Abstract
Being able to comprehend communicative intentions and to recognize whether such intentions are directed toward us or not is extremely important in social interaction. Two brain systems, the mentalizing and the mirror neuron system, have been proposed to underlie intention recognition. However, little is still known about how the systems cooperate within the process of communicative intention understanding and to what degree they respond to self-directed and other-directed stimuli. To investigate the role of the mentalizing and the mirror neuron system, we used functional magnetic resonance imaging with four types of action sequence: communicative and private intentions as well as other-directed and self-directed intentions. Categorical and functional connectivity analyses showed that both systems contribute to the encoding of communicative intentions and that both systems are significantly stronger activated and more strongly coupled in self-directed communicative actions.
Keywords: communicative intentions, mentalizing, mirror system, second-person interaction
INTRODUCTION
From observing other people’s actions, we can readily detect their focus of attention and draw inferences regarding their intentions: does she intend to drink or to offer the glass? Is the action directed at me or toward another person?
Despite the fact that non-linguistic communication contributes considerably to social cognition (Bara et al., 2011), the neural processes involved in the ability to understand intentions from action observation remain controversial (Van Overwalle and Baetens, 2009). It has been proposed that intention understanding is accomplished by means of a motor simulation within the so-called ‘mirror neuron system’ (Rizzolatti and Sinigaglia, 2010). This system includes the premotor cortex (PMC) and the anterior intraparietal sulcus (aIPS) and is involved in tasks requiring the understanding of intention conveyed by body motion (Iacoboni et al., 2005; Vingerhoets et al., 2010; Becchio et al., 2012). However, it remains unclear to what extent mirror areas might contribute to the recognition of more complex intentions (Figure 1), such as communicative intentions (Montgomery et al., 2007).
Fig. 1.
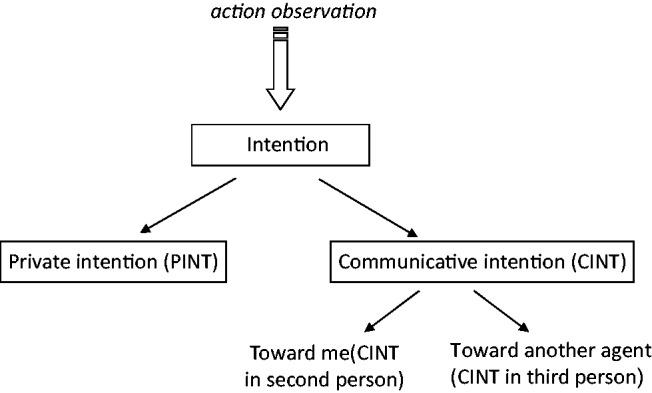
Varieties of intentions. Starting from the observation of others’ action, we can infer two kinds of intentions: private intentions (PInt) and communicative intentions (CInt). Within communicative intentions we can further distinguish if the action is directed at me (CInt0°) or toward another person (CInt30°). Figure adapted from Ciaramidaro et al. (2007).
On the other hand, intention understanding has been related to inferential processes based on a so-called ‘theory of mind’ (Amodio and Frith, 2006), also referred to as ‘mentalizing’. Mentalizing processes have been consistently linked to a set of regions outside the motor system, including the medial prefrontal cortex (MPFC) and the temporo-parietal junction (TPJ) as well as the adjacent posterior superior-temporal-sulcus (pSTS) (Frith and Frith, 2006; Saxe, 2006). This system is typically recruited when people reflect on others intentions in the absence of detailed information on biological motion, for example, when reading stories or watching cartoons implying goals, beliefs or morality (Walter et al., 2004; Young and Saxe, 2008). During action observation, activation of the mentalizing network is noted when subjects are explicitly instructed to identify the intentions of actors they observe (Grezes et al., 2004; De Lange et al., 2008; Liew et al., 2010; Spunt et al., 2010; Centelles et al., 2011), or the actions themselves are atypical (Brass et al., 2007). However, little is known about the contribution of these areas to the implicit encoding of intention during the observation of daily communicative actions (Frith and Frith, 2008). Moreover, no study has so far elucidated the possibility that self-involvement affects the contribution and integration of mentalizing and mirror areas during the observation of communicative actions. Social cognition has been proposed to be substantially different when we are in interaction with others (second-person interaction) rather than merely observing them (third-person interaction; Schilbach et al., in press). Second-person interaction is closely related to feelings of engagement and emotional responses to others and is characterized by intricate reciprocity dynamics not involved in merely observing someone else interacting. In terms of the underlying neural substrates, such differences might be reflected in overlapping vs distinct neural circuits or could be related to differences in connectivity between mirror and mentalizing regions (Schilbach et al., in press).
In this study, we used functional magnetic resonance imaging (fMRI), within the framework of cognitive pragmatics (Bara, 2010) to investigate (i) how mirror and mentalizing regions contribute to the implicit encoding of communicative intentions and (ii) whether activity in these regions is shaped and modulated by self-involvement. To this aim, fMRI data were interrogated through a comprehensive approach that incorporated conventional univariate and multivariate analysis of psychophysiological interactions (PPIs).
