Abstract
The observation of goal-directed actions performed by another individual allows one to understand what that individual is doing and why he/she is doing it. Important information about others’ behaviour is also carried out by the dynamics of the observed action. Action dynamics characterize the ‘vitality form’ of an action describing the cognitive and affective relation between the performing agent and the action recipient. Here, using the fMRI technique, we assessed the neural correlates of vitality form recognition presenting participants with videos showing two actors executing actions with different vitality forms: energetic and gentle. The participants viewed the actions in two tasks. In one task (what), they had to focus on the goal of the presented action; in the other task (how), they had to focus on the vitality form. For both tasks, activations were found in the action observation/execution circuit. Most interestingly, the contrast how vs what revealed activation in right dorso-central insula, highlighting the involvement, in the recognition of vitality form, of an anatomical region connecting somatosensory areas with the medial temporal region and, in particular, with the hippocampus. This somatosensory-insular-limbic circuit could underlie the observers’ capacity to understand the vitality forms conveyed by the observed action.
Keywords: vitality form, social cognition, action observation, insula, fMRI
INTRODUCTION
Surviving in social groups depends on one’s ability to deal with others, to interpret correctly their behaviours and to anticipate timely their actions. When socially interacting with other individuals, we are typically able to understand their action goals as well as their intentions. There is evidence that a basic mechanism subserving such an ability is related to the existence of a class of neurons endowed with mirror properties, that is of a set of neurons that discharge both during action observation and action execution (Rizzolatti and Craighero, 2004; Fabbri-Destro and Rizzolatti, 2008; Keysers and Fadiga, 2008; Iacoboni, 2009; Caspers et al., 2010; Rizzolatti and Sinigaglia, 2010; Grosbras et al., 2012; Molenberghs et al., 2012).
Besides the goal and the intention of the performing agent, there is a third aspect that an observer may capture when viewing an action done by another individual: its vitality form (Stern, 1985, 2010). Vitality forms characterize the style of an action and are detected on the basis of movement dynamics. They are made of four basic components of movement: time profile (start, duration and the end of an action), force, space and direction. Vitality forms lay in between ‘cold’ actions, that is actions devoid of an emotional content, where the crucial information is related to the action goal or meaning, and ‘hot’ actions that is actions expressing emotions, where the crucial information is related to the emotional dimension of an action, like actions made with fear, anger, etc. Vitality forms rest on a third dimension of action processing that describes both the affective and cognitive style of an action. By recognizing the vitality form of an action, one can appraise the affective/cognitive state of an agent as well as his/her relationship with the action recipient. For example, if an action is performed energetically or gently, one can understand if the agent’s mood is aggressive or kind, whether the agent performs the action with willingness or hesitancy.
The expression and the capacity to understand the vitality forms is already present in infants, a finding indicating their importance for the development of social attunement (Condon and Sander, 1974; Nadel and Butterworth, 1999; Flom and Bahrick, 2007; Rochat, 2009). Stern (1984, 1985, 2004) proposed that, well before developing linguistic abilities, infants are actively engaged in non-verbal exchanges with their caregivers. This ability denotes a primordial way to relate to and understand others and presumably represents a constitutive element of interpersonal relations (Trevarthen, 1998; Trevarthen and Aitken, 2001; Stern, 2010). In support of this idea is evidence suggesting that parents are able to implicitly comprehend and extrapolate the infants’ mental states from their whole-body kinesthetic patterns (Shai and Belsky, 2011).
In this study, we used functional magnetic resonance imaging (fMRI) to investigate the neural correlates underpinning the recognition of vitality forms. To this purpose, we presented participants with short videos showing interactions between two actors performing different actions (i.e. giving a mug, etc.) that could be executed with different vitality forms (energetic or gentle). Participants were instructed to focus either on the type of action (the what of the action, e.g. passing a bottle) or on the action vitality form (the how of the action, e.g. gently). The anatomical regions specifically involved in the processing of vitality forms have been highlighted through the comparison between tasks (how vs what).
MATERIALS AND METHODS
Participants
Nineteen healthy right-handed volunteers [10 females (mean age = 24.1, s.d. = 2, range = 21–28) and nine males (mean age = 24.4, s.d. = 2.18, range = 22–29)] participated in this study. All participants had normal or corrected-to-normal visual acuity. None reported a history of psychiatric or neurological disorders, or current use of any psychoactive medications. They gave their written informed consent to the experimental procedure, which was approved by the Local Ethics