mediated influences. In addition to the effects of GAG sulfation on COL1A2 expression, GAG-dependent downregulation of the TNC gene, which encodes for a glycoprotein found in developing and mature tendon, was seen (Fig. 4B). These results are consistent with prior work that demonstrated a tradeoff between tenocyte proliferation and maintenance of the tendon phenotype [3,45].
Following the investigation of tenocyte bioactivity at different serum concentrations, the examination of tenocyte response to the proliferative growth factor (IGF-1) was undertaken. Prior work using the C:CS scaffold demonstrated dose-dependent increases in tenocyte proliferation with IGF-1 supplementation (10, 50, 200 ng/mL) [3]. It was hypothesized that this dose-dependent increase in tenocyte metabolic activity could be replicated with a single dose of IGF-1 (50 ng/mL) and differential levels of GAG-mediated IGF-1 sequestration within the matrix. Indeed, dose-dependent trends and significant (p < 0.01) increases in tenocyte metabolic activity at days 4, 7, and 14 in the CG scaffolds with the highest degree of GAG-sulfation were seen (Fig. 5A). In addition, GAG-dependent upregulation of COL1A2 and downregulation of TNC and SCXB was observed (Fig. 5B–D). Critically, in enhanced proliferative conditions established in scaffolds of increasing GAG-sulfation, COL1A2 expression increased while markers of tenogenic phenotype decreased.
Finally, the use of GDF-5 to alter hMSC bioactivity within the CG scaffold variants was studied (Fig. 6). To specifically interrogate the addition of GDF-5, these experiments were completed in serum-free conditions, leading to a shortened experimental time (7 days) and overall reduced metabolic activity for all conditions. As seen with tenocyte cultures (Fig. 3), hMSC metabolic activity was enhanced in the most highly-sulfated C:HP scaffolds. These results confirm that even without serum or a pro-proliferative factor the highly sulfated GAGs can improve cellular bioactivity, likely through local amplification of biomolecule signals [22]. Examining expression of a characteristic tenogenic gene SCXB, global increases over time for all groups were observed with no significant differences between the GAG variants, perhaps due to the reduced metabolic support from the media or the overall GDF-5 dose.
Taken together, this work illustrates the application of bioinspired proteoglycan design within a model collagen–GAG scaffold with focus on creating a 3D biomaterial construct able to leverage concepts taken from GAG-mediated local sequestration of biomolecular signals within the native ECM. The incorporation of three distinct GAGs into a model CG scaffold was examined to determine the ability for each scaffold variant to sequester growth factors from the media. Further, scaffolds with increasing degrees of GAG sulfation were used to replicate previously observed dose-dependent effects of growth factor supplementation on cell bioactivity using single factor dosages. Notably, there was enhanced sequestration of IGF-1 within the CG scaffold structure with moderately or highly sulfated GAGs. While an increased degree of sulfation led to increased tenocyte metabolic activity in low serum and IGF-1 supplemented media conditions, this increase in metabolic activity is accompanied by a decrease in tendon phenotypic markers. Although this effort used a single collagen:GAG ratio previously described in the literature as optimal for a range of tissue regeneration studies [23,46], future work will include modifying the overall collagen:GAG ratio or gradually desulfating a particular GAG of interest (e.g., CS, HP [47,48]) to investigate the impact of GAG concentration and specific degrees of sulfation on cell bioactivity. Future endeavors may also consider more specific alteration to the carbohydrate backbone or GAG sulfation code to gain improved control over factor sequestration and release.
5. Conclusions
Recognizing the significance of GAGs in the native ECM for transiently sequestering and amplifying biomolecular signals, this manuscript demonstrated the use of bioinspired GAG design within a collagen–GAG scaffold to impact cell bioactivity for a tendon tissue engineering application. This study showed that increasing scaffold GAG sulfation increases equine tenocyte metabolic activity in low serum and IGF-1 supplemented media conditions, but at the expense of tendon phenotypic markers. Previously described dose-dependent effects of IGF-1 and GDF-5 supplementation on equine tenocytes and hMSCs in CG scaffolds were replicated by using a single dose of IGF-1 or GDF-5 and the series of CG scaffolds with increasing degree of GAG sulfation. These results suggest ways that scaffold chemistry may be optimized to reduce required dosages of growth factors in the cell culture media.