MATERIALS AND METHODS
Participants
Twenty-three right-handed volunteers (12 female), age 24 (±3.98) with no history of neurological or psychiatric disorder were recruited via local newspapers and campus advertisements. The study was conducted in accordance to the regulations of the local Ethics Committee and the declaration of Helsinki (De Roy, 2004) and approved by the local institutional review board. Participants gave written informed consent after the experimental procedure had been explained to them.
Experimental procedure
Participants were shown short video clips of every day action sequences. The video clips depicted an actor standing in the proximity of a table on which two objects were placed. To create the stimulus material, we filmed four types of action sequence (Figure 2).
Fig. 2.
Activation paradigm showing the four types of action sequences in a 2 × 2 factorial design, in which the factors were the type of Intention (communicative vs private) and the Orientation of the observed action (0° vs 30°).
Communicative intention in second person, 0°oriented
The actor reached toward, grasped an object and performed a communicative action (show the object or offer the object) directed straight at the camera (CInt0°) using a frontal view from the participant’s perspective. Direct gaze at the camera signaled the intention to communicate.
Communicative intention in third person, 30°oriented
This action sequence was similar to the CInt0° sequence, except that the communicative action was directed toward a co-experimenter located outside the recorded area at an angular distance of ∼30° to the right (CInt30°). To signal the intention to communicate, the actor looked straight ahead toward the co-experimenter.
Private intention, 0°oriented
The actor reached toward, grasped an object and performed an individual action (move the object or look at the object). In performing the individual action, the model’s body was oriented straight to the camera (PInt0°), but the model never looked directly at the camera.
Private intention, 30°oriented
This action sequence was similar to the PInt0° sequence, except that in performing the individual action, the model’s body was oriented 30° to the right (PInt30°). As for the Pint0° condition, the model never looked straight ahead.
To obtain a large sample of every day action sequences, we employed six actors (three females) and six different objects (apple, key, book, picture frame, cup and alarm clock). Each actor performed 24 actions (4 action × 6 objects) for a total of 144 original video sequences (48 per condition, 12 videos were seen twice).
The four types of action sequences were embedded in a 2 × 2 factorial design, in which the factors were the type of Intention (communicative vs private) and the Orientation of the observed action (0° vs 30°). Before participation, all participants received standardized instructions. They were told they would observe an agent performing a brief action sequence. In some cases, the agent’s action would be oriented toward the participant himself/herself (0°), in other cases, toward a second agent, not visible in the video clip. Intention coding was assessed implicitly using a gender categorization task. Participants were instructed to observe each action sequence carefully and to make a right index button press when the model was a female. Trials were arranged in 48 blocks of four video clips displaying the same type of action sequence for a total of 192 trials. Each video was presented for 2.75 s, so that a block lasted ∼11 s. After each block, a blank screen was shown for a period varying between 6 and 11.5 s. Blocks were presented in randomized order during one session lasting ∼23 min. Before scanning, participants received outside-scanner training with videos for each category. Stimuli were presented by means of Presentation software (Neurobehavioral Systems, Albany, CA, USA) using binocular LCD-Goggles (Nordic Neurolab, Bergen, Norway) connected to the head coil. The responses were recorded with fiber-optic response devices (Nordic Neurolab).
Post-scan questionnaire
After scanning, individual differences in trait empathy were assessed using a self-report empathy questionnaire: the Empathy Quotient (EQ) (Baron-Cohen and Wheelwright, 2004). The EQ contains 40 empathy items and 20 filler/control items and on each item a person can score 2, 1 or 0. High scores correspond to more emphatic behavior.
Behavioral data analysis
Participant’s reaction times and response accuracy were measured during scanning. Data were analyzed using SPSS Statistics 17.0 in a one-way ANOVA with subsequent comparisons between means, using Bonferroni’s correction.
fMRI data acquisition and data analysis
Imaging was performed on a 1.5-T Siemens Sonata. Functional images were acquired using an echoplanar imaging (EPI) sequence. A total of 473 whole-brain scans were obtained. One volume consisted of 26 slices [slice thickness 4 mm+ 1 mm gap, field of view (FOV) 210 mm, repetition time (TR) 2.25 s, echo time (TE) 50, 64 × 64 matrix and flip angle 90°]. In addition, anatomical whole-brain images were obtained using a T1-weighted magnetization-prepared, 3D gradient-echo pulse sequence with the following parameters: TR = 1660 ms; TE = 3.09 ms; flip angle 15°; FOV = 256 × 256 mm and 160 sagittal slices with 1 mm thickness.
Data preprocessing
Data preprocessing and statistical analyses were carried out with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/). Individual functional images were corrected for motion with a fourth degree B-spline realignment. For normalization, a transformation matrix between the mean image of realigned volumes and the SPM2-EPI (MNI) template was generated with a trilinear algorithm and applied to resliced volumes with a voxel size of 2 × 2 × 2 mm. For spatial smoothing, a Gaussian Kernel of 8 mm full width at half maximum was chosen to increase sensitivity for cortical activations in group inference. A high-pass filter of two TR times the longest period between two subsequent trials of the same condition was used to filter out systematic low-frequency activation unrelated to the task. The standard hemodynamic response function (HRF) was used for convolution with the covariates of the experimental design.
