Abstract
Neuroimaging studies show a correlation between activity of the ventromedial prefrontal cortex (vmPFC) and skin conductance measurements. However, little is known whether this brain region plays a causal role in regulating physiological arousal. To address this question, we employed Granger causality analysis (GCA) to establish causality between cerebral blood oxygenation level-dependent and skin conductance signals in 24 healthy adults performing a cognitive task during functional magnetic resonance imaging. The results showed that activity of the vmPFC not only negatively correlated with skin conductance level (SCL) but also Granger caused SCL, thus establishing the direction of influence. Importantly, across participants, the strength of Granger causality was negatively correlated to phasic skin conductance responses elicited by external events during the behavioral task. In contrast, activity of the dorsal anterior cingulate cortex positively correlated with SCL but did not show a causal relationship in GCA. These new findings indicate that the vmPFC plays a causal role in regulating physiological arousal. Increased vmPFC activity leads to a decrease in skin conductance. The findings may also advance our understanding of dysfunctions of the vmPFC in mood and anxiety disorders that involve altered control of physiological arousal.
Keywords: ventromedial prefrontal cortex, arousal, skin conductance, Granger causality
INTRODUCTION
Changes in physiological arousal accompany attention, decision-making, affective regulation and other motivated behaviors (Frith and Allen, 1983; Damasio, 1994; Bechara et al., 1997; Critchley, 2002, 2009; Dolan, 2002). Skin conductance, which increases via sympathetic innervations of the sweat glands, is often used as a physiological index of arousal (Critchley, 2002; Naqvi and Bechara, 2006). Skin conductance comprises a tonic and phasic component. Tonic skin conductance, or skin conductance level (SCL), reflects the overall conductivity of the skin over a long period of time; in contrast, the phasic component—skin conductance response (SCR)—refers to a discrete and short fluctuation in skin conductance, as elicited by a cognitive event. Numerous imaging studies have investigated the neural correlates of SCL and SCR.
The medial prefrontal cortex, including anterior cingulate cortex (ACC) and ventromedial prefrontal cortex (vmPFC), is consistently involved in cerebral responses to physiological arousal (Table 1). For instance, in a decision-making task, activity of the dorsal ACC (dACC) was positively modulated by the SCR during a delay period between reward-related decisions and their outcomes (Critchley et al., 2001). In contrast, the vmPFC showed task independent negative correlations with the SCL. When participants controlled their arousal via biofeedback by increasing SCL during an arousal condition while decreasing SCL during a relaxation condition, vmPFC activity was negatively correlated with SCL in both conditions (Nagai et al., 2004). A recent study demonstrated that the negative correlation between the vmPFC activity and SCL also occurs during resting sate (Fan et al., 2012). It has been postulated that while the dACC responds to changes in skin conductance, the vmPFC may play a role in regulating physiological arousal (Damasio, 1994; Critchley et al., 2000, 2001).
Table 1.
A summary of brain regions within the medial prefrontal cortex that respond to skin conductance signals
| Study | MNI coordinate (mm) |
Identified region and approximate Brodmann area (BA) | ||
|---|---|---|---|---|
| x | y | z | ||
| Decision making task (Critchley et al., 2000) | ||||
| Activity preceding SCR | −12 | 50 | 10 | Rostral ACC; BA 10 |
| Activity subsequent to SCR | 10 | 52 | −10 | vmPFC; BA 10 |
| Decision making task (Critchley et al., 2001) | ||||
| Positive correlation with SCR | 4 | 32 | 24 | Dorsal ACC; BA 32 |
| Biofeedback relaxation task (Nagai et al., 2004) | ||||
| Positive correlation with SCL | −12 | 32 | 36 | Dorsal ACC; BA 32 |
| Negative correlation with SCL | −2 | 58 | −10 | vmFPC; BA 11 |
| Emotional faces viewing task (Williams et al., 2005) | ||||
| Activation for fear and SCR | 14 | 42 | 7 | Rostral ACC; BA 32 |
| Activation for anger and SCR | 7 | 31 | 28 | Dorsal ACC; BA 32 |
| 7 | 28 | −7 | vmPFC; BA 11 | |
| Activation for disgust and SCR | 11 | 20 | 21 | Dorsal ACC; BA 32 |
| Resting state (Fan et al., 2012) | ||||
| Positive correlation with SCL | 6 | 6 | 44 | Dorsal ACC; BA 24 |
| Negative correlation with SCL | 2 | 28 | −18 | vmPFC; BA 11 |
However, the results of these earlier studies were based on correlation and it is not clear whether the vmPFC plays a direct role in regulating physiological arousal. Elucidation of this relationship is critical to our understanding of the functions and dysfunctions of vmPFC and the etiologies of mood and anxiety disorders that implicate altered emotional regulation. We sought to address this issue by examining the causal relationship between cerebral activities and skin conductance with Granger causality analysis (GCA; Granger, 1969). In functional magnetic resonance imaging (fMRI), GCA has been widely used to investigate the causal relationship between regional activities during cognitive performance (Ding et al., 2006; Stilla et al., 2007; Deshpande et al., 2008, 2009; Duann et al., 2009). For instance, a recent study employed GCA to elucidate the flow of information between ventral and dorsal attention networks during a trial-by-trial cued visual spatial attention task (Wen et al., 2012). Here, GCA would be a useful tool to explore the casual relationship between cerebral activity and skin conductance, two time series recorded continuously throughout a cognitive task. We hypothesized that blood oxygenation level-dependent (BOLD) signals of the vmPFC would not only correlate negatively with but also Granger cause SCL. Furthermore, the strength of causality or regulatory influence of the vmPFC would negatively correlate with events-evoked SCR across subjects.
MATERIALS AND METHODS
Subjects and behavioral tasks
Twenty-four adult healthy subjects (10 males, 30 ± 11 years of age, all right-handed and using their right hand to respond) participated in this study. All participants denied medical including neurological illnesses, history of head injuries, current use of any medications or use of any psychotropic medications in the past year. They were also free of any psychiatric diagnoses as assessed with the Structured Clinical Interview for Diagnostic and Statistical Manual Disorders (First et al., 1995), denied use of illicit substances and showed a negative urine toxicology test on the day of fMRI. All subjects were paid to participate and signed a written consent after details of the study were explained, in accordance to institute guidelines and procedures approved by the Yale Human Investigation Committee.
