Abstract
Adolescents view thousands of food commercials annually, but the neural response to food advertising and its association with obesity is largely unknown. This study is the first to examine how neural response to food commercials differs from other stimuli (e.g. non-food commercials and television show) and to explore how this response may differ by weight status. The blood oxygen level-dependent functional magnetic resonance imaging activation was measured in 30 adolescents ranging from lean to obese in response to food and non-food commercials imbedded in a television show. Adolescents exhibited greater activation in regions implicated in visual processing (e.g. occipital gyrus), attention (e.g. parietal lobes), cognition (e.g. temporal gyrus and posterior cerebellar lobe), movement (e.g. anterior cerebellar cortex), somatosensory response (e.g. postcentral gyrus) and reward [e.g. orbitofrontal cortex and anterior cingulate cortex (ACC)] during food commercials. Obese participants exhibited less activation during food relative to non-food commercials in neural regions implicated in visual processing (e.g. cuneus), attention (e.g. posterior cerebellar lobe), reward (e.g. ventromedial prefrontal cortex and ACC) and salience detection (e.g. precuneus). Obese participants did exhibit greater activation in a region implicated in semantic control (e.g. medial temporal gyrus). These findings may inform current policy debates regarding the impact of food advertising to minors.
Keywords: marketing, adolescents, obesity, fMRI
INTRODUCTION
Individuals are exposed to a vast amount of food advertising, particularly adolescents, who are frequently targeted as a key advertising demographic (Federal Trade Commission, 2012). The average adolescent was exposed to ∼6000 television food advertisements in 2010 (Rudd Center for Food Policy and Obesity, 2011), with most commercials promoting products high in calories, sugar, sodium and/or fat (Powell et al., 2011). Yet, little is known about how the brain responds to these advertisements, which may be of importance for individuals at-risk for obesity. Individual differences in the response to food advertisements may contribute to problematic food consumption, but the food images used in prior studies of obesity differ in meaningful ways from food commercials. Thus, our understanding of how food advertisements impact brain reward and attention regions is limited, as is our knowledge of how this may differ based on body mass. This study was designed to address these two questions.
Meso-limbic-cortico regions (e.g. ventral striatum and insula) appear to encode the reward value of food images and cues (Stoeckel et al., 2008) and obese relative to lean participants have been found to show greater neural activation in brain regions implicated in reward [e.g. orbitofrontal cortex (OFC)], visual attention (e.g. parietal lobe), memory (e.g. hippocampus), cognition (e.g. temporal lobe) and somatosensory processing (e.g. postcentral gyrus) in response to food cues (Carnell and Wardle, 2007; Rothemund et al., 2007; Stoeckel et al., 2008; Martin et al., 2009; Bruce et al., 2010; Stice et al., 2010). Elevated nucleus accumbens response to high-fat/sugar food images (Demos et al., 2012) and OFC response to cues signaling impending unhealthy food image presentation predicted future weight gain (Yokum et al., 2012). Furthermore, activation in reward, visual and attention areas (e.g. insula, OFC, parietal and occipital lobe) during exposure to food cues is associated with less successful weight loss and increased weight regain (Murdaugh et al., 2012).
Although these results highlight the potential role of food-cue responsivity in obesity, the stimuli used in these studies are typically a picture of food without branding and without context, limiting ecological validity. Thus, these findings provide limited information about how food advertisements in the current environment may contribute to problematic eating. In contrast to food pictures used in prior studies, food commercials are specifically designed to induce the desire to consume the advertised product (Council of American Research Survey Organizations, 2005). Not only do food commercials present enticing images of unhealthy and highly palatable foods but successful advertising also creates positive associations with brands and reinforces them every time an advertisement is viewed (Heath, 2001). Brands associated with basic human motivations (e.g. happiness, attractiveness and accomplishment) encourage product sales (Wansink, 2003) and food advertising to young people typically utilizes appeals to these attributes (Schor and Ford, 2007). Consumption of a preferred brand (e.g. Coca-Cola) is related to increased activation in the hippocampus, dorsolateral prefrontal cortex (dlPFC) and midbrain (McClure et al., 2004). Furthermore, healthy-weight children have shown greater activation in the OFC, temporal cortex and visual cortex during exposure to food logos (e.g. McDonald’s arches) relative to control images (Bruce et al., in press); exposure to food logos relative to non-food logos was also related to greater activation in the occipital cortex, paracentral lobule, parietal gyrus, lingual gyrus and posterior cingulate cortex. Furthermore, obese relative to lean children exhibit greater activation in somatosensory and reward-related regions (i.e. postcentral gyrus and midbrain) for food logos compared with control images (Bruce et al., 2012).
Thus, participants may respond more strongly to food commercials (which contain branded food images) relative to non-food commercials or a television show. This study is the first to examine the neural correlates of food commercials relative to control stimuli. The major aims of this study are (i) to examine whether food commercials relative to non-food commercials and television viewing are related to differential patterns of activation in brain regions implicated in visual attention, somatosensory response, reward and motivation (e.g. OFC, postcentral gyrus and occipital lobe) and (ii) to evaluate whether neural response to these stimuli differ by weight class (e.g. obesity vs normal weight). Although a number of strategies to choose commercial stimuli were considered for this study (e.g. matching food and non-food commercials on visual characteristics, price, participant preferences, etc.), we focused on real-world exposure by choosing commercials based on data from Nielsen on television and advertising exposure for 12- to 17-year olds. To further increase the generalizability of our paradigm to settings where food commercials are typically encountered, commercials breaks were embedded in the context of a television show. Finally, we conduct this study in adolescent participants, as this is a target demographic for food advertisements (Federal Trade Commission, 2012) and a risk period for the development of obesity (Ogden et al., 2012).