Conventional analysis
First-level analysis of fMRI data was performed according to the general linear model. Regressors were defined based on the timing of presentation of each of the four experimental conditions. To model response events (see ‘Experimental procedure’ section), separate regressors were defined for female and male actor videos. The first-level regression model consisted, therefore, of a set of eight regressors (CInt0° with male actor, CInt0° with female actor, CInt30° with male actor, CInt30° with female actor, PInt0° with male actor, PInt0° with female actor, PInt30° with male actor and PInt30° with female actor) convolved with the HRF and six regressors describing residual motion. Second-level analysis utilized the individual contrast images for simple effects from the first-level analysis. The differential effects of the experimental tasks were assessed with a repeated measures ANOVA model. All reported results of statistical comparisons multiple testing across the whole brain were thresholded at a voxel level of P < 0.001 uncorrected (using an extent voxel size of k = 10). To assess regional overlap between the main effect of Intention and the interaction of Intention by Orientation, an additional conjunction analysis was conducted.
For regression analyses, individual peak voxel data were extracted from the respective contrast and region and analyzed externally using SPSS Statistics 17.0.
Psychophysiological interaction analysis
To assess coupling between the mentalizing and the mirror neuron areas, we estimated a PPI analysis (Friston et al., 1997). PPI allows inference as to whether region-to-region co-activation changes significantly as a function of task. We extracted the subject-specific time course of activity in the MPFC (a mentalizing region) with an 8 mm radial sphere centered at the voxel displaying peak activity for the contrast CINT0° > CInt30°. Taking as reference independent studies (Gilbert et al., 2007; Burnett and Blakemore, 2009), the specific region of interest (ROI) for MPFC was defined as the volume from 8 to +8 on the x-axis, from +40 to +56 on the y-axis and from −12 to +30 on the z-axis. We then calculated the product of this activation time course with the interaction term of the CInt0° > CInt30° action sequences to create the PPI term. PPI analyses were carried out for each subject, and then entered into a random effects group analysis using a one-sample t-test. For PPI analysis, threshold was set to P < 0.05, corrected for false discovery rate (FDR), using an extent voxel size of k = 70.
Correlation analysis
To assess correlations between brain activation and individual empathic abilities (as measured by the EQ), we calculated a one-sample t-tests for the contrast CInt0° > CInt30°. T-statistics for each voxel were thresholded at P < 0.001 corrected for multiple comparisons across the whole brain. Individual data were extracted from this group maximum for each individual at (−2 64 12) activation. Data were analyzed externally using SPSS Statistics 17.0, and correlation analysis was performed with subjects’ empathic traits (EQ).
RESULTS
Behavioral data
Response times during scanning
A repeated measures ANOVA with within-subject factors Intention (communicative vs private) and Orientation (0° vs 30°) showed a significant main effect of Intention [F(1, 22) = 11.049; P = 0.003]. Participants were slower to respond during observation of communicative actions relative to individual actions [CInt0° 563.88 ms (± 189.64); CInt30° 544.45 ms (± 161.46); PInt0° 518.84 ms (± 152.64) and PInt30° 528.66 ms (± 159.58)]. There was no main effect of Orientation [F(1, 22) = 0.248; P = 0.623] and no interaction Intention by Orientation [F(1, 22) = 3.421; P = 0.07].
Response accuracy during scanning
A repeated measures ANOVA on response accuracy with within-subject factors Intention and Orientation yielded a significant main effect of Intention [F(1,22) = 14.817; P > 0.001] and a significant interaction effect [F(1,22) = 11.563; P = 0.002]. Participants were more accurate during observation of communicative actions relative to private actions [CInt0° 22.61 (± 1.95); CInt30° 23.65 (± 0.49); PInt0° 23.26 (± 0.96) and PInt30° 22.17 (± 2.48)]. Post hoc (Bonferroni) tests indicated that response accuracy was higher for CInt30° than for PInt30° (P = 0.01). There was no main effect of Orientation [F(1,22) = 0.323; P = 0.575].
Neuroimaging data
Categorical analysis
A whole-brain analysis was carried out to identify brain regions implicated in the understanding of communicative and private intentions during second- and third-person interaction. The peak activity and stereotaxic coordinates for activations are listed in Table 1.
Table 1.