We employed a simple reaction time (RT) task in this stop-signal paradigm, as described in details in our previous studies (Chao et al., 2009; Li et al., 2009; Zhang and Li, 2012a). Briefly, there were two trial types: ‘go’ (∼75%) and ‘stop’ (∼25%), randomly intermixed. A small dot appeared on the screen to engage attention at the beginning of a go trial. After a randomized time interval (fore-period) between 1 and 5 s, the dot turned into a circle, prompting the subjects to quickly press a button. The circle vanished at button press or after 1 s had elapsed, whichever came first, and the trial terminated. A premature button press prior to the appearance of the circle also terminated the trial. In a stop trial, an additional ‘X’, the ‘stop’ signal, appeared after the go signal and instructed the subjects to withhold button press. Likewise, a trial terminated at button press or after 1 s had elapsed. There was an inter-trial-interval of 8 s to allow adequate spacing between events of interest and identification of SCR associated with these events. The time interval between go and stop signals or stop signal delay (SSD) started at 200 ms and varied from one stop trial to the next according to a staircase procedure, increasing and decreasing by 64 ms, each after a successful and failed stop trial (Levitt, 1971; De Jong et al., 1990). With the staircase procedure, a ‘critical’ SSD could be computed that represents the time delay required for the subject to succeed in half of the stop trials (Levitt, 1971). Subjects were instructed to respond to the go signal quickly while keeping in mind that a stop signal could come up in a small number of trials. With the staircase procedure, we anticipated that the subjects would succeed in withholding their response in approximately half of the stop trials. Prior to the fMRI study, each subject had a practice session outside the scanner. Each subject completed six 10 min runs of the task. Across 24 subjects, we had an average of 205 ± 14 go and 69 ± 7 stop including 38 ± 4 stop success and 31 ± 4 stop error trials.
Skin conductance acquisition and analysis
With a Biopac MP150 system, skin conductance was continuously recorded during fMRI from the palmer surfaces of the index and middle fingers of the left hand. The biopac system used a AcqKnowledge 4.1 software (Biopac Systems, USA) and the Biopac electrodermal activity amplifier module (Galvanic Skin Response 100c) set at a channel sampling rate of 31 Hz and a gain of 5 µSiemens (µS) per volt (resulting in a resolution of 0.0015 µS). Recording of skin conductance is synchronized with behavioral task and image acquisition. A smoothing function with a moving average of 500 ms was applied in order to eliminate high-frequency noise (Figner and Murphy, 2011). The SCL was computed by resampling the skin conductance waveform to match the TR (2 s) used in the functional imaging data acquisition and analysis (Critchley et al., 2000; Patterson et al., 2002). Because all trials were longer than 10 s, we used a 10 s window aligned with go signal onset to compute the SCR associated with each trial. Thus, the SCR of each trial was computed as the onset-to-peak amplitude difference in skin conductance in this 10 s window as in a previous study (Zhang et al., 2012).
Imaging protocol
Conventional T1-weighted spin echo sagittal anatomical images were acquired for slice localization using a 3T scanner (Siemens Trio). Anatomical images of the functional slice locations were next obtained with spin echo imaging in the axial plane parallel to the AC–PC line with TR = 300 ms, TE = 2.5 ms, bandwidth = 300 Hz/pixel, flip angle = 60°, field of view = 220 × 220 mm, matrix = 256 × 256, 32 slices with slice thickness = 4 mm and no gap. Functional, BOLD signals were then acquired with a single-shot gradient echo echoplanar imaging (EPI) sequence. Thirty-two axial slices parallel to the AC–PC line covering the whole brain were acquired with TR = 2000 ms, TE = 25 ms, bandwidth = 2004 Hz/pixel, flip angle = 85°, field of view = 220 × 220 mm, matrix = 64 × 64, 32 slices with slice thickness = 4 mm and no gap.
Imaging data preprocessing
Brain imaging data were preprocessed using Statistical Parametric Mapping version 8 (Wellcome Department of Imaging Neuroscience, University College London, UK). Images from the first five TRs at the beginning of each session/run were discarded to enable the signal to achieve steady-state equilibrium between RF pulsing and relaxation. Images of each individual subject were first corrected for slice timing and realigned (motion corrected). A mean functional image volume was constructed for each subject for each run from the realigned image volumes. These mean images were normalized to an Montreal Neurological Institute (MNI) EPI template with affine registration followed by non-linear transformation (Ashburner and Friston, 1999). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each subject. Finally, images were smoothed with a Gaussian kernel of 8 mm at full width at half maximum.
Additional preprocessing was applied to reduce spurious BOLD variances that were unlikely to reflect neuronal activity (Fox et al., 2005; Zhang and Li, 2012b). The sources of spurious variance were removed through linear regression by including the signal from the ventricular system, the white matter and the whole brain, in addition to the six parameters obtained by rigid body head motion correction. First-order derivatives of the whole brain, ventricular and white matter signals were also included in the regression.
Linear correlation with skin conductance
We computed for individual subjects the correlation coefficient between the SCL and the time courses of each voxel for the whole brain. Note that the skin conductance impulse response function is very close in shape and latency to that of the canonical hemodynamic response function (HRF) (Patterson et al., 2002; Nagai et al., 2004). Thus, SCL could be cross correlated with BOLD signals without additional processing. We then converted these individual correlation maps, which were not normally distributed, to z score maps by Fisher’s z transform (Charles F. Bond and Richardson, 2004): 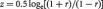 . The z maps were used in the second-level random effects analysis (Penny et al., 2004). A one-sample t-test was applied to the ‘z maps’ across 24 subjects to identify regional activities correlated to skin conductance.
. The z maps were used in the second-level random effects analysis (Penny et al., 2004). A one-sample t-test was applied to the ‘z maps’ across 24 subjects to identify regional activities correlated to skin conductance.
In region of interest (ROI) analysis, we used MarsBar (http://marsbar.sourceforge.net/) to derive for each individual subject the effect size of activity change for the ROIs. Functional ROIs were defined based on activated clusters from whole-brain analysis. All voxel activations were presented in MNI coordinates.
Granger causality analysis
BOLD and skin conductance signals were examined with GCA (Granger, 1969), which has been widely used to describe ‘causal’ influence between sets of EEG or fMRI time series (Ding et al., 2000; Roebroeck et al., 2005; Abler et al., 2006; Stilla et al., 2007; Deshpande et al., 2008; Sato et al., 2009; Wen et al., 2012), as described in details in our previous studies (Duann et al., 2009; Ide and Li, 2011a, 2011b). Briefly, we used multivariate autoregressive (MAR) modeling (Harrison et al., 2003; Sato et al., 2009) to perform GCA. In an unrestricted model of the BOLD time series
| (1) |
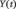 is a column vector
is a column vector 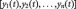 in which each element
in which each element 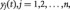 is the average time series of a ROI at time point t; T is the number of time points; n is the number of ROIs and
is the average time series of a ROI at time point t; T is the number of time points; n is the number of ROIs and 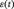 is a column vector
is a column vector 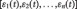 of residuals at time point t. The model order is represented by p and
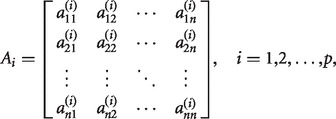
of residuals at time point t. The model order is represented by p and  is a n-by-n matrix given by
is a n-by-n matrix given by
 |
(2) |
estimated by ordinary least squares (Seth, 2010). To determine the model order, we employed the Bayesian Information Criterion (Schwarz, 1978; Gentle et al., 2004). The application of MAR modeling required that each ROI or SCL was covariance stationary, which we examined with the Augmented Dickey Fuller (ADF) test (Hamilton, 1994). The ADF test verified that there was no unit root in the modeled time series. To test whether variable x Granger causes y, where 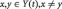 , we computed the regression equation (1) without variable x (the restricted model) and obtained the residual sum of squares
, we computed the regression equation (1) without variable x (the restricted model) and obtained the residual sum of squares 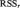 of variable y. The residual sum of squares of y is given by
of variable y. The residual sum of squares of y is given by 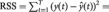
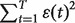 , where
, where 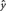 represents the predicted value of y. These residuals were used to compute the Granger causality strength measures (F-values) of each possible connection between ROIs and skin conductance (Hamilton, 1994):
represents the predicted value of y. These residuals were used to compute the Granger causality strength measures (F-values) of each possible connection between ROIs and skin conductance (Hamilton, 1994):
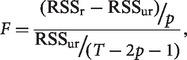 |
(3) |
where  is the residual sum of squares of variable y in the unrestricted model. We tested the significance of the Granger causality between time series by an F test and used binomial test to assess statistical significance in group analysis as described in details earlier (Duann et al., 2009; Ide and Li, 2011a, 2011b). For each connection, we counted the number of subjects that had significant connections and estimated its significance using a binomial distribution with parameters n = 24 trials, and P = q = 0.5 (same probability to observe a connection or not). For each subject, we had a total of 1770 (295 × 6) time points for GCA.