MATERIALS AND METHODS
Participants
Participants were 30 healthy adolescents [mean age = 15.20, s.d. = 1.06, range = 14–17 years old; mean body mass index (BMI) = 26.92, s.d. = 5.43; 17 females] recruited from the community via ads. To examine how neural response to food commercials differs by weight class, we enrolled a roughly equivalent number of participants in each weight category: 10 normal weight (mean BMI = 21.20, s.d. = 0.90), 8 overweight (mean BMI = 25.53, s.d. = 1.41) and 12 obese (mean BMI = 32.64, s.d. = 5.43). Exclusion criteria were current regular use of psychotropic medication or illicit drugs, pregnancy, head injury with a loss of consciousness or current axis I psychiatric disorder. In total, 6.7% reported being Hispanic, 63.3% European Americans, 3.3% Native Americans and 26.7% mixed race/ethnicity. There were no significant differences in age [F(2,27) = 3.12, P = 0.06], or parental education level [F(2,27) = 0.157, P = 0.85) for obese, overweight and lean participants. The local Institutional Review Board approved this project. Participants and parents provided written informed consent.
fMRI media paradigm
Participants were asked to consume a typical breakfast/lunch, but to refrain from eating or drinking (except water) 5 h immediately preceding their scan in an effort to standardize hunger. To motivate participants to attend to the clips, participants were told they would complete a commercial-recognition task after the scan. Prior to scanning, participants rated hunger levels on a visual analog scale (not hungry at all to never been more hungry). Hunger was included as a control variable in all analyses. All participants were scanned in the afternoon (mean time onset scan = 4 p.m., s.d. = 1.5, range = 1 p.m.–6 p.m.) (all main effects remained significant when time of day that scanning occurred was controlled for in analyses.).
Data were obtained from Nielsen to measure the number of television ads viewed by 12- to 17-year-old individuals in 2009 for all food brands. After eliminating brands that are clearly aimed at younger children (e.g. Chuck ‘E Cheese), the 10 food brands advertised most frequently to this age group were identified. Commercials for these 10 brands were chosen as food commercial stimuli. For non-food commercial stimuli, Nielsen data were used to identify the weekly television programs that appeared during the first quarter of 2009 with the largest audience of 12- to 17-year olds (‘American Idol’, ‘Family Guy’, ‘Simpsons’, ‘George Lopez’ and ‘Secret Life of the American Teenager’). During January 2010, each of these programs, including the commercials, was recorded twice. Commercials for the 10 most frequently featured non-food brands were selected for inclusion as study stimuli (Table 1).
Table 1.
Food and non-food brands featured in commercial breaksa
Food brands
|
Non-food brands
|
aAll commercials were 15 s in length.
During scanning, participants saw a video of the television show ‘Mythbusters’ that was edited to include 20 food commercials and 20 non-food commercials (two commercials from each brand, see Table 1). The commercials were shown over four breaks (10 commercials per break, 15 s per commercial). This number of commercials in the paradigm was chosen to provide an adequate number of opportunities to capture blood oxygenation level-dependent (BOLD) activation during the commercials. Order of the commercials was randomized over the four breaks, and the order of the four breaks was randomized over the participants. The duration of each break was 2 min and 30 s. Total paradigm duration was 34 min.
Measures
Body mass index
The BMI (BMI = kg/m2) was used to reflect adiposity. To compute BMI, height was measured to the nearest millimeter, and weight was assessed to the nearest 0.1 kg (after removal of shoes and coats). Obesity was defined using the 95th percentiles of BMI for age and sex, based on historical nationally representative data because this definition corresponds closely to the BMI cut-point that is associated with increased risk for weight-related health problems (Cole et al., 2000). Adolescents with BMI scores between the 25th and 75th percentile using these historical norms were defined as lean, and adolescents with a BMI score between the 75th and 95th percentile were defined as overweight.
Pubertal development
Adolescents were asked to report on their current state of pubertal development using a standardized series of line drawings of youth at various states of pubertal development (Bonat et al., 2002).
Commercial recall measures
Participants were asked to list five commercials that they had seen during the television program they just viewed to measure top-of-mind recall. In addition, participants were given a list of 40 different products, including products that were and were not included in the television program, and asked to indicate whether they had seen commercials for these products to assess aided recall.
Commercial liking and familiarity measures
Participants were asked to rate how much they liked the products/companies featured in the advertisements on a five-point Likert scale (dislike extremely to like extremely) and how familiar they were with the advertisements on a five-point Likert scale (not at all familiar to extremely familiar).
Statistical analyses
fMRI data acquisition, preprocessing and statistical analysis
Scanning was performed with a Siemens Allegra 3 T head-only MRI scanner using a standard birdcage coil. Functional scans used a T2*-weighted gradient single-shot echo planar imaging sequence (echo time = 30 ms, repetition time = 2000 ms, flip angle = 80°) with an in plane resolution of 3.0 × 3.0 mm2 (64 × 64 matrix; 192 × 192 mm2 field of view). To cover the whole brain, 32 interleaved, no skip, 4 mm slices were acquired along the AC-PC transverse oblique plane, as determined by the midsagittal section. Prospective acquisition correction (PACE) was applied to adjust slice position and orientation, as well as to re-grid residual volume-to-volume motion in real-time during data acquisition for the purpose of reducing motion-induced effects (Thesen et al., 2000). No participant’s data set failed to meet the movement inclusion criteria, which were that within-run movement before correction did not exceed 2 mm in translational movement and 2° in rotational movement. For smaller movements, PACE adjusts slice position, orientation and regrids the residual volume-to-volume motion during data acquisition. Anatomical scans were acquired using a high-resolution inversion recovery T1-weighted sequence (Magnetization Prepared Rapid Acquisition Gradient Echo; Field of View = 256 × 256 mm2, 256 × 256 matrix, thickness = 1.0 mm, slice number ≈ 160).