The voxels with the highest value for the main effect Intention and Orientation and for the interaction and conjunction analysis
| Region | x | y | z | Z | Cluster size | |
|---|---|---|---|---|---|---|
| Main effect Intention | ||||||
| pSTSa | R | 44 | −48 | 14 | 5.18 | 343 |
| L | −50 | −62 | 12 | 4.50 | 331 | |
| MPFCa | L | −4 | 24 | 52 | 4.66 | 452 |
| TPJa | L | −48 | −60 | 24 | 3.52 | 226 |
| PMCa | R | 44 | 12 | 28 | 3.75 | 221 |
| L | −36 | 14 | 32 | 4.67 | 267 | |
| aIPSa | R | 34 | −40 | 52 | 3.57 | 44 |
| L | −36 | −46 | 48 | 4.02 | 100 | |
| FFAa | R | 40 | −52 | −16 | 5.07 | 424 |
| L | −42 | −48 | −12 | 4.99 | 384 | |
| Occipital lobea | R | 18 | −88 | 26 | 4.57 | 282 |
| Inferior occipital lobea | L | −32 | −86 | −8 | 3.83 | 82 |
| Main effect Orientation | ||||||
| Lingual gyrusa | R | 18 | −84 | −4 | 5.84 | 340 |
| L | −10 | −82 | −6 | 6.25 | 694 | |
| Medial occipital lobe | L | −28 | −86 | 4 | 3.56 | 53 |
| Interaction of Intention by Orientation | ||||||
| PMC | R | 40 | 22 | 28 | 3.58 | 143 |
| L | −42 | 26 | 14 | 4.73 | 208 | |
| MPFC | L | −6 | 58 | 24 | 3.36 | 31 |
| Medial temporal gyrus | L | −58 | −16 | −8 | 3.76 | 43 |
| Superior frontal gyrus | L | −22 | 62 | 14 | 3.70 | 40 |
| Inferior occipital lobe | L | −34 | −84 | −10 | 3.24 | 48 |
| Conjunction of Intention and Interaction of Intention by Orientation | ||||||
| PMC | L | −42 | 28 | 20 | 3.67 | 32 |
| Inferior occipital lobe | L | −34 | −84 | −10 | 3.44 | 28 |
The threshold was set at P < 0.001 uncorrected (using an extent voxel size of k = 10). R, right; L, left; x, y, z, respective MNI coordinates of peak voxel activation; Z, Z-value.
aRegions that survive the FDR set at P < 0.05 correction.
Main effect of intention
Observing actions performed with a communicative intent relative to actions performed with a private intent (CInt > PInt) revealed activity in typical mentalizing areas, namely bilateral pSTS (44 −48 14 and −50 −46 10), in the left TPJ (−46 −58 26) and the MPFC (−4 24 52) for the mentalizing network, and the bilaterally PMC (44 12 28 and −36 14 32) and bilaterally aIPS (34 −40 52 and −36 −46 48) for the mirror system. Furthermore, an additional cluster of activation was observed in the fusiform face area (FFA) (40 −52 −16 and −42 −48 −12). The reverse contrast (PInt > CInt) did not reveal any activation. For detailed results, see Figure 3 and Table 1.
Fig. 3.
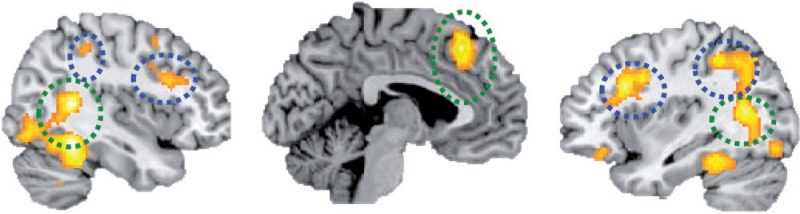
Brain responses of the main effect intention. Green circles indicate mentalizing brain regions, and blue circles mirror brain areas. All results are thresholded at P < 0.05 FDR corrected for display purposes (using an extent voxel size of k = 10).
Main effect of orientation
Observing 0° oriented actions relative to 30° oriented actions (0° > 30°) revealed activations in visual areas (20 −90 4 and −10 −82 −6). No activation was found for the reverse contrast (30° > 0°) (Table 1).
Interaction of intention by orientation
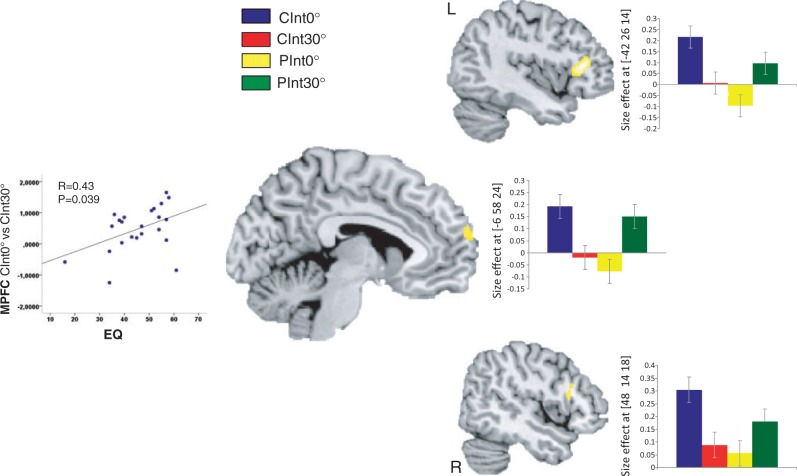
A significant effect of interaction [(CInt0° > CInt30°) > (PInt0° > Pint30°)] was observed within the MPFC (−6 58 24) and the bilateral PMC (40 22 28 and −42 26 14). For detailed results, see Figure 4 and Table 1.
Fig. 4.