is the residual sum of squares of variable y in the unrestricted model. We tested the significance of the Granger causality between time series by an F test and used binomial test to assess statistical significance in group analysis as described in details earlier (Duann et al., 2009; Ide and Li, 2011a, 2011b). For each connection, we counted the number of subjects that had significant connections and estimated its significance using a binomial distribution with parameters n = 24 trials, and P = q = 0.5 (same probability to observe a connection or not). For each subject, we had a total of 1770 (295 × 6) time points for GCA.
To assess how the strength of Granger causality relate to event-evoked arousal, we examined the correlation across subjects between the causality strength measures (F-values) and stimulus-evoked SCR with a linear regression.
RESULTS
Behavioral performance and SCRs
Behavioral results of the SST are listed in Table 2(a). Participants succeeded in about half of the stop trials, indicating the success of the staircase procedure in tracking their performance.
Table 2.
Behavioral performance and trial-specific SCR
| a. General performance in the stop signal task | ||||||
|---|---|---|---|---|---|---|
| Go RT (ms) | Coefficient of variation in go RT |
% go |
% stop |
SSRT (ms) |
Critical SSD (ms) |
Post-error slowing (effect size) |
| 641 ± 74 | 0.21 ± 0.04 | 94.3 ± 6.5 | 55.3 ± 3.5 | 235 ± 42 | 411 ± 77 | 1.78 ± 1.29 |
| b. SCR of go, stop, stop success and stop error trials | ||||||
|---|---|---|---|---|---|---|
| Go | Stop | Stop success | Stop error | |||
| SCR (µSiemens) | 0.16 ± 0.18 | 0.33 ± 0.34 | 0.22 ± 0.22 | 0.49 ± 0.51 | ||
Note: %go and %stop, percentage of successful go and stop trials; RT, reaction time; SSRT, stop-signal reaction time; SSD, stop signal delay; all numbers are mean ± s.d.
SCRs during the SST are shown in Table 2(b). As described in the ‘Materials and Methods’ section, we quantified the change of skin conductance or SCR by subtracting the amplitude at the baseline from the amplitude at the peak in a 10 s window after stimulus onset for each trial. Across all 24 subjects, go (G), stop success (SS) and stop error (SE) trials showed significant differences in SCR (P = 0.003, one-way ANOVA), as did planned comparisons: G vs SS (P = 0.01), G vs SE (P = 0.0002) and SS vs SE (P = 0.0003), with two-sample t-tests.
Arousal related brain activation
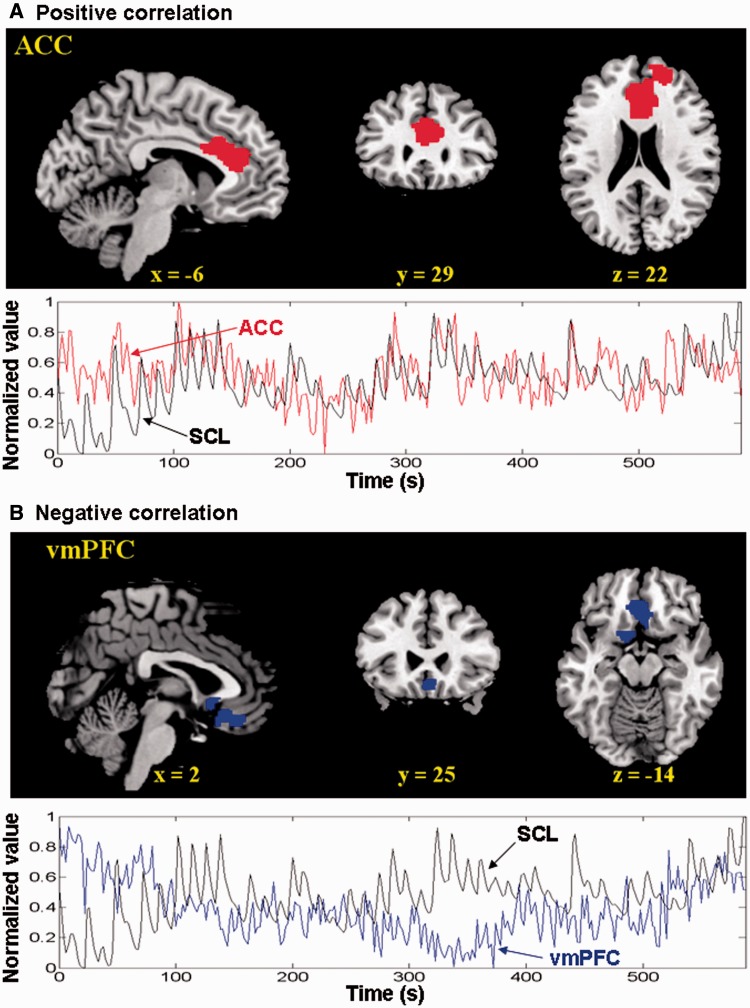
We computed for individual subjects the correlation coefficient between the SCL and the time courses of each voxel for the whole brain, and normalized these correlation maps with Fisher’s z transformation for a one-sample t-test (see ‘Materials and Methods’ section). The ACC as well as right superior frontal gyrus showed significant (cluster level P < 0.05, corrected for family-wise error or FWE of multiple comparisons) positive correlation, whereas the vmPFC showed significant (cluster level P < 0.05, FWE corrected) negative correlation, with skin conductance (Figure 1 and Table 3). This activity of the ACC and vmPFC did not correlate across subjects (P > 0.1).
Fig. 1.
Brain regions showed positive (top of A) and negative (top of B) correlations with the SCL across 24 subjects at voxel P < 0.0001 uncorrected and cluster P < 0.05 corrected for FWE of multiple comparisons. (Bottom of A and B) Data from a typical participant show that the time series of the ACC and vmPFC are each correlated and anti-correlated with SCL in a 10 min session.