Images were manually reoriented to the AC-PC line and skull stripped using the Brain Extraction Tool function in FMRIB's Software Library (Smith, 2002). Data were pre-processed and analyzed using SPM8 (Wellcome Department of Imaging Neuroscience) in MATLAB (Mathworks Inc.; Worsley et al., 1996). Functional images were realigned to the mean, and both the anatomical and functional images were normalized to the standard Montreal Neurological Institute (MNI) T1 template brain (ICBM152). Normalization resulted in a voxel size of 3 mm3 for functional images and a voxel size of 1 mm3 for high-resolution anatomical images. Functional images were smoothed with a 6-mm FWHM isotropic Gaussian kernel.
We contrasted BOLD activation during food commercials vs non-food commercials, food commercials vs a television show and non-food commercials vs a television show. Because there were 20 food commercials and 20 non-food commercials, we also included 20 randomly selected segments of the television show. Condition-specific effects at each voxel were estimated using general linear models. Vectors of the onsets for each event of interest were compiled and entered into the design matrix so that event-related responses could be modeled by the canonical hemodynamic response function, as implemented in SPM8. The event consisted of the entirety of the 15-s commercial and television segment. A 128 s high-pass filter was used to remove low-frequency noise and slow drifts in the signal.
Individual maps were constructed to compare the activations within each participant for food commercials, non-food commercials and television show. Consistent effects across subjects were then tested using the contrast images in one-sample t-tests (conforming a random effects model). We then created three groups based on weight status (obese, overweight and lean) and conducted second-level 3 (group: obese, overweight and lean) × 2 (stimulus type: food commercials, non-food commercials and television show) random-effects analysis of variance. As this study uses a novel paradigm (i.e. commercials imbedded in the context of a television show), whole-brain analyses were conducted throughout to allow for the identification of peaks in brain regions outside the classic reward regions (e.g. visual processing, attention) that may play a role advertising response. Cluster level thresholds corrected for multiple comparisons were derived using a Monte Carlo simulations (10 000 iterations) of random noise distribution in the whole-brain mask (3 × 3 × 3 mm) using the 3dClustSim and 3dFWHMx modules in AFNI (Forman et al., 1995; Cox, 1996). Using intrinsic smoothness, the Monte Carlo simulation combines individual voxel probability threshold and minimum cluster size to estimate the probability of a false positive. The threshold resulted in P < 0.001 with a cluster (k) ≥ 19, which is equal to P < 0.05 corrected for multiple comparisons across the whole brain. All contrasts were run in both directions (e.g. food commercials > non-food commercials and non-food commercials > food commercials) and only significant peaks are reported. Effect sizes (r) were derived from the Z-values (Z/√N).
RESULTS
Behavioral results
Overall, participants recalled more food (mean = 2.69, s.d. = 0.92) than non-food commercials [mean = 2.0, s.d. = 0.88; t(29) = 2.25, P = 0.03] and recognized more food commercials (mean = 1.78, s.d. = 0.32) than non-food commercials [mean = 1.60, s.d. = 0.33; t(29) = 3.13, P = 0.004]. Participants reported liking the food commercials better (mean = 3.52, s.d. = 0.49) than non-food commercials [mean = 3.24, s.d. = 0.36; t(29) = 2.29, P = 0.03] and reported to be more familiar with food (mean = 4.08, s.d. = 0.75) than non-food commercials [mean = 3.72, s.d. = 0.99; t(29) = 3.13, P = 0.004]. Hunger ratings suggest that participants were on average in a neutral hunger state (mean hunger = 0.63, s.d. = 3.69) prior to their scan session.
There were no significant differences between obese, overweight and lean individuals on pubertal development [F(2,27) = 1.44, P = 0.26), hunger ratings [F(2,27) = 1.58, P = 0.22], aided recall of food commercials [F(2,27) = 0.07, P = 0.94], aided recall of non-food commercials [F(2,27) = 0.06, P = 0.95], top-of-mind recall of food commercials [F(2,27) = 0.08, P = 0.92], top-of-mind recall of non-food commercials [F(2,27) = 0.17, P = 0.85], liking ratings of non-food commercials [F(2,27) = 0.40, P = 0.67], familiarity of food commercials [F(2,27) = 0.29, P = 0.75] and familiarity of non-food commercials [F(2,27) = 0.29, P = 0.76] (Table 2). However, there was a significant difference among the three groups in liking ratings of the food commercials [F(2,27) = 4.57, P = 0.03]. Post hoc tests showed that obese participants (mean = 3.26, s.d. = 0.43) reported lower liking ratings of food commercials than overweight participants (mean = 3.83, s.d. = 0.33).
Table 2.
Pubertal development, hunger and commercial ratings of obese, overweight and lean participants
| Obese (n = 12) | Overweight (n = 8) | Lean (n = 10) | F (28) | P | |
|---|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | Mean (s.d.) | |||
| Pubertal developmenta | 4.63 (0.50) | 4.19 (0.53) | 4.56 (0.73) | 1.44 | 0.26 |
| Hunger | 8.8 (4.3) | 10.7 (1.9) | 11.9 (5.1) | 1.58 | 0.22 |
| Top-of-mind recall food commercials | 8.2 (1.3) | 8.4 (1.5) | 8.6 (1.3) | 0.07 | 0.94 |
| Top-of-mind recall non-food commercials | 7.0 (2.9) | 6.9 (1.9) | 7.3 (2.0) | 0.06 | 0.95 |
| Aided recall food commercials | 1.8 (0.4) | 1.8 (0.3) | 1.8 (0.1) | 0.08 | 0.92 |
| Aided recall non-food commercials | 1.6 (0.4) | 1.5 (0.3) | 1.6 (0.3) | 0.17 | 0.85 |
| Liking food commercials | 3.26 (0.43) | 3.83 (0.33) | 3.66 (0.49) | 4.57 | 0.03b |
| Liking non-food commercials | 3.29 (0.5) | 3.26 (0.16) | 3.13 (0.19) | 0.40 | 0.67 |
| Familiarity food commercials | 4.01 (0.65) | 4.27 (0.92) | 4.01 (0.81) | 0.29 | 0.75 |
| Familiarity non-food commercials | 3.66 (0.81) | 3.97 (1.34) | 3.59 (0.98) | 0.29 | 0.76 |
aGirls were given line drawings of the five stages of breast and female pubic hair development with appropriate written descriptions of each stage. Boys were given line drawings of boys showing the five stages of pubic hair and external genitalia development and appropriate written descriptions of each stage. For girls, we averaged the scores on breast and pubic hair development. For boys, we averaged the scores on pubic hair and external genitalia development. The pubertal development scale ranges from prepubertal = 1 to adult stage = 5.
bPost hoc analyses identified that obese participants reported liking food commercials significantly less than overweight participants.