Brain responses of the interaction effect intention × orientation. Bar plots indicate size of the effect at the maximum activated voxel for MPFC and bilateral PMC (P = 0.001 uncorrected, k = 10). The amount of MPFC activation (between condition effect CInt0° vs CInt30°) depended on self-reported trait empathy (EQ) (r = 0.43, P = 0.039).
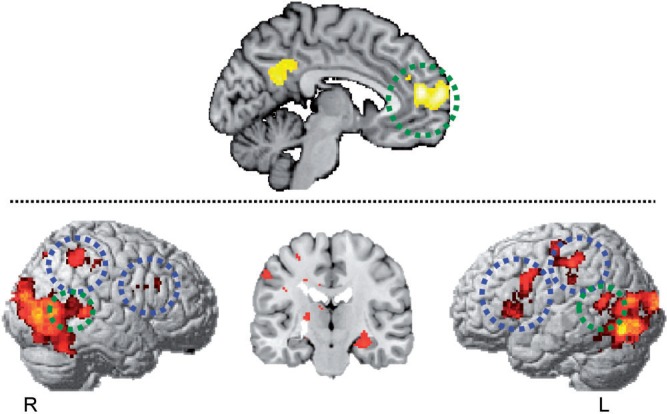
Psychophysiological interaction analysis
The PPI analysis showed increasing coupling of the MPFC with both mentalizing and mirror areas during second-person perspective communication. In particular, with bilateral pSTS (42 −50 8 and −46 −56 14) for the mentalizing system and with left PMC (−32 18 4) and bilateral aIPS (38 −48 48 and −46 −28 30) for the mirror system. Additional increased coupling was shown in bilateral FFA (38 −44 −18 and −40 −40 −16) and right amygdala (28 −22 −14). See also Figure 5.
Fig. 5.
Results of PPI analysis. Participants showed increased coupling between MPFC with bilateral pSTS (42 −50 8 and −46 −56 14) for the mentalizing system (green circle) and bilateral left PMC (−32 18 4) and bilateral aIPS (38 −48 48 and −46 −28 30) in the MNS (blue circle) (P < 0.05 corrected for FDR, using an extent voxel size of k = 70).
Correlation with empathic traits and MPFC
The correlation analysis revealed a positive correlation (r = 0.43, P = 0.039) between self-reported trait empathy (EQ) and the bold signal in the MPFC (Figure 4).
DISCUSSION
In spite of the remarkable progress made in the field of social neuroscience, the neural mechanisms that underlie social encounters still represent a ‘dark matter’ (Becchio et al., 2010; Schilbach et al., in press). In this fMRI study, we assessed the contribution of mirror and mentalizing to the understanding of communicative intention. Based on the premise that social interaction is fundamentally different when we are in interaction with others rather than merely observe them (Schilbach et al., in press), we contrasted the implicit encoding of communicative intentions during second-person interaction and third-person interaction.
Encoding of communicative intention within both mirror and mentalizing areas
Although looking at a book or showing a book to someone may involve similar movements, the intentions conveyed by these actions are clearly different: whereas looking at a book entails a private intention, showing a book is directed toward another agent and entails a communicative intention.
Contrasting these two types of intentions revealed differential activations within both mirror areas, including the PMC and aIPS, and mentalizing areas, including the MPFC, bilateral pSTS and the left TPJ, while the mirror system and the mentalizing system are rarely concomitantly activated (Van Overwalle and Baetens, 2009). These findings indicate that both systems contribute to the encoding of communicative intentions during action observation (Figure 3).
So far, evidence that the mirror system contributes to the understanding of communicative intentions has been sparse using video clips of hand gestures (Montgomery et al., 2007; Liew et al., 2010) or social scenes conveyed through point-light stimuli (Centelles et al., 2011). However, as clearly different actions sets were employed to portray social and non-social scenes, starkly contrasting configural stimulus properties might be responsible for the results. Our data provide the first evidence that hand gestures directed at the same objects may recruit the PMC to a different degree depending on whether they convey a private or a communicative intention.
Evidence that areas within the mentalizing system are sensitive to the type of intention was first provided by Walter et al. (2004) and Ciaramidaro et al. (2007). Using cartoons, they found that an increasing number of mentalizing areas was involved as cartoons progressed along a dimension of increasing social interaction, starting with private intentions, moving to social prospective intentions (preparing future social interactions) and ending with communicative intentions. Whereas the right TPJ was activated in the comprehension of all three types of intentions, the MPFC was specifically activated in the comprehension of social prospective and communicative intentions, the left TPJ in the comprehension of communicative intentions only.
Anatomically MPFC cortex activation revealed for the main effect of Intention in this study was more dorsal as compared with MPFC activations reported by Walter et al. (2004) and Ciaramidaro et al. (2007). Modulation of dorsal regions within the MPFC has been reported, for example, during mentalizing about dissimilar others (Mitchell et al., 2006), thinking about friends (Kumaran and Maguire, 2005) and reasoning about false beliefs (e.g. Sommer et al., 2007). On tasks that involve action observation, activity in the dorsal MPFC is noted when participants are explicitly told to try to figure out the intention motivating the observed action (x = −7, y = 34, z = 44; Iacoboni et al., 2005), during observation of unintended actions (x = 9, y = 35, z = 56; Buccino et al., 2007) and during observation of grasping actions conveying a social intention (x = 0, y = 28, z = 40; Becchio et al., 2012). Thus, one possibility is that more dorsal regions of the MPFC are specifically involved in the encoding of intention during movement observation.