Table 3.
Brain regions showing significant correlations with skin conductance time series (voxel P < 0.0001 uncorrected and cluster-level threshold of P < 0.05, FWE corrected)
| Cluster size (mm3) | Voxel z value | MNI coordinate (mm) |
Identified region and approximate Brodmann area (BA) | ||
|---|---|---|---|---|---|
| X | y | z | |||
| Positive correlation | |||||
| 21 222 | 5.07 | −6 | 29 | 22 | Anterior cingulated cortex; BA 24/32 |
| 4.72 | 24 | 59 | 13 | Right superior frontal gyrus; BA 10 | |
| Negative correlation | |||||
| 6372 | 4.68 | 3 | 32 | −17 | Ventromedial prefrontal cortex; BA 11 |
| 4.49 | −12 | 8 | −17 | Left olfactory cortex; BA 25 | |
Granger causality analysis
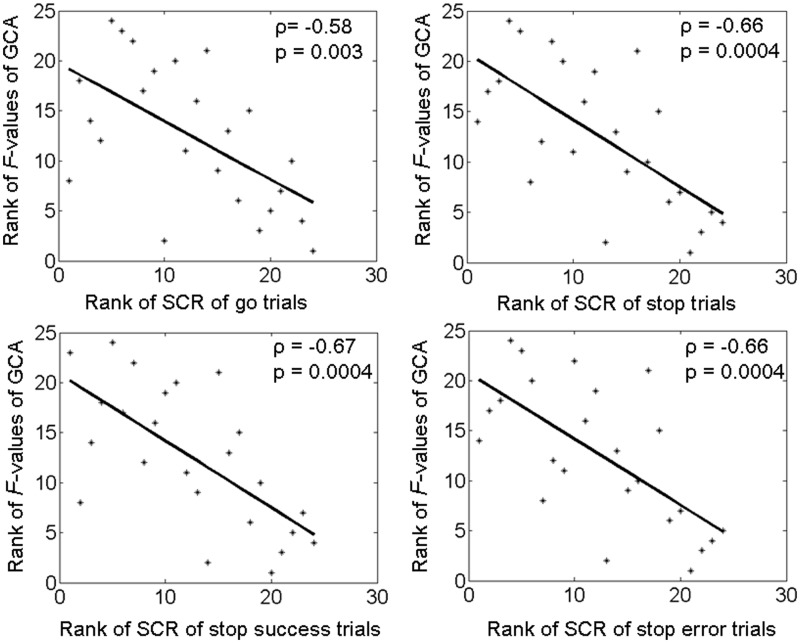
The results of GCA showed that BOLD signals of the vmPFC Granger caused the SCL (P < 0.05 for individual GCA and P = 0.03, binomial test for group analysis), but the SCL did not Granger cause vmPFC activity (P = 0.08). In contrast, there was no significant Granger causality between dACC and SCL in either direction (P = 0.85 and 0.27). Moreover, individuals varied in the strength of Granger causality as indexed by the F-value (mean ± s.d. = 5.0 ± 4.5; range 1.5–18.6). Across the 24 participants, Spearman regressions showed that higher Granger causality strength (F-values) of the vmPFC was associated with less SCRs elicited by go (P = 0.003, ρ = −0.58), stop (P = 0.0004, ρ = −0.66), stop success (P = 0.0004, ρ = −0.67) and stop error (P = 0.0004, ρ = −0.66) trials during the stop signal task (Figure 2).
Fig. 2.
The strength of Granger causality (F-value) of the vmPFC in regulating skin conductance is negatively correlated with SCR elicited by go (P = 0.003, ρ = −0.58; Spearman regression), stop (P = 0.0004, ρ = −0.66), as well stop success (P = 0.0004, ρ = −0.67) and stop error (P = 0.0004, ρ = −0.66) trials. That is, the stronger the regulatory influence of vmPFC, the less the SCR is elicited. Assuming linearity between the F-value and SCR, Pearson regressions also showed significant correlation between the two variables: go (P = 0.01, r = −0.50); stop (P = 0.01, r = −0.50); stop success (P = 0.0098, r = −0.52) and stop error (P = 0.016, r = −0.49) trials.
Because previous studies used a smaller window (0.5–4.5 s) following stimulus onset to compute the event-related SCR (Delgado et al., 2008; Schiller et al., 2008; Nili et al., 2010), we recomputed the SCR by the onset-to-peak amplitude within a 5 s window and reran the regression analysis. The results were similar: significant negative correlations were observed between the Granger causality strength and the SCR elicited by go (P = 0.0004, ρ = −0.66), stop (P = 0.0003, ρ = −0.68), stop success (P = 0.0001, ρ = −0.70) and stop error (P = 0.0004, ρ = −0.67) trials. Furthermore, to eliminate the confound of individual variability in mean SCL, we removed the mean value of the skin conductance time series before computing the SCR, and reran the regression analysis. The results were similar: significant negative correlations were observed between the Granger causality strength and the SCR elicited by go (P = 0.0002, ρ = −0.69), stop (P = 0.0002, ρ = −0.69), stop success (P = 0.01, ρ = −0.50) and stop error (P = 0.0001, ρ = −0.73) trials.
DISCUSSION
vmPFC and the regulation of physiological arousal
We showed that activation of a distinct region in the vmPFC is negatively correlated with moment to moment changes in SCL. Importantly, Granger causality analyses demonstrated that activity of the vmPFC Granger causes skin conductance signals, thus establishing the direction of influence. Furthermore, across the 24 participants, higher Granger causality strength is associated with less SCRs elicited by cognitive events during the stop signal task. These findings confirmed our hypotheses and are important on two fronts. First, extending previous findings of a negative correlation between the vmPFC and SCL, we demonstrated a direct, causal relationship between the activity of the vmPFC and the changes in physiological arousal. Second, the stronger the regulatory influence of the vmPFC, the less the SCRs to cognitive events. Together, these results support a critical role of this ventromedial prefrontal structure in the control of arousal during cognitive performance.
The finding of a significant negative association between vmPFC activity and SCL replicates the results of biofeedback relaxation (Nagai et al., 2004) and resting state (Fan et al., 2012). Studies using paradigms of fear conditioning similarly demonstrated less activity of the vmPFC when participants anticipated an electric shock and exhibited higher SCR than when they did not expect to receive the shock (Milad et al., 2007; Delgado et al., 2008; Schiller et al., 2008). In another study, the vmPFC increased activity when subjects tried to overcome a real-life fear (Nili et al., 2010); the vmPFC activity was enhanced, whereas the SCR was attenuated, as the level of subjective fear increased during the ‘overcome’ period. The investigators posited that activity of the vmPFC may be related to the inhibition of fear-related arousal, an interpretation supported by our current findings. Together, it appears that the negative association between the vmPFC and skin conductance activity is task independent.