Acknowledgments
The authors would like to acknowledge Dr. Allison Stewart (Veterinary Sciences, UIUC) for the equine tenocytes, Karen Doty (Veterinary Sciences, UIUC) for sectioning for pore size analysis, Dr. Sandra McMasters (SCS, UIUC) for culture media preparation, and the IGB Core Facilities for assistance with real-time PCR. This research was carried out in part in the Frederick Seitz Materials Research Laboratory Central Facilities, University of Illinois, which is partially supported by the U.S. Department of Energy under grants DE-FG02-07ER46453 and DE-FG02-07ER46471. This material is based upon work supported by the National Science Foundation under Grant No.1105300.We are grateful for the funding for this study provided by the NSF Graduate Research Fellowship DGE 11-44245 FLLW(RAH), the Chemical and Biomolecular Engineering Dept. (BAH), and the Institute for Genomic Biology (BAH) at the University of Illinois at Urbana-Champaign.
References
- 1.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–470. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 2.Farrell E, O’Brien FJ, Doyle P, Fischer J, Yannas I, Harley BA, et al. A collagen–glycosaminoglycan scaffold supports adult rat mesenchymal stem cell differentiation along osteogenic and chondrogenic routes. Tissue Eng. 2006;12:459–468. doi: 10.1089/ten.2006.12.459. [DOI] [PubMed] [Google Scholar]
- 3.Caliari SR, Harley BA. Composite growth factor supplementation strategies to enhance tenocyte bioactivity in aligned collagen–GAG scaffolds. Tissue Eng Part A. 2013;19:1100–1112. doi: 10.1089/ten.tea.2012.0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulotta LV, Rodeo SA. Growth factors for rotator cuff repair. Clin Sports Med. 2009;28:13–23. doi: 10.1016/j.csm.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 5.Molloy T, Wang Y, Murrell G. The roles of growth factors in tendon and ligament healing. Sports Med. 2003;33:381–394. doi: 10.2165/00007256-200333050-00004. [DOI] [PubMed] [Google Scholar]
- 6.Shen W, Chen X, Chen J, Yin Z, Heng BC, Chen W, et al. The effect of incorporation of exogenous stromal cell-derived factor-1 alpha within a knitted silk-collagen sponge scaffold on tendon regeneration. Biomaterials. 2010;31:7239–7249. doi: 10.1016/j.biomaterials.2010.05.040. [DOI] [PubMed] [Google Scholar]
- 7.Martin TA, Caliari SR, Williford PD, Harley BA, Bailey RC. The generation of biomolecular patterns in highly porous collagen–GAG scaffolds using direct photolithography. Biomaterials. 2011;32:3949–3957. doi: 10.1016/j.biomaterials.2011.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberti K, Davey RE, Onishi K, George S, Salchert K, Seib FP, et al. Functional immobilization of signaling proteins enables control of stem cell fate. Nat Methods. 2008;5:645–650. doi: 10.1038/nmeth.1222. [DOI] [PubMed] [Google Scholar]
- 9.Klenkler BJ, Sheardown H. Characterization of EGF coupling to aminated silicone rubber surfaces. Biotechnol Bioeng. 2006;95:1158–1166. doi: 10.1002/bit.21083. [DOI] [PubMed] [Google Scholar]
- 10.Mann BK, Schmedlen RH, West JL. Tethered-TGF-beta increases extracellular matrix production of vascular smooth muscle cells. Biomaterials. 2001;22:439–444. doi: 10.1016/s0142-9612(00)00196-4. [DOI] [PubMed] [Google Scholar]
- 11.Hanson JA, Chang CB, Graves SM, Li Z, Mason TG, Deming TJ. Nanoscale double emulsions stabilized by single-component block copolypeptides. Nature. 2008;455:85–88. doi: 10.1038/nature07197. [DOI] [PubMed] [Google Scholar]
- 12.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–1034. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 13.Sohier J, Vlugt TJH, Cabrol N, Van Blitterswijk C, de Groot K, Bezemer JM. Dual release of proteins from porous polymeric scaffolds. J Control Release. 2006;111:95–106. doi: 10.1016/j.jconrel.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Raman R, Sasisekharan V, Sasisekharan R. Structural insights into biological roles of protein–glycosaminoglycan interactions. Chem Biol. 2005;12:267–277. doi: 10.1016/j.chembiol.2004.11.020. [DOI] [PubMed] [Google Scholar]
- 15.Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M, Vaidehi N, et al. Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol. 2006;2:467–473. doi: 10.1038/nchembio810. [DOI] [PubMed] [Google Scholar]