Outside the mirror system and the mentalizing system, the neural representation of the communicative and private intentions also differed in the FFA suggesting that the processing of face, crucial to grasp the significance of a social scene (for review, see Kanwisher and Yovel, 2006), may itself be modulated by the type of intention. In line with the view that communicative intentions call for more complex representations than private intentions, the reverse contrast, private vs communicative intention, failed to reveal any differential activation. The increased relational complexity of communicative relative to private intentions was further confirmed by behavioral assessment during scanning. Participants were slower, but more accurate to respond during observation of communicative actions relative to individual actions, suggesting that they were more engaged during the encoding of communicative intention compared with private intentions.
Second vs third person perspective in communicative intention encoding
Whereas the orientation of the action revealed no differential mirror or mentalizing activations, we found a significant interaction effect between type of intention and orientation within the MPFC and the bilateral PMC (Figure 4). Inspection of activity specifically related to communicative intentions revealed that the MPFC and bilateral PMC were more active for second-person communicative intention than for third-person communicative intention. A conjunction analysis showed that the left PMC, but not right PMC or MPFC overlapped with the main effect of intention (Table 1). In line with preliminary neuroscientific evidence, these findings support the hypothesis of differences in neural processing of social stimuli depending on whether they are directed toward oneself or another person (Schilbach et al., in press). In both the CInt0° and the CInt30° action sequences, the actor reached toward and grasped an object with the communicative intent either to show or to offer the object to another person. The key difference was that in CInt0° action sequence, the gesture was directed toward the participant, whereas in CInt30° sequence, the action was directed toward another person not visible in the scene. Only in the encoding of CInt0° intentions, but not during the encoding CInt30°, the participant was therefore self-involved in the ongoing interaction.
Differences in the processing of self-related and other-related social stimuli have been previously reported for gaze processing. Social gaze shifts, i.e. gaze shift directed at another person, have been shown to activate the MPFC as a function of personal involvement (see also, Kampe et al., 2003; Schilbach et al., 2006; Bristow et al., 2007). Furthermore, in gaze-based social interactions, increased activity in MPFC has been observed when participants follow the gaze of another person to engage in joint attention (Schilbach, 2010). During action observation, indirect evidence that self-involvement modulates mirror activity is provided by the finding that mu wave suppression—an index of mirror neuron activity—is greater for self-directed social actions compared with non-social actions (Oberman et al., 2007; Perry et al., 2010; see also Kourtis et al., 2010). Our results add to these findings suggesting that self-involvement impacts on the recruitment of both the mirror and the mentalizing system during the implicit encoding of communicative intentions. Most importantly, they indicate that self-involvement may result in changes in functional connectivity between mirror and mentalizing regions.
Increased functional connectivity among ‘social brain’ regions has been previously reported by Lombardo et al. (2010) during reflective mentalistic judgments about self and other. Spunt and Liberman (2012a, 2012b) found that mirror and mentalizing areas are functionally coupled when participants make attributions about the cause of an action or emotion, but not when they consider how the action or emotion is implemented. This functional coupling has been proposed to support an integrational model of mirror and mentalizing contributions to action/emotion understanding, wherein the mirror system translates sensory input about motor behavior into a format that is relevant to attribution process carried out within the mentalizing (Keysers and Gazzola, 2007).
In this study, increased functional connectivity within the mentalizing seed region (MPFC) was observed during CInt0° > CInt30° in a widely distributed neural network including the left PMC and the bilateral aIPS, as well as the bilateral pSTS, the bilateral FFA and the right amygdala (Figure 5). This demonstrates that coupling among ‘social brain’ areas is stronger during the implicit encoding of second-person communicative intention compared with third-person communicative intention. This finding provides new insights into the integration of the mirror and the mentalizing system during intention understanding, suggesting that self-involvement may modulate the degree to which these systems work in concert.
It is also notable that activations within the MPFC (from the contrast CInt0° > CInt30°) positively correlated with individual differences in empathy as measured by EQ (Figure 4). In addition to self-involvement, a second-person grasping of other minds has been proposed to be closely related to feelings of engagement and emotional response to others (Schilbach et al., in press). While emotional engagement may also occur during observation (such as watching an emotionally charged movie scene), it would seem plausible that emotional-embodied responses could facilitate the understanding of other minds during second-person social interactions. The finding that people scoring higher in empathy show higher MPFC activity supports this hypothesis, suggesting that being able to perceive what others feel may indeed facilitate the implicit encoding of communicative intention during second-person interaction.
In summary, our study confirms the co-activation of the mirror and mentalizing system to decode complex intentions such as communicative intentions. We provide evidence that both systems work in synergy to recognize communicative gestures and that their reciprocal interaction increases when gestures are directed toward the self. These results shed new light on the role of personal involvement in social interaction and on the basic neural mechanisms that enable two minds to communicate.