Posterior vs anterior vmPFC: responses to SCR and the effects of a lesion
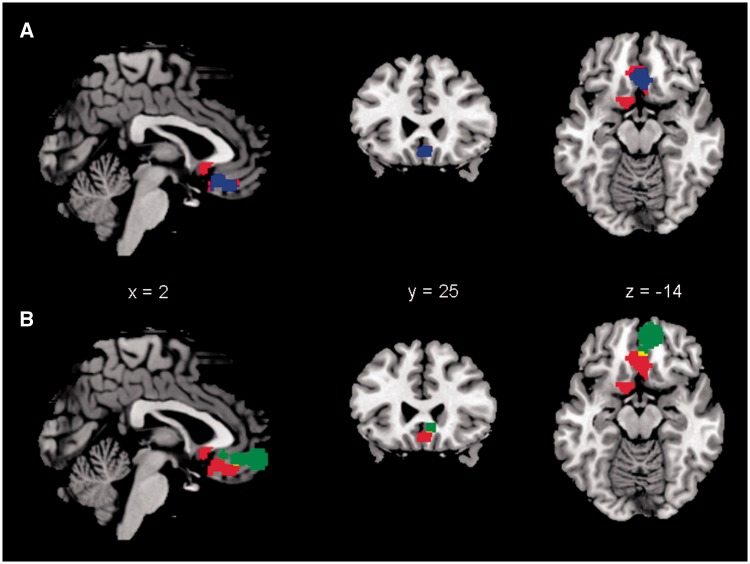
Another consideration is the anatomical location of the vmPFC as implicated in the regulation of arousal. In addition to a negative correlation with SCL, a few studies have shown a positive correlation between activity of the vmPFC and SCR (Critchley et al., 2000; Patterson et al., 2002; Williams et al., 2005). To address this issue, we delineated the anatomical boundary of the vmPFC cluster identified here and those of other studies (Figure 3). Our vmPFC cluster clearly overlapped the regions where activities were negatively associated with SCL (Nagai et al., 2004; Fan et al., 2012). In contrast, activities that showed positive correlations with the SCR were located in the anterior part of the vmPFC (Critchley et al., 2000; Patterson et al., 2002; Williams et al., 2005). Notably, a recent review suggested a functional differentiation between anterior and posterior vmPFC in relation to mood and anxiety disorders (Myers-Schulz and Koenigs, 2012). The posterior vmPFC was thought to be associated with negative affect and mood (Simpson et al., 2001; Zald et al., 2002; Paradiso et al., 2003; Masten et al., 2009), whereas the anterior vmPFC was associated with positive affect and related emotional states (Somerville et al., 2006, 2010; Gianaros et al., 2007; Glascher et al., 2009; Kim et al., 2010). Patients with major depression exhibit increased posterior vmPFC activity but decreased anterior vmPFC activity (Drevets et al., 1997; Mayberg et al., 2005; Greicius et al., 2007), a pattern of response that reversed after successful treatment (Mayberg et al., 2000, 2005; Kennedy et al., 2007). Thus, it is important to distinguish the exact locales of activity when associating vmPFC with the regulation of arousal and emotion.
Fig. 3.
The anatomical relationship between the vmPFC identified in the current study and brain regions identified in earlier studies that negatively correlated with SCL or positively correlated with SCR (Table 1). Only regions with MNI coordinate z < 0 were included. (A) Our vmPFC (red) overlaps brain regions negatively correlated with SCL during biofeedback (Nagai et al., 2004) and resting state (Fan et al., 2012). Blue color represents an area that overlapped across all studies. (B) Our vmPFC (red) show little overlap (yellow) with brain regions (green, combined from all studies) positively correlated with SCR. Anatomical boundary of the brain regions identified from the other studies was manually drawn according to the figures, coordinates and number of voxels reported.
The current findings also need to be considered along with lesion studies that implicated the vmPFC in the generation of arousal (Damasio et al., 1990, 1991; Damasio, 1994; Tranel and Damasio, 1994; Zahn et al., 1999). Patients with lesions in the vmPFC have deficits in generating electrodermal responses and showed a reduction in anticipatory arousal, which is the opposite of what would be expected if the vmPFC down-regulates arousal. A possible explanation is that the lesions examined in these studies encompass a much larger area of the medial prefrontal cortex that involves both the anterior and posterior parts of the vmPFC and rostral/dACC. Given that activity of the rostral/dACC correlates positively with the SCR and plays a potential role in the generation of skin conductance (Critchley et al., 2000), lesioning of this and adjacent structures could lead to impaired arousal. As selective lesions of these neighboring brain regions are rare, experimental lesioning of these specific areas in non-human primates may be required to pursue this issue.
Potential implications for clinical neuroscience
An increasing number of neuroimaging studies that sought to identify the brain anomalies associated with mood and anxiety disorders have implicated the vmPFC and neural circuits involving vmPFC (Savitz and Drevets, 2009; Hamani et al., 2011; Myers-Schulz and Koenigs, 2012). Increased blood flow or metabolism of the subgenual ACC (sgACC)—a part of the posterior vmPFC—together with gray matter volume loss in this region, is a well-replicated finding in major depression (Savitz and Drevets, 2009). Deep brain stimulation of the sgACC ameliorates depressive symptoms in treatment-resistant patients (Drevets et al., 2008; Holtzheimer et al., 2012). FMRI studies presented a more diverse picture of vmPFC activity, depending on behavioral tasks, models and contrasts, as well as genotypes, patient populations and medication status (Keedwell et al., 2005; Johnstone et al., 2007; O’Nions et al., 2011; Holsen et al., 2012; Tao et al., 2012). For instance, during evaluation of emotional words, depression patients showed a decreased response to negative compared with positive stimuli in the anterior vmPFC (Brassen et al., 2008). Moreover, this altered pattern was positively correlated with symptom severity and ‘normalized’, accompanied by a significant improvement in symptoms, after treatment. It is posited that vmPFC participates in an extended ‘visceromotor network’ of structures that modulates autonomic/neuroendocrine responses during the neural processing of reward, fear and stress (Drevets et al., 2008). Thus, the current finding of vmPFC regulation of physiological arousal adds an important dimension to this empirical literature.
Regulation of arousal: other brain regions and vmPFC connectivity
Amygdala is another key region involved in regulating electrodermal response (Phelps et al., 2001; Williams et al., 2001, 2005; Spoormaker et al., 2011). Sweat responses failed to be evoked in a female patient with bilateral amygdala lesions caused by idiopathic encephalitis (Asahina et al., 2003). Numerous non-human and human studies indicated that the vmPFC is both functionally and anatomically connected with the amygdala (Milad and Quirk, 2002; Ghashghaei et al., 2007; Hare et al., 2008; Myers-Schulz and Koenigs, 2012). A recent study of diffusion tensor imaging showed that the structural integrity of the pathway between the amygdala and vmPFC was inversely correlated with trait anxiety levels across subjects (Kim and Whalen, 2009). Although vmPFC control of amygdala activity is posited as a pathway to regulate physiological arousal and affect, the pattern of activations and functional connectivities seems to show a more complicated pattern of interaction between the two areas (Ochsner and Gross, 2005; Li et al., 2009; Burghy et al., 2012). Furthermore, midbrain and limbic structures other than the amygdala respond to salient stimuli and interact with the vmPFC to sustain an optimal level of physiological arousal for cognitive control (Brooks et al., 2012; Sara and Bouret, 2012; Szabadi, 2012). It remains a challenge to understand the complexity of this interaction.