Main neural responses to food commercials compared with non-food commercials
On average participants exhibited greater activation in bilateral posterior cerebellar lobe (declive) (r left > 0.9 and r right > 0.9; Figure 1A), bilateral middle occipital gyrus (MOG; r left > 0.9 and r right = 0.87), right precentral gyrus (r > 0.9), right inferior temporal gyrus (ITG; r > 0.9), bilateral inferior parietal lobe (IPL; r left = 0.88 and r right = 0.75), left postcentral gyrus (r = 0.78), right precuneus (r = 0.74) and right superior parietal lobe (SPL; r = 0.69) (Table 3). The areas of greater neural response for non-food commercials and the television show are included in the Supplementary Table S1.
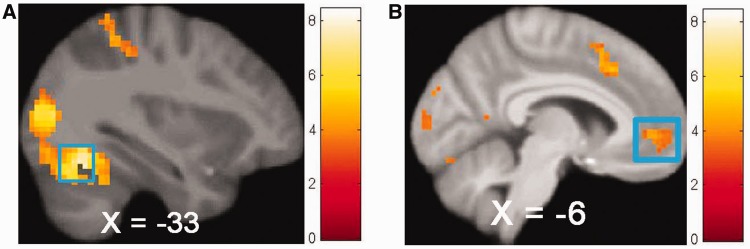
Fig. 1.
Participants (N = 30) exhibited greater activation in (A) bilateral posterior cerebellar lobe (MNI: −33, −64, −20, Z = 5.95, k = 811) in response to food commercials vs non-food commercials and greater activation in (B) the right vmPFC (MNI: 0, 56, −5, Z = 3.94, k = 106) in response to food commercials vs television show.
Table 3.
Average comparisons (N = 30) contrasting differences in brain responses to food commercials vs non-food commercials and food commercials vs television show
| Contrast and region | BA | k | Z-value | MNI coordinates | Effect size |
|---|---|---|---|---|---|
| Food > non-food | |||||
| Posterior cerebellar lobe (declive) | 813 | 5.96 | −33, −64, −20 | >0.90 | |
| MOG | 5.09 | −30, −88, 7 | >0.90 | ||
| Precentral gyrus | 4 | 78 | 5.62 | 57, −19, 37 | >0.90 |
| Posterior cerebellar lobe (declive) | 382 | 5.34 | 30, −52, −20 | >0.90 | |
| ITG | 5.29 | 48, −58, −14 | >0.90 | ||
| IPL | 118 | 4.81 | −42, −40, 52 | 0.88 | |
| Postcentral gyrus | 2 | 4.27 | 4.19 | −45, −28, 46 | 0.76 |
| MOG | 213 | 4.79 | 33, −79, 10 | 0.87 | |
| Precuneus | 4.03 | 24, −79, 34 | 0.74 | ||
| IPL | 40 | 48 | 4.18 | 36, −46, 52 | 0.76 |
| SPL | 37 | 3.71 | 27, −55, 58 | 0.68 | |
| Food > television show | |||||
| Cuneus | 19 | 820 | 5.95 | −12, −91, 25 | >0.90 |
| Posterior cerebellar lobe (declive) | 5.28 | −27, −58, −17 | >0.90 | ||
| Anterior cerebellar lobe (culmen) | 510 | 5.69 | 24, −43, −23 | >0.90 | |
| Lingual gyrus | 18 | 5.23 | 21, −79, −14 | >0.90 | |
| MOG | 227 | 5.32 | 36, −79, 13 | >0.90 | |
| Cingulate gyrus | 32 | 108 | 4.60 | −3, 17, 40 | 0.84 |
| MOG | 24 | 4.09 | −42, −73, −14 | 0.75 | |
| vmPFC | 97 | 3.90 | 0, 56, −5 | 0.71 | |
| ACC | 32 | 3.82 | −6, 47, −2 | 0.70 | |
| OFC | 11 | 3.66 | 3, 50, −11 | 0.67 | |
For all contrasts, activated regions, Brodmann areas (BA), Z-values and coordinates within the MNI coordinate system are displayed. Number of contiguous voxels (k) are shown for peak coordinates. Peaks within the regions were considered significant at k ≥ 19, P < 0.05, corrected for multiple comparisons across the entire brain.
Main neural responses to food commercials compared with television show
Participants exhibited greater activation in the left cuneus (r > 0.9), bilateral posterior cerebellar lobe (r left > 0.9 and r right > 0.9), right anterior cerebellar lobe (culmen) (r > 0.9), right lingual gyrus (r > 0.9), bilateral MOG (r right > 0.9 and r left = 0.74), left cingulate gyrus (r = 0.85), right ventromedial prefrontal cortex (vmPFC; r = 0.72; Figure 1B), left anterior cingulate cortex (ACC; r = 0.71) and right ventromedialPFC/medial OFC (vmPFC/medial OFC; r = 0.68).
Relation between main neural responses and self-report ratings of commercials
Because participants recalled more food commercials than non-food commercials, reported greater familiarity with food vs non-food commercials and reported greater liking of food commercials vs non-food commercials, we examined the relations between these variables and the main neural responses. We extracted the main effect parameter estimates at the individual level and calculated the Pearson correlation coefficients in SPSS (SPSS for Windows, version 19.0, IBM-SPSS, Chicago, IL, USA). Activation in the left posterior cerebellar lobe in response to food commercials relative to non-food commercials was positively correlated with familiarity ratings of food commercials (r = 0.46, P = 0.03). Activation in the midcingulate cortex in response to non-food commercials relative to food commercials was negatively correlated with liking ratings of non-food commercials (r = −0.49, P = 0.02). There were no significant correlations between the main neural responses and recall measures.