Acknowledgments
The authors would like to thank Pietro Santoro for his help in preparing the designs material. A.C., L.C. and B.B. were supported by the San Paolo Foundation (Neuroscience Programme: Action representations and their impairment, 2009-2012). C.B. was supported by a grant from the Regione Piemonte, bando Scienze Umane e Sociali 2008, L.R. n.4/2006.
REFERENCES
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Review Neuroscience. 2006;7:268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Bara BG. Cognitive Pragmatics. The Mental Processes of Communication. Cambridge, MA: The MIT Press; 2010. [Google Scholar]
- Bara BG, Ciaramidaro A, Walter H, Adenzato M. Intentional minds: a philosophical analysis of intention tested through fMRI experiments involving people with schizophrenia, people with autism, and healthy individuals. Frontiers in Human Neuroscience. 2011;5:7. doi: 10.3389/fnhum.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S, Wheelwright S. The Empathy Quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. Journal of Autism and Developmental Disorders. 2004;34:163–75. doi: 10.1023/b:jadd.0000022607.19833.00. [DOI] [PubMed] [Google Scholar]
- Becchio C, Cavallo A, Begliomini C, Sartori L, Feltrin G, Castiello U. Social grasping: from mirroring to mentalizing. Neuroimage. 2012;61:240–8. doi: 10.1016/j.neuroimage.2012.03.013. [DOI] [PubMed] [Google Scholar]
- Becchio C, Sartori L, Castiello U. Toward you: the social side of actions. Current Directions in Psychological Science. 2010;19:183–8. [Google Scholar]
- Brass M, Schmitt R, Spengler S, Gergely G. Investigating action understanding: inferential processes versus action simulation. Current Biology. 2007;17:1783–9. doi: 10.1016/j.cub.2007.11.057. [DOI] [PubMed] [Google Scholar]
- Bristow D, Rees G, Frith CD. Social interaction modifies neural response to gaze shifts. Social Cognitive and Affective Neuroscience. 2007;2:52–61. doi: 10.1093/scan/nsl036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buccino G, Baumgaertner A, Colle L, Buechel C, Rizzolatti G, Binkofski F. The neural basis for understanding non- intended actions. Neuroimage. 2007;2:119–27. doi: 10.1016/j.neuroimage.2007.03.036. [DOI] [PubMed] [Google Scholar]
- Burnett S, Blakemore S. Functional connectivity during a social emotion task in adolescents and in adults. European Journal of Neuroscience. 2009;29:1294–301. doi: 10.1111/j.1460-9568.2009.06674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centelles L, Assaiante C, Nazarian B, Anton JL, Schmitz C. Recruitment of both the mirror and the mentalizing networks when observing social interactions depicted by point-lights: a neuroimaging study. Plos One. 2011;6:15749–9. doi: 10.1371/journal.pone.0015749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaramidaro A, Adenzato M, Enrici I, et al. The intentional network: how the brain reads varieties of intentions. Neuropsychologia. 2007;45:3105–13. doi: 10.1016/j.neuropsychologia.2007.05.011. [DOI] [PubMed] [Google Scholar]
- De Lange FP, Spronk M, Willems RM, Toni I, Bekkering H. Complementary systems for understanding action intentions. Current Biology. 2008;18:454–7. doi: 10.1016/j.cub.2008.02.057. [DOI] [PubMed] [Google Scholar]
- De Roy PG. Helsinki and the Declaration of Helsinki. World Medical Journal. 2004;50:9–11. [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6:218–29. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Frith C, Frith U. How we predict what other people are going to do. Brain Research. 2006;1079:36–46. doi: 10.1016/j.brainres.2005.12.126. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Implicit and explicit processes in social cognition. Neuron. 2008;60:503–10. doi: 10.1016/j.neuron.2008.10.032. [DOI] [PubMed] [Google Scholar]
- Gilbert SJ, Williamson IDM, Dumontheil I, Simons JS, Frith CD, Burgess PW. Distinct regions of medial rostral prefrontal cortex supporting social and nonsocial functions. Social Cognitive of Affective Neuroscience. 2007;2:217–26. doi: 10.1093/scan/nsm014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grezes J, Frith CD, Passingham RE. Brain mechanisms for inferring deceit in the actions of others. Journal of Neuroscience. 2004;24:5500–5. doi: 10.1523/JNEUROSCI.0219-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacoboni M, Molnar-Szakacs I, Gallese V, Buccino G, Mazziotta JC. Grasping the intentions of others with one's own mirror neuron system. PLoS Biology. 2005;3:529–35. doi: 10.1371/journal.pbio.0030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampe KKW, Frith CD, Frith U. “Hey John”: signals conveying communicative intention toward the self activate brain regions associated with “mentalizing”, regardless of modality. Journal of Neuroscience. 2003;23:5258–63. doi: 10.1523/JNEUROSCI.23-12-05258.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwisher N, Yovel G. The fusiform face area: a cortical region specialized for the perception of faces. Philosophical Transactions of the Royal Society of London Series B, Biological sciences. 