Methodological considerations
One potential issue concerns whether the SCR function (SCRF) and HRF are close enough, particularly in their latency, as suggested by previous studies (Patterson et al., 2002; Nagai et al., 2004), so that one can apply time series analyses such as correlation as well as GCA to these signals. More work clearly needs to be done to address this issue. On the other hand, previous studies have correlated BOLD and skin conductance time series with similar results, on the basis of this assumption (Patterson et al., 2002; Nagai et al., 2004). Furthermore, the latencies of SCRF derived in previous studies (Lim et al., 1997; Alexander et al., 2005; Bach et al., 2010; Boucsein, 2011) were all close to and minimally faster than HRF. If the current finding of Granger causality were determined by the latency difference, we would have observed an opposite direction of causality.
It has also been questioned whether skin conductance could be used to quantify changes in sympathetic nerve activity since the relationship between skin conductance and sudomotor activity is non-linear (Kirno et al., 1991). Direct recordings of skin sympathetic nerve activity (SSNA) with participants exposed to arousing visual images showed that, while the increases in SSNA were often coupled with sweat release and cutaneous vasoconstriction, these markers were not always consistent with the SSNA increases (Brown et al., 2012). Concurrent brain imaging demonstrated that, during increases in SSNA, BOLD signals intensified in the central and lateral amygdala, dorsolateral pons, thalamus, nucleus accumbens and cerebellar cortex and decreased in the left orbitofrontal, frontal and right precuneus cortices (Henderson et al., 2012). The pattern of brain activations overlapped but was not identical in association with SSNA and electrodermal response. Thus, it would be important to examine the role of the vmPFC in regulating physiological arousal by directly recording SSNA in future work.
Conclusions
To conclude, we reported a direct regulatory relationship between the activity of the vmPFC and skin conductance as a physiological index of arousal. Future work is warranted to investigate how this regulatory process may be compromised in patients with anxiety and mood disorders.
Conflict of Interest
None declared.
Acknowledgments
The authors thank Sarah Bednarski and Emily Erdman in subject recruitment and assessment as well as running of some of the imaging studies. This work was supported by National Institute of Health grants R01DA023248, R21AA018004, K02DA026990, R03CA138121, Tourette Syndrome Association, William O. Seery Foundation and a Yale Cancer Center translational pilot grant. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
REFERENCES
- Abler B, Roebroeck A, Goebel R, et al. Investigating directed influences between activated brain areas in a motor-response task using fMRI. Magnetic Resonance Imaging. 2006;24(2):181–5. doi: 10.1016/j.mri.2005.10.022. [DOI] [PubMed] [Google Scholar]
- Alexander DM, Trengove C, Johnston P, Cooper T, August JP, Gordon E. Separating individual skin conductance responses in a short interstimulus-interval paradigm. Journal of Neuroscience Methods. 2005;146(1):116–23. doi: 10.1016/j.jneumeth.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Asahina M, Suzuki A, Mori M, Kanesaka T, Hattori T. Emotional sweating response in a patient with bilateral amygdala damage. International Journal of Psychophysiology. 2003;47(1):87–93. doi: 10.1016/s0167-8760(02)00123-x. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Nonlinear spatial normalization using basis functions. Human Brain Mapping. 1999;7(4):254–66. doi: 10.1002/(SICI)1097-0193(1999)7:4<254::AID-HBM4>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach DR, Flandin G, Friston KJ, Dolan RJ. Modelling event-related skin conductance responses. International Journal of Psychophysiology. 2010;75(3):349–56. doi: 10.1016/j.ijpsycho.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275(5304):1293–5. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Boucsein W. Electrodermal Activity. New York: Springer; 2011. [Google Scholar]
- Brassen S, Kalisch R, Weber-Fahr W, Braus DF, Buchel C. Ventromedial prefrontal cortex processing during emotional evaluation in late-life depression: a longitudinal functional magnetic resonance imaging study. Biological Psychiatry. 2008;64(4):349–55. doi: 10.1016/j.biopsych.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Brooks SJ, Savov V, Allzen E, Benedict C, Fredriksson R, Schioth HB. Exposure to subliminal arousing stimuli induces robust activation in the amygdala, hippocampus, anterior cingulate, insular cortex and primary visual cortex: a systematic meta-analysis of fMRI studies. Neuroimage. 2012;59(3):2962–73. doi: 10.1016/j.neuroimage.2011.09.077. [DOI] [PubMed] [Google Scholar]
- Brown R, James C, Henderson LA, Macefield VG. Autonomic markers of emotional processing: skin sympathetic nerve activity in humans during exposure to emotionally charged images. Frontiers in Physiology. 2012;3:394. doi: 10.3389/fphys.2012.00394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, et al. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1736–41. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao HH, Luo X, Chang JL, Li CS. Activation of the pre-supplementary motor area but not inferior prefrontal cortex in association with short stop signal reaction time—an intra-subject analysis. BMC Neuroscience. 2009;10:75. doi: 10.1186/1471-2202-10-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles F, Bond J, Richardson K. Seeing the fisher z-transformation. Psychometrika. 2004;69(2):291–303. [Google Scholar]
- Critchley HD. Electrodermal responses: what happens in the brain. Neuroscientist. 2002;8(2):132–42. doi: 10.1177/107385840200800209. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Psychophysiology of neural, cognitive and affective integration: fMRI and autonomic indicants. International Journal of Psychophysiology. 2009;73(2):88–94. doi: 10.1016/j.ijpsycho.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Elliott R, Mathias CJ, Dolan RJ. Neural activity relating to generation and representation of galvanic skin conductance responses: a functional magnetic resonance imaging study. Journal of Neuroscience. 2000;20(8):3033–40. doi: 10.1523/JNEUROSCI.20-08-03033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Damasio AR. Descartes’ Error: Emotion, Reason and the Human Brain. New York: Putnam; 1994. [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41(2):81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio HC. Somatic markers and the guidance of behavior: theory and preliminary testing. In: Levin HS, Eisenberg HM, Benton LB, editors. Frontal Lobe Function and Dysfunction. New York: Oxford University Press; 1991. pp. 217–29. [Google Scholar]
- De Jong R, Coles MG, Logan GD, Gratton G. In search of the point of no return: the control of response processes. Journal of Experimental Psychology. Human Perception and Performance. 1990;16(1):164–82. doi: 10.1037/0096-1523.16.1.164. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, Ledoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59(5):829–38. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, Hu X, Stilla R, Sathian K. Effective connectivity during haptic perception: a study using Granger causality analysis of functional magnetic resonance imaging data. Neuroimage. 2008;40(4):1807–14. doi: 10.1016/j.neuroimage.2008.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande G, LaConte S, James GA, Peltier S, Hu X. Multivariate Granger causality analysis of fMRI data. Human Brain Mapping. 2009;30(4):1361–73. doi: 10.1002/hbm.20606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding M, Bressler SL, Yang W, Liang H. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biological Cybernetics. 2000;83(1):35–45. doi: 10.1007/s004229900137. [DOI] [PubMed] [Google Scholar]