- 16.Rawat M, Gama CI, Matson JB, Hsieh-Wilson LC. Neuroactive chondroitin sulfate glycomimetics. J Am Chem Soc. 2008;130:2959–2961. doi: 10.1021/ja709993p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spillmann D, Lindahl U. Glycosaminoglycan protein interactions – a question of specificity. Curr Opin Struct Biol. 1994;4:677–682. doi: 10.1016/j.sbi.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 18.Hudalla GA, Kouris NA, Koepsel JT, Ogle BM, Murphy WL. Harnessing endogenous growth factor activity modulates stem cell behavior. Integr Biol (Camb) 2011;3:832–842. doi: 10.1039/c1ib00021g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hempel U, Hintze V, Moller S, Schnabelrauch M, Scharnweber D, Dieter P. Artificial extracellular matrices composed of collagen I and sulfated hyaluronan with adsorbed transforming growth factor beta 1 promote collagen synthesis of human mesenchymal stromal cells. Acta Biomaterialia. 2012;8:659–666. doi: 10.1016/j.actbio.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 20.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide–polysaccharide interactions. J Control Release. 2006;114:130–142. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakiyama-Elbert SE, Hubbell JA. Controlled release of nerve growth factor from a heparin-containing fibrin-based cell ingrowth matrix. J Control Release. 2000;69:149–158. doi: 10.1016/s0168-3659(00)00296-0. [DOI] [PubMed] [Google Scholar]
- 22.Hudalla GA, Koepsel JT, Murphy WL. Surfaces that sequester serum-borne heparin amplify growth factor activity. Adv Mater. 2011;23:5415–5418. doi: 10.1002/adma.201103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yannas IV, Lee E, Orgill DP, Skrabut EM, Murphy GF. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc Natl Acad Sci U S A. 1989;86:933–937. doi: 10.1073/pnas.86.3.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harley BA, Freyman TM, Wong MQ, Gibson LJ. A new technique for calculating individual dermal fibroblast contractile forces generated within collagen–GAG scaffolds. Biophys J. 2007;93:2911–2922. doi: 10.1529/biophysj.106.095471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harley BA, Kim HD, Zaman MH, Yannas IV, Lauffenburger DA, Gibson LJ. Microarchitecture of three-dimensional scaffolds influences cell migration behavior via junction interactions. Biophys J. 2008;95:4013–4024. doi: 10.1529/biophysj.107.122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harley BA, Spilker MH, Wu JW, Asano K, Hsu HP, Spector M, et al. Optimal degradation rate for collagen chambers used for regeneration of peripheral nerves over long gaps. Cells Tissues Organs. 2004;176:153–165. doi: 10.1159/000075035. [DOI] [PubMed] [Google Scholar]
- 27.O’Brien FJ, Harley BA, Yannas IV, Gibson LJ. The effect of pore size on cell adhesion in collagen–GAG scaffolds. Biomaterials. 2005;26:433–441. doi: 10.1016/j.biomaterials.2004.02.052. [DOI] [PubMed] [Google Scholar]
- 28.Torres DS, Freyman TM, Yannas IV, Spector M. Tendon cell contraction of collagen–GAG matrices in vitro: effect of cross-linking. Biomaterials. 2000;21:1607–1619. doi: 10.1016/s0142-9612(00)00051-x. [DOI] [PubMed] [Google Scholar]
- 29.Ellis DL, Yannas IV. Recent advances in tissue synthesis in vivo by use of collagen–glycosaminoglycan copolymers. Biomaterials. 1996;17:291–299. doi: 10.1016/0142-9612(96)85567-0. [DOI] [PubMed] [Google Scholar]
- 30.Harley BA, Leung JH, Silva EC, Gibson LJ. Mechanical characterization of collagen–glycosaminoglycan scaffolds. Acta Biomater. 2007;3:463–474. doi: 10.1016/j.actbio.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 31.James R, Kesturu G, Balian G, Chhabra AB. Tendon: biology, biomechanics, repair, growth factors, and evolving treatment options. J Hand Surg Am. 2008;33:102–112. doi: 10.1016/j.jhsa.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Ramanath HS, Wang DA. Tendon tissue engineering using scaffold enhancing strategies. Trends Biotechnol. 2008;26:201–209. doi: 10.1016/j.tibtech.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 33.Wang JH. Mechanobiology of tendon. J Biomech. 2006;39:1563–1582. doi: 10.1016/j.jbiomech.