Differences in brain activation in response to food commercials vs non-food commercials between obese, overweight and lean individuals
Obese individuals showed greater activation in the middle temporal gyrus (MTG; r = 0.77) and less activation in the left cuneus (r = −0.74; Figure 2A) and left posterior cerebellar lobe (r = 0.70) compared with overweight individuals (Table 4). Overweight individuals showed greater activation in the left cuneus (r = 0.73) and left posterior cerebellar lobe (r = 0.73) compared with lean individuals (Table 4).
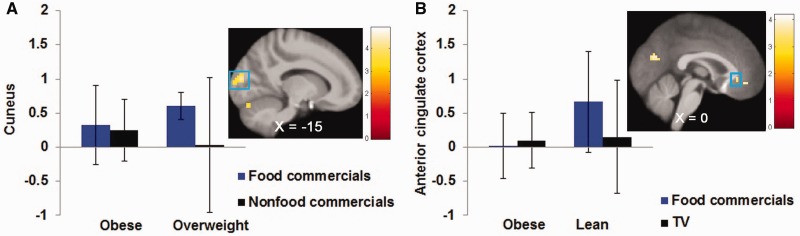
Fig. 2.
Overweight participants exhibited greater activation in (A) the left cuneus (MNI: −12, −91, 13, Z = 4.06, k = 47) in response to food commercials vs non-food commercials compared with obese participants. Obese participants exhibited less activation in (B) the right ACC (MNI: 0, 32, −5, Z = 3.31) in response to food commercials vs television show compared with lean participants.
Table 4.
Group differences in brain activation in response to food commercials vs non-food commercials and food commercials vs television show between obese (n = 12), overweight (n = 8) and lean (n = 10) individuals
| Contrast and region | BA | k | Z-value | MNI coordinates | Effect size |
|---|---|---|---|---|---|
| Food > non-food | |||||
| Obese > overweight | |||||
| MTG | 20 | 4.24 | 57, −46, −5 | 0.77 | |
| Overweight > obese | |||||
| Cuneus | 18 | 47 | 4.06 | −12, −91, 13 | 0.74 |
| Posterior cerebellar lobe | 24 | 3.86 | −21, −76, −26 | 0.70 | |
| Overweight > lean | |||||
| Cuneus | 18 | 53 | 4.02 | −15, −88, 16 | 0.73 |
| Posterior cerebellar lobe | 31 | 4.01 | −21, −76, −23 | 0.73 | |
| Food > television show | |||||
| Obese > overweight | |||||
| MTG | 22 | 4.06 | 60, −46, −5 | 0.74 | |
| Lean > obese | |||||
| vmPFC | 21 | 3.97 | −3, 41, −11 | 0.72 | |
| ACC | 3.31 | 0, 32, −5 | 0.60 | ||
| Precuneus | 31 | 32 | 3.84 | 3, −70, 19 | 0.70 |
For all contrasts, activated regions, BA, Z-values and coordinates within the MNI coordinate system are displayed. Number of contiguous voxels (k) are shown for peak coordinates. Peaks within the regions were considered significant at k ≥ 19, P < 0.05, corrected for multiple comparisons across the entire brain.
Differences in brain activation in response to food commercials vs television show between obese, overweight and lean individuals
Obese individuals showed greater activation in the MTG (r = 0.74) compared with overweight individuals and less activation in the vmPFC (r = 0.73), ACC (r = 0.60; Figure 2B) and precuneus (r = 0.70) compared with lean individuals.
DISCUSSION
In this study, adolescents generally exhibited greater activation in regions implicated in visual processing (e.g. MOG), attention (e.g. parietal lobes), cognitive processing (e.g. ITG and posterior cerebellar lobe), movement (e.g. anterior cerebellar lobe), somatosensory response (postcentral gyrus) and reward (i.e. OFC and ACC) during food commercials relative to non-food commercials and the television show. This pattern of results is consistent with participants’ greater recall of food commercials compared with non-food commercials.
Viewing of food commercials vs non-food commercials and the television show was related to greater activation in the occipital gyrus. This finding extends previous evidence that suggest that activation in the occipital gyrus is greater during exposure to food pictures relative to non-food pictures (Schur et al., 2009). Frank et al. (2010) also found that the occipital gyrus displayed greater activation than traditional reward-related regions (e.g. OFC and insula) in response to high-calorie food pictures (compared with non-food pictures that were matched on physical features). Similarly, the occipital gyrus was also the most active brain region during exposure to food logos (relative to control images) in children (Bruce et al., 2012a). The lingual gyrus and precuneus were also more active during food commercials relative to other stimuli, and these regions (in addition to the occipital lobe) are thought to be related to identifying the salience of appetitive cues (Tang et al., 2012). The lingual gyrus has been found to be more active during food relative to non-food logos (Bruce et al., 2012a). Thus, participants in this study may have found food commercials to be more salient and may have been visually attended more to food commercials relative to the other stimuli in the paradigm. In contrast, television viewing relative to food and non-food commercials was related to greater activation in neural regions associated with semantic processing and language (e.g. superior temporal gyrus and middle frontal gyrus) (Binder et al., 1997; Buchsbaum et al., 2001), which may reflect the more complicated nature of the discussions occurring in the television segments.