- Ding M, Chen Y, Bressler SL. Granger causality: basic theory and application to neuroscience. In: Schelter B, Winderhalder M, Timmer J, editors. Handbook of Time Series Analysis. Berlin: Wiley-VCH; 2006. pp. 451–74. [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298(5596):1191–4. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386(6627):824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Savitz J, Trimble M. The subgenual anterior cingulate cortex in mood disorders. CNS Spectrums. 2008;13(8):663–81. doi: 10.1017/s1092852900013754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duann JR, Ide JS, Luo X, Li CS. Functional connectivity delineates distinct roles of the inferior frontal cortex and presupplementary motor area in stop signal inhibition. Journal of Neuroscience. 2009;29(32):10171–9. doi: 10.1523/JNEUROSCI.1300-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J, Xu P, Van Dam NT, et al. Spontaneous brain activity relates to autonomic arousal. Journal of Neuroscience. 2012;32(33):11176–86. doi: 10.1523/JNEUROSCI.1172-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figner B, Murphy RO. Using skin conductance in judgment and decision making research. In: Schulte-Mecklenbeck M, Kuehberger A, Ranyard R, editors. A Handbook of Process Tracing Methods for Decision Research. New York: Psychology Press; 2011. pp. 163–84. [Google Scholar]
- First MB, Spitzer RL, Williams JBW, Gibbon M. Structured Clinical Interview for DSM-IV (SCID) Washington, DC: American Psychiatric Association; 1995. [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(27):9673–8. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frith CD, Allen HA. The skin conductance orienting response as an index of attention. Biological Psychology. 1983;17(1):27–39. doi: 10.1016/0301-0511(83)90064-9. [DOI] [PubMed] [Google Scholar]
- Gentle JE, Härdle W, Mori Y. Handbook of Computational Statistics: Concepts and Methods. Berlin: Springer; 2004. [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34(3):905–23. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Horenstein JA, Cohen S, et al. Perigenual anterior cingulate morphology covaries with perceived social standing. Social Cognitive and Affective Neuroscience. 2007;2(3):161–73. doi: 10.1093/scan/nsm013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glascher J, Hampton AN, O’Doherty JP. Determining a role for ventromedial prefrontal cortex in encoding action-based value signals during reward-related decision making. Cerebral Cortex. 2009;19(2):483–95. doi: 10.1093/cercor/bhn098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger CWJ. Investigating causal relations by econometric models and cross-spectral methods. Econometrica. 1969;37:424. [Google Scholar]
- Greicius MD, Flores BH, Menon V, et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biological Psychiatry. 2007;62(5):429–37. doi: 10.1016/j.biopsych.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamani C, Mayberg H, Stone S, Laxton A, Haber S, Lozano AM. The subcallosal cingulate gyrus in the context of major depression. Biological Psychiatry. 2011;69(4):301–8. doi: 10.1016/j.biopsych.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Hamilton JD. Time Series Analysis. Princeton, NJ: Princeton University Press; 1994. [Google Scholar]
- Hare TA, Tottenham N, Galvan A, Voss HU, Glover GH, Casey BJ. Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry. 2008;63(10):927–34. doi: 10.1016/j.biopsych.2008.03.015015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison L, Penny WD, Friston K. Multivariate autoregressive modeling of fMRI time series. Neuroimage. 2003;19(4):1477–91. doi: 10.1016/s1053-8119(03)00160-5. [DOI] [PubMed] [Google Scholar]
- Henderson LA, Stathis A, James C, Brown R, McDonald S, Macefield VG. Real-time imaging of cortical areas involved in the generation of increases in skin sympathetic nerve activity when viewing emotionally charged images. Neuroimage. 2012;62(1):30–40. doi: 10.1016/j.neuroimage.2012.04.049. [DOI] [PubMed] [Google Scholar]
- Holsen LM, Lee JH, Spaeth SB, et al. Brain hypoactivation, autonomic nervous system dysregulation, and gonadal hormones in depression: a preliminary study. Neuroscience Letters. 2012;514(1):57–61. doi: 10.1016/j.neulet.2012.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzheimer PE, Kelley ME, Gross RE, et al. Subcallosal cingulate deep brain stimulation for treatment-resistant unipolar and bipolar depression. Archives of General Psychiatry. 2012;69(2):150–8. doi: 10.1001/archgenpsychiatry.2011.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS. A cerebellar thalamic cortical circuit for error-related cognitive control. Neuroimage. 2011a;54(1):455–64. doi: 10.1016/j.neuroimage.2010.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide JS, Li CS. Error-related functional connectivity of the habenula in humans. Frontiers in Human Neuroscience. 2011b;5:25. doi: 10.3389/fnhum.2011.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27(33):8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keedwell PA, Andrew C, Williams SC, Brammer MJ, Phillips ML. The neural correlates of anhedonia in major depressive disorder. Biological Psychiatry. 2005;58(11):843–53. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kennedy SH, Konarski JZ, Segal ZV, et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. American Journal of Psychiatry. 2007;164(5):778–88. doi: 10.1176/ajp.2007.164.5.778. [DOI] [PubMed] [Google Scholar]
- Kim H, Shimojo S, O’Doherty JP. Overlapping responses for the expectation of juice and money rewards in human ventromedial prefrontal cortex. Cerebral Cortex. 2010;21(4):769–76. doi: 10.1093/cercor/bhq145. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Whalen PJ. The structural integrity of an amygdala-prefrontal pathway predicts trait anxiety. Journal of Neuroscience. 2009;29(37):11614–8. doi: 10.1523/JNEUROSCI.2335-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirno K, Kunimoto M, Lundin S, Elam M, Wallin BG. Can galvanic skin response be used as a quantitative estimate of sympathetic nerve activity in regional anesthesia? Anesthesia and Analgesia. 1991;73(2):138–42. [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. The Journal of the Acoustical Society of America. 1971;49(2):467+. [PubMed] [Google Scholar]