2006;361:2109–28. doi: 10.1098/rstb.2006.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Gazzola V. Integrating simulation and theory of mind: from self to social cognition. Trends Cognitive Science. 2007;11:194–6. doi: 10.1016/j.tics.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Kourtis D, Sebanz N, Knoblich G. Favouritism in the motor system: social interaction modulates action simulation. Biology Letters. 2010;23:758–61. doi: 10.1098/rsbl.2010.0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumaran D, Maguire EA. The human hippocampus: congnitive maps or relational memory? The Journal of Neuroscience. 2005;25:7254 –9. doi: 10.1523/JNEUROSCI.1103-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew S, Han S, Aziz-Zadeh L. Familiarity modulates mirror neuron and mentalizing regions during intention understanding. Human Brain Mapping. 2010;32:1986–97. doi: 10.1002/hbm.21164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombardo MV, Chakrabarti B, Bullmore ET, et al. Shared neural circuits for mentalizing about the self and others. Journal of Cognitive Neuroscience. 2010;22:1623–35. doi: 10.1162/jocn.2009.21287. [DOI] [PubMed] [Google Scholar]
- Mitchell JP, Macrae CN, Banaji MR. Dissociable medial prefrontal contributions to judgments of similar and dissimilar others. Neuron. 2006;50:655–63. doi: 10.1016/j.neuron.2006.03.040. [DOI] [PubMed] [Google Scholar]
- Montgomery KJ, Isenberg N, Haxby JV. Communicative hand gestures and object-directed hand movements activated the mirror neuron system. Social Cognitive of Affective Neuroscience. 2007;2:114–22. doi: 10.1093/scan/nsm004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberman LM, Pineda JA, Ramachandran VS. The human mirror neuron system: a link between action observation and social skills. Social Affective of Neuroscience. 2007;2:62–6. doi: 10.1093/scan/nsl022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry A, Trojeb NF, Bentin S. Exploring motor system contributions to the perception of social information: evidence from EEG activity in the mu/alpha frequency range. Social Neuroscience. 2010;5:272–84. doi: 10.1080/17470910903395767. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Review Neuroscience. 2010;11:264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Saxe R. Uniquely human social cognition. Current Opinion Neurobiology. 2006;16:235–9. doi: 10.1016/j.conb.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Schilbach L. A second-person approach to other minds. Nature Reviews Neuroscience. 2010;11:449. doi: 10.1038/nrn2805-c1. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Timmermans B, Reddy V, et al. Toward a second-person neuroscience. Behavioural and Brain Science. in press doi: 10.1017/S0140525X12000660. [DOI] [PubMed] [Google Scholar]
- Schilbach L, Wohlschlaeger AM, Kraemer NC, et al. Being with virtual others: Neural correlates of social interaction. Neuropsychologia. 2006;44:718–30. doi: 10.1016/j.neuropsychologia.2005.07.017. [DOI] [PubMed] [Google Scholar]
- Sommer M, Döhnel K, Sodian B, Meinhardt J, Thoermer C, Hajaka G. Neural correlates of true and false belief reasoning. NeuroImage. 2007;15:1378–84. doi: 10.1016/j.neuroimage.2007.01.042. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. Dissociating modality-specific and supramodal neural systems for action understanding. The Journal of Neuroscience. 2012a;32:3575–83. doi: 10.1523/JNEUROSCI.5715-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spunt RP, Lieberman MD. An integrative model of the neural systems supporting the comprehension of observed emotional behavior. NeuroImage. 2012b;59:3050–9. doi: 10.1016/j.neuroimage.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Spunt RP, Satpute AB, Lieberman MD. Identifying the what, why, and how of an observed action: an fMRI study of mentalizing and mechanizing during action observation. Journal of Cognitive Neuroscience. 2010;23:63–74. doi: 10.1162/jocn.2010.21446. [DOI] [PubMed] [Google Scholar]
- Van Overwalle F, Baetens K. Understanding others' actions and goals by mirror and mentalizing systems: a meta-analysis. Neuroimage. 2009;48:564–84. doi: 10.1016/j.neuroimage.2009.06.009. [DOI] [PubMed] [Google Scholar]
- Vingerhoets G, Honoré P, Vandekerckhove E, Nys J, Vandemaele P, Achten E. Multifocal intraparietal activation during discrimination of action intention in observed tool grasping. Neuroscience. 2010;169:1158–67. doi: 10.1016/j.neuroscience.2010.05.080. [DOI] [PubMed] [Google Scholar]
- Walter H, Adenzato M, Ciaramidaro A, Enrici I, Pia L, Bara BG. Understanding intentions in social interactions: the role of the anterior paracingulate cortex. Journal of Cognitive Neuroscience. 2004;16:1854–63. doi: 10.1162/0898929042947838. [DOI] [PubMed] [Google Scholar]
- Young L, Saxe R. The neural basis of belief encoding and integration in moral judgment. Neuroimage. 2008;40:1912–20. doi: 10.1016/j.neuroimage.2008.01.057. [DOI] [PubMed] [Google Scholar]