The IPL and SPL, which are related to mediating attentional processes (Pessoa et al., 2002), were more active during food relative to non-food commercials. Greater activation in the SPL has been related to initial orientation to food cues (Yokum et al., 2012), and greater regional cerebral blood flow in the parietal lobe during exposure to food pictures has been linked to feelings of hunger in obese women (Karhunen et al., 1997). The ITG was also more active during food relative to non-food commercials and has been linked to a variety of cognitive processes, including semantic memory, language, visual perception and sensory integration (Ojemann et al., 2001; Noppeney and Price, 2002; Price, 2002). Both the parietal lobe and temporal gyrus have been found to be more active in healthy children during food logo exposure (Bruce et al., 2012a). The cerebellar lobe was also more active during food relative to non-food commercials and the television show, which is consistent with prior research that found greater cerebellar activation in response to food stimuli (Killgore et al., 2003). While the anterior cerebellar lobe has been associated with motor responses, the posterior cerebellar lobe has been linked to cognitive and attention processes (Stoodley et al., 2012) and activation in this region may reflect a ‘hyper-attentive state’ (Anderson et al., 2005). Therefore, these findings suggest that participants’ attention may have been more fully captured by the food commercials (relative to the non-food commercials) and greater cognitive processing regarding these commercials may have occurred. This is consistent with participants’ greater recall of food commercials and the association between activation in the posterior cerebellar lobe and food-commercial familiarity.
Somatosensory, motor and reward-related regions were more active during food commercials relative to other stimuli. The postcentral gyrus is implicated in taste perception, and food cues exposure is related to activation in this region (Killgore et al., 2003; Frank et al., 2010). Increased activation in motor-related regions (i.e. anterior cerebellum, precentral gyrus) (Stoodley et al., 2012) in response to binge-type food cues for obese binge eaters has been interpreted as reflecting planning to acquire of consume food (Geliebter et al., 2006). The ACC is a region associated with reward-related decision making, motivation and attention (Bush et al., 2002; Tang et al., 2012; Totah et al., 2013). Greater activation in this area is related to high (vs low) calorie food stimuli (Bruce et al., 2010) and increased response in the ACC to high-calorie food images (relative to control pictures) is predictive of greater difficulty losing weight (Murdaugh et al., 2012). Activation of the medial OFC is thought to reflect the intensity of desire (Kawabata and Zeki, 2008) and subjective evaluation of reward (Berridge et al., 2010). Increased activation in the medial OFC is related to higher ratings of food pleasantness (Kringelbach, 2005) and elevated hunger (Siep et al., 2009; Bruce et al., 2010), as well as food logo exposure in children (Bruce et al., 2012a). The vmPFC is also thought to encode value (Hare et al., 2009), guide reward-related behaviors (Miller et al., 2007) and is more active during exposure to food (relative to neutral stimuli) (Killgore et al., 2003). Thus, in this study, food commercials relative to other stimuli may have triggered increased subjective pleasure and intensified motivation to seek out the featured products.
Contrary to our hypothesis, obese participants exhibited less activation during food commercials relative to non-food commercials in neural regions implicated in visual processing (i.e. cuneus) (Meyer et al., 2007) and attention (i.e. posterior cerebellar lobe) (Stoodley et al., 2012). Obese relative to normal-weight participants also exhibited less activation in regions related to reward (i.e. vmPFC and ACC) (Hare et al., 2009; Tang et al., 2012) and salience detection (i.e. precuneus) (Tang et al., 2012). Although previous research has typically found obese participants to be more responsive to food cues (Rothemund et al., 2007; Stoeckel et al., 2008; Martin et al., 2009; Bruce et al., 2010; Stice et al., 2010), a recent study examining neural response to food logos (relative to non-food logos) in children found that healthy weight compared with obese children exhibited greater activation in a number of regions (e.g. frontal gyrus, precuneus, parietal lobe and insula) (Bruce et al., 2012b). Thus, branded food items may differ from the type of food cues used in prior studies in a manner that alters the pattern of neural response for lean and obese participants. Prior research also found that obese compared with normal-weight participants exhibited greater activation in multiple brain regions in response to food cues, but only prior to eating a meal (Dimitropoulos et al., 2012). Following the meal, obese participants exhibited greater activation in prefrontal and corticolimbic regions relative to normal-weight participants. The obese participants’ hypo-activation in pre-meal condition was thought to reflect the use of control strategies to reduce food desire during cue exposure. Obese relative to overweight adolescents in this study exhibited greater activation in the MTG during food commercials relative to overweight participants. The MTG has been related to the implementation of semantic control used in executively demanding semantic decisions (Whitney et al., 2011). In other words, semantic control is associated with focusing on one target response (e.g. avoiding the advertised product), when multiple response options are available (e.g. attending to the advertised product). Thus, it is possible that obese participants were using control strategies to reduce their response during food commercials.
Interestingly, overweight participants showed increased activation in the regions associated with attention/cognition (i.e. posterior cerebellum) (Stoodley et al., 2012) and visual processing (i.e. cuneus) (Meyer et al., 2007) relative to both obese and lean participants. This pattern of results suggests a non-linear relationship between body weight and neural response to food advertisements. These findings are consistent with the hypothesis that risk for obesity (i.e. being overweight) may be related to hyper-responsivity to food-related reward, but the development of obesity may lead to a reduction in reward circuitry functioning (Stice and Burger, 2012). Consistent with this interpretation, obese relative to overweight participants reported decreased liking of the food commercials.
It is important to consider the limitations of this study. First, this study was designed to most accurately capture exposure to food commercials in real-world settings. This goal led us to embed commercial breaks in the context of television viewing and to choose commercial stimuli based on the frequency that adolescents were exposed to these commercials types. Thus, the commercials types likely differ in meaningful ways (e.g. color intensity and emotional response). As these variables may differ in certain ways in a manner that increases the effectiveness of marketing for the different product types, we choose not to match commercials on these characteristics. The greater recall of food commercials relative to non-food commercials suggests that food advertising might have been more effective in this study. It will be important for future research to identify how attributes that differ by commercial type may impact neural response, memory and eating behavior. Second, the sample size of this study is relatively small, thus there may have been limited power to detect other effects between weight classes, such as individual differences in the midbrain or striatum. This may be even more likely given the complicated nature of the stimuli used in this paradigm (e.g. commercials). Finally, this study is cross-sectional, which does not provide information regarding the time course of eating-related problems and the pattern of neural activation associated with food commercials. It may be especially important to conduct longitudinal studies on this topic, as lean relative to obese participants in this study exhibited greater activation in the ACC, cuneus and cerebellum. Greater neural response in these areas during exposure to high-calorie food images (relative to control pictures) is implicated in difficulties with weight loss/maintenance (Murdaugh et al., 2012). Thus, neural response to food commercials might prospectively predict weight gain, especially in normal-weight adolescents.