- Li CS, Chao HH, Lee TW. Neural correlates of speeded as compared with delayed responses in a stop signal task: an indirect analog of risk taking and association with an anxiety trait. Cerebral Cortex. 2009;19(4):839–48. doi: 10.1093/cercor/bhn132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim CL, Rennie C, Barry RJ, et al. Decomposing skin conductance into tonic and phasic components. Int J Psychophysiol. 1997;25(2):97–109. doi: 10.1016/s0167-8760(96)00713-1. [DOI] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, et al. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience. 2009;4(2):143–57. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biological Psychiatry. 2000;48(8):830–43. doi: 10.1016/s0006-3223(00)01036-2. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45(5):651–60. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420(6911):70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007;62(5):446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B, Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Molecular Psychiatry. 2012;17(2):132–41. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y, Critchley HD, Featherstone E, Trimble MR, Dolan RJ. Activity in ventromedial prefrontal cortex covaries with sympathetic skin conductance level: a physiological account of a “default mode” of brain function. Neuroimage. 2004;22(1):243–51. doi: 10.1016/j.neuroimage.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Naqvi NH, Bechara A. Skin conductance: a psychophysiological approach to the study of decision making. In: Senior C, Russell T, Gazzaniga MS, editors. Methods in Mind. Cambridge, MA, US: The MIT Press; 2006. pp. 103–22. [Google Scholar]
- Nili U, Goldberg H, Weizman A, Dudai Y. Fear thou not: activity of frontal and temporal circuits in moments of real-life courage. Neuron. 2010;66(6):949–62. doi: 10.1016/j.neuron.2010.06.009. [DOI] [PubMed] [Google Scholar]
- O’Nions EJ, Dolan RJ, Roiser JP. Serotonin transporter genotype modulates subgenual response to fearful faces using an incidental task. J Cogn Neurosci. 2011;23(11):3681–93. doi: 10.1162/jocn_a_00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Science. 2005;9(5):242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Paradiso S, Robinson RG, Boles Ponto LL, Watkins GL, Hichwa RD. Regional cerebral blood flow changes during visually induced subjective sadness in healthy elderly persons. J Neuropsychiatry Clin Neurosci. 2003;15(1):35–44. doi: 10.1176/jnp.15.1.35. [DOI] [PubMed] [Google Scholar]
- Patterson JC, 2nd, Ungerleider LG, Bandettini PA. Task-independent functional brain activity correlation with skin conductance changes: an fMRI study. Neuroimage. 2002;17(4):1797–806. doi: 10.1006/nimg.2002.1306. [DOI] [PubMed] [Google Scholar]
- Penny WD, Holmes AP, Friston K. Random-effects analysis. In: Frackowiak R, Frith C, Dolan RJ, et al., editors. Human Brain Function. San Diego, California, US: Academic Press; 2004. pp. 843–50. [Google Scholar]
- Phelps EA, O’Connor KJ, Gatenby JC, Gore JC, Grillon C, Davis M. Activation of the left amygdala to a cognitive representation of fear. Nature Neuroscience. 2001;4(4):437–41. doi: 10.1038/86110. [DOI] [PubMed] [Google Scholar]
- Roebroeck A, Formisano E, Goebel R. Mapping directed influence over the brain using Granger causality and fMRI. Neuroimage. 2005;25(1):230–42. doi: 10.1016/j.neuroimage.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S. Orienting and reorienting: the locus coeruleus mediates cognition through arousal. Neuron. 2012;76(1):130–41. doi: 10.1016/j.neuron.2012.09.011. [DOI] [PubMed] [Google Scholar]
- Sato JR, Takahashi DY, Arcuri SM, Sameshima K, Morettin PA, Baccala LA. Frequency domain connectivity identification: an application of partial directed coherence in fMRI. Human Brain Mapping. 2009;30(2):452–61. doi: 10.1002/hbm.20513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitz JB, Drevets WC. Imaging phenotypes of major depressive disorder: genetic correlates. Neuroscience. 2009;164(1):300–30. doi: 10.1016/j.neuroscience.2009.03.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller D, Levy I, Niv Y, LeDoux JE, Phelps EA. From fear to safety and back: reversal of fear in the human brain. Journal of Neuroscience. 2008;28(45):11517–25. doi: 10.1523/JNEUROSCI.2265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz G. Estimating the dimension of a model. Annals of Statistics. 1978;6(2):461–4. [Google Scholar]
- Seth AK. A MATLAB toolbox for Granger causal connectivity analysis. Journal of Neuroscience Methods. 2010;186(2):262–73. doi: 10.1016/j.jneumeth.2009.11.020. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Jr, Drevets WC, Snyder AZ, Gusnard DA, Raichle ME. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(2):688–93. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Heatherton TF, Kelley WM. Anterior cingulate cortex responds differentially to expectancy violation and social rejection. Nature Neuroscience. 2006;9(8):1007–8. doi: 10.1038/nn1728. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Kelley WM, Heatherton TF. Self-esteem modulates medial prefrontal cortical responses to evaluative social feedback. Cerebral Cortex. 2010;20(12):3005–13. doi: 10.1093/cercor/bhq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spoormaker VI, Andrade KC, Schroter MS, et al. The neural correlates of negative prediction error signaling in human fear conditioning. Neuroimage. 2011;54(3):2250–6. doi: 10.1016/j.neuroimage.2010.09.042. [DOI] [PubMed] [Google Scholar]
- Stilla R, Deshpande G, LaConte S, Hu X, Sathian K. Posteromedial parietal cortical activity and inputs predict tactile spatial acuity. Journal of Neuroscience. 2007;27(41):11091–102. doi: 10.1523/JNEUROSCI.1808-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabadi E. Modulation of physiological reflexes by pain: role of the locus coeruleus. Frontiers in Integrative Neuroscience. 2012;6:94. doi: 10.3389/fnint.2012.00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao R, Calley CS, Hart J, et al. Brain activity in adolescent major depressive disorder before and after fluoxetine treatment. American Journal of Psychiatry. 2012;169(4):381–8. doi: 10.1176/appi.ajp.2011.11040615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranel D, Damasio H. Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology. 1994;31(5):427–38. doi: 10.1111/j.1469-8986.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Wen X, Yao L, Liu Y, Ding M. Causal interactions in attention networks predict behavioral performance. Journal of Neuroscience. 2012;32(4):1284–92. doi: 10.1523/JNEUROSCI.2817-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams LM, Das P, Liddell B, et al. BOLD, sweat and fears: fMRI and skin conductance distinguish facial fear signals. Neuroreport. 2005;16(1):49–52. doi: 10.1097/00001756-200501190-00012. [DOI] [PubMed] [Google Scholar]
- Williams LM, Phillips ML, Brammer MJ, et al. Arousal dissociates amygdala and hippocampal fear responses: evidence from simultaneous fMRI and skin conductance recording. Neuroimage. 2001;14(5):1070–9. doi: 10.1006/nimg.2001.0904. [DOI] [PubMed] [Google Scholar]
- Zahn TP, Grafman J, Tranel D. Frontal lobe lesions and electrodermal activity: effects of significance. Neuropsychologia. 1999;37(11):1227–41. doi: 10.1016/s0028-3932(99)00020-2. [DOI] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV. Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences of the United States of America. 2002;99(4):2450–4. doi: 10.1073/pnas.042457199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Hu S, Chao HH, Luo X, Farr OM, Li CS. Cerebral correlates of skin conductance responses in a cognitive task. Neuroimage. 2012;62(3):1489–98. doi: 10.1016/j.neuroimage.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional networks for cognitive control in a stop signal task: Independent component analysis. Human Brain Mapping. 2012a;33(1):89–104. doi: 10.1002/hbm.21197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Li CS. Functional connectivity mapping of the human precuneus by resting state fMRI. Neuroimage. 2012b;59(4):3548–62. doi: 10.1016/j.neuroimage.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]