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 34.Caliari SR, Harley BAC. The effect of anisotropic collagen–GAG scaffolds and growth factor supplementation on tendon cell recruitment, alignment, and metabolic activity. Biomaterials. 2011;32:5330–5340. doi: 10.1016/j.biomaterials.2011.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caliari SR, Weisgerber DW, Ramirez MA, Kelkhoff DO, Harley BAC. The influence of collagen–glycosaminoglycan scaffold relative density and microstructural anisotropy on tenocyte bioactivity and transcriptomic stability. J Mech Behav Biomed Mater. 2012;11:27–40. doi: 10.1016/j.jmbbm.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caliari SR, Ramirez MA, Harley BA. The development of collagen–GAG scaffold-membrane composites for tendon tissue engineering. Biomaterials. 2011;32:8990–8998. doi: 10.1016/j.biomaterials.2011.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olde Damink LHH, Dijkstra PJ, Van Luyn MJA, Van Wachem PB, Nieuwenhuis P, Feijen J. Cross-linking of dermal sheep collagen using a water-soluble carbodiimide. Biomaterials. 1996;17:765–773. doi: 10.1016/0142-9612(96)81413-x. [DOI] [PubMed] [Google Scholar]
- 38.O’Brien FJ, Harley BA, Yannas IV, Gibson L. Influence of freezing rate on pore structure in freeze-dried collagen–GAG scaffolds. Biomaterials. 2004;25:1077–1086. doi: 10.1016/s0142-9612(03)00630-6. [DOI] [PubMed] [Google Scholar]
- 39.Kapoor A, Caporali EH, Kenis PJ, Stewart MC. Microtopographically patterned surfaces promote the alignment of tenocytes and extracellular collagen. Acta Biomater. 2010;6:2580–2589. doi: 10.1016/j.actbio.2009.12.047. [DOI] [PubMed] [Google Scholar]
- 40.Taylor SE, Vaughan-Thomas A, Clements DN, Pinchbeck G, Macrory LC, Smith RK, et al. Gene expression markers of tendon fibroblasts in normal and diseased tissue compared to monolayer and three dimensional culture systems. BMC Musculoskelet Disord. 2009;10:27. doi: 10.1186/1471-2474-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pauly S, Klatte F, Strobel C, Schmidmaier G, Greiner S, Scheibel M, et al. Characterization of tendon cell cultures of the human rotator cuff. Eur Cell Mater. 2010;20:84–97. doi: 10.22203/ecm.v020a08. [DOI] [PubMed] [Google Scholar]
- 42.Park A, Hogan MV, Kesturu GS, James R, Balian G, Chhabra AB. Adipose-derived mesenchymal stem cells treated with growth differentiation factor-5 express tendon-specific markers. Tissue Eng Part A. 2010;16:2941–2951. doi: 10.1089/ten.tea.2009.0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.James R, Kumbar SG, Laurencin CT, Balian G, Chhabra AB. Tendon tissue engineering: adipose-derived stem cell and GDF-5 mediated regeneration using electrospun matrix systems. Biomed Mater. 2011;6:025011. doi: 10.1088/1748-6041/6/2/025011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Costa MA, Wu C, Pham BV, Chong AK, Pham HM, Chang J. Tissue engineering of flexor tendons: optimization of tenocyte proliferation using growth factor supplementation. Tissue Eng. 2006;12:1937–1943. doi: 10.1089/ten.2006.12.1937. [DOI] [PubMed] [Google Scholar]
- 45.Thomopoulos S, Harwood FL, Silva MJ, Amiel D, Gelberman RH. Effect of several growth factors on canine flexor tendon fibroblast proliferation and collagen synthesis in vitro. J Hand Surg Am. 2005;30:441–447. doi: 10.1016/j.jhsa.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Chamberlain LJ, Yannas IV, Hsu H-P, Strichartz G, Spector M. Collagen–GAG substrate enhances the quality of nerve regeneration through collagen tubes up to level of autograft. Exper Neurol. 1998;154:315–329. doi: 10.1006/exnr.1998.6955. [DOI] [PubMed] [Google Scholar]
- 47.Schubert M. Chondroitin from chondroitin sulfate. In: Whistler R, BeMiller J, Wolfrom M, editors. Methods in carbohydrate chemistry: general polysaccharides. New York: Academic Press; 1965. pp. 109–110. [Google Scholar]
- 48.Lim JJ, Temenoff JS. The effect of desulfation of chondroitin sulfate on interactions with positively charged growth factors and upregulation of cartilaginous markers in encapsulated MSCs. Biomaterials. 2013;34:5007–5018. doi: 10.1016/j.biomaterials.2013.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]