CONCLUSIONS
Despite these limitations, this study has a number of strengths and implications. This is the first study, to our knowledge, to examine how the brain responds to food commercials. Relative to prior research on food pictures, the stimuli in this study were designed to evoke desire and prominently featured well-known food brands (e.g. McDonalds) that could also influence neural response (Bruce et al., 2012a). Further, the study was designed to recreate the environment that represents how adolescents are often exposed to advertising (e.g. commercials chosen based on age-group exposure and viewed during television commercial breaks). Thus, the study provides some insight into how the ubiquitous nature of food advertising may play a role in the obesity epidemic. Interestingly, regardless of weight class, participants recalled food commercials more than non-food commercials. This is consistent with greater activation across a number of domains (e.g. attention, cognition and reward) in response to food commercials relative to other stimuli. Also, lean relative to obese adolescents exhibited greater neural response to food commercials in regions related to greater difficulty with weight loss/maintenance. This suggests that even adolescents who are not currently exhibiting signs of pathology (e.g. normal-weight) may be impacted by commercials in a manner that might shape future eating tendencies. These findings might inform current policy debates about food advertising to minors.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
The work described in the manuscript has not been published previously and is not under consideration for publication elsewhere. The submission is approved by all authors. This research was supported by the Rudd Foundation, National Institute of Health grant DK080760 and the Robert Wood Johnson Foundation.
REFERENCES
- Anderson CM, Maas LC, deB Frederick B, et al. Cerebellar vermis involvement in cocaine-related behaviors. Neuropsychopharmacology. 2005;31(6):1318–26. doi: 10.1038/sj.npp.1300937. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho C-Y, Richard JM, DiFeliceantonio AG. The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Research. 2010;1350(20388498):43–64. doi: 10.1016/j.brainres.2010.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. The Journal of Neuroscience. 1997;17(1):353–62. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonat S, Pathomvanich A, Keil MF, Field AE, Yanovski JA. Self-assessment of pubertal stage in overweight children. Pediatrics. 2002;110(4):743–7. doi: 10.1542/peds.110.4.743. [DOI] [PubMed] [Google Scholar]
- Bruce AS, Bruce JM, Black WR, et al. Branding and a child’s brain: an fMRI study of neural responses to logos. Social Cognitive and Affective Neuroscience. In press doi: 10.1093/scan/nss109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Holsen L, Chambers R, et al. Obese children show hyperactivation to food pictures in brain networks linked to motivation, reward and cognitive control. International Journal of Obesity. 2010;34(10):1494–500. doi: 10.1038/ijo.2010.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce AS, Lepping RJ, Bruce JM, et al. Brain responses to food logos in obese and healthy weight children. The Journal of Pediatrics. 2012;162:759–764. doi: 10.1016/j.jpeds.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Hickok G, Humphries C. Role of left posterior superior temporal gyrus in phonological processing for speech perception and production. Cognitive Science. 2001;25(5):663–78. [Google Scholar]
- Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(1):523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnell S, Wardle J. Measuring behavioural susceptibility to obesity: validation of the child eating behaviour questionnaire. Appetite. 2007;48(1):104–13. doi: 10.1016/j.appet.2006.07.075. [DOI] [PubMed] [Google Scholar]
- Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320(7244):1240. doi: 10.1136/bmj.320.7244.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Council of American Research Survey Organizations. CASRO's Data Trends Survey: 2005 Survey Results. 2005. http://www.casro.org/pdfs/CASRO%202005%20Data%20Trends%20Results.pdf. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Demos KE, Heatherton TF, Kelley WM. Individual differences in nucleus accumbens activity to food and sexual images predict weight gain and sexual behavior. The Journal of Neuroscience. 2012;32(16):5549–52. doi: 10.1523/JNEUROSCI.5958-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitropoulos A, Tkach J, Ho A, Kennedy J. Greater corticolimbic activation to high-calorie food cues after eating in obese vs. normal-weight adults. Appetite. 2012;58:303–12. doi: 10.1016/j.appet.2011.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Trade Commission. A Review of Food Marketing to Children and Adolescents: Follow-up Report. 2012. http://www.ftc.gov/os/2012/12/121221foodmarketingreport.pdf. [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magnetic Resonance in Medicine. 1995;33(5):636–47. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Research. 2010;1350:159–166. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Ladell T, Logan M, Schweider T, Sharafi M, Hirsch J. Responsivity to food stimuli in obese and lean binge eaters using functional MRI. Appetite. 2006;46(1):31–5. doi: 10.1016/j.appet.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Hare TA, Camerer CF, Rangel A. Self-control in decision-making involves modulation of the vmPFC valuation system. Science. 2009;324(19407204):646–8. doi: 10.1126/science.1168450. [DOI] [PubMed] [Google Scholar]
- Heath R. Low involvement processing-a new model of brand communication. Journal of Marketing Communications. 2001;7(1):27–33. [Google Scholar]
- Karhunen L, Lappalainen R, Vanninen E, Kuikka J, Uusitupa M. Regional cerebral blood flow during food exposure in obese and normal-weight women. Brain. 1997;120(9):1675–84. doi: 10.1093/brain/120.9.1675. [DOI] [PubMed] [Google Scholar]
- Kawabata H, Zeki S. The neural correlates of desire. PLoS One. 2008;3(8):e3027. doi: 10.1371/journal.pone.0003027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Young AD, Femia LA, Bogorodzki P, Rogowska J, Yurgelun-Todd DA. Cortical and limbic activation during viewing of high-versus low-calorie foods. NeuroImage. 2003;19(4):1381. doi: 10.1016/s1053-8119(03)00191-5. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Martin LE, Holsen LM, Chambers RJ, et al. Neural mechanisms associated with food motivation in obese and healthy weight adults. Obesity. 2009;18(2):254–60. doi: 10.1038/oby.2009.220. [DOI] [PubMed] [Google Scholar]
- MATLAB 7.1, The Mathworks Inc., Natick MA, 2005.
- McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR. Neural correlates of behavioral preference for culturally familiar drinks. Neuron. 2004;44(2):379–87. doi: 10.1016/j.neuron.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Meyer M, Baumann S, Marchina S, Jancke L. Hemodynamic responses in human multisensory and auditory association cortex to purely visual stimulation. BMC Neuroscience. 2007;8(1):14. doi: 10.1186/1471-2202-8-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JL, James GA, Goldstone AP, et al. Enhanced activation of reward mediating prefrontal regions in response to food stimuli in Prader–Willi syndrome. Journal of Neurology, Neurosurgery & Psychiatry. 2007;78(6):615–9. doi: 10.1136/jnnp.2006.099044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murdaugh DL, Cox JE, Cook Iii EW, Weller RE. fMRI reactivity to high-calorie food pictures predicts short- and long-term outcome in a weight-loss program. NeuroImage. 2012;59(3):2709–21. doi: 10.1016/j.neuroimage.2011.10.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U, Price C. Retrieval of visual, auditory, and abstract semantics. NeuroImage. 2002;15(4):917–26. doi: 10.1006/nimg.2001.1016. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–90. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann G, Schoenfield-McNeill J, Corina D. Anatomic subdivisions in human temporal cortical neuronal activity related to recent verbal memory. Nature Neuroscience. 2001;5(1):64–71. doi: 10.1038/nn785. [DOI] [PubMed] [Google Scholar]
- Pessoa L, Gutierrez E, Bandettini PA, Ungerleider LG. Neural correlates of visual working memory: fMRI amplitude predicts task performance. Neuron. 2002;35(5):975–87. doi: 10.1016/s0896-6273(02)00817-6. [DOI] [PubMed] [Google Scholar]
- Powell LM, Schermbeck RM, Szczypka G, Chaloupka FJ, Braunschweig CL. Trends in the nutritional content of television food advertisements seen by children in the United States: analyses by age, food categories, and companies. Archives of Pediatrics and Adolescent Medicine, archpediatrics. 2011;165:1078–1086. doi: 10.1001/archpediatrics.2011.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2002;197(3):335–59. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothemund Y, Preuschhof C, Bohner G, et al. Differential activation of the dorsal striatum by high-calorie visual food stimuli in obese individuals. NeuroImage. 2007;37(2):410. doi: 10.1016/j.neuroimage.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Rudd Center for Food Policy and Obesity. Trends in television food advertising to young people: 2010 update. 2011 http://www.yaleruddcenter.org/resources/upload/docs/what/reports/RuddReport_TVFoodAdvertising_6.11.pdf. [Google Scholar]
- Schor JB, Ford M. From tastes great to cool: children's food marketing and the rise of the symbolic. The Journal of Law, Medicine & Ethics. 2007;35(1):10–21. doi: 10.1111/j.1748-720X.2007.00110.x. [DOI] [PubMed] [Google Scholar]
- Schur E, Kleinhans N, Goldberg J, Buchwald D, Schwartz M, Maravilla K. Activation in brain energy regulation and reward centers by food cues varies with choice of visual stimulus. International Journal of Obesity. 2009;33(6):653–61. doi: 10.1038/ijo.2009.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte ML, Jansen A. Hunger is the best spice: an fMRI study of the effects of attention, hunger and calorie content on food reward processing in the amygdala and orbitofrontal cortex. Behavioural Brain Research. 2009;198(1):149–58. doi: 10.1016/j.bbr.2008.10.035. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Burger KS. Neurobiology of Overeating In: eLS. Chichester: John Wiley & Sons, Ltd; 2012. DOI: 10.1002/9780470015902.a0024012. [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in body mass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50(4):1618–25. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoeckel LE, Weller RE, Cook E, 3rd, Twieg DB, Knowlton RC, Cox JE. Widespread reward-system activation in obese women in response to pictures of high-calorie foods. NeuroImage. 2008;41(2):636–47. doi: 10.1016/j.neuroimage.2008.02.031. [DOI] [PubMed] [Google Scholar]
- Stoodley CJ, Valera EM, Schmahmann JD. Functional topography of the cerebellum for motor and cognitive tasks: an fMRI study. NeuroImage. 2012;59(2):1560–70. doi: 10.1016/j.neuroimage.2011.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D, Fellows L, Small D, Dagher A. Food and drug cues activate similar brain regions: a meta-analysis of functional MRI studies. Physiology & Behavior. 2012;106:317–324. doi: 10.1016/j.physbeh.2012.03.009. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magnetic Resonance in Medicine. 2000;44(3):457–65. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Totah NKB, Jackson ME, Moghaddam B. Preparatory attention relies on dynamic interactions between prelimbic cortex and anterior cingulate cortex. Cerebral Cortex. 2013;23:729–738. doi: 10.1093/cercor/bhs057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink B. Using laddering to understand and leverage a brand’s equity. Qualitative Market Research: An International Journal. 2003;6(2):111–8. [Google Scholar]
- Wellcome Department of Imaging Neuroscience. Institute of Neurology, University College of London, London UK.
- Whitney C, Kirk M, O'Sullivan J, Ralph MAL, Jefferies E. The neural organization of semantic control: TMS evidence for a distributed network in left inferior frontal and posterior middle temporal gyrus. Cerebral Cortex. 2011;21(5):1066–75. doi: 10.1093/cercor/bhq180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4(1):58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Yokum S, Ng J, Stice E. Attentional bias to food images associated with elevated weight and future weight gain: an FMRI study. Obesity. 2012;19(9):1775–83. doi: 10.1038/oby.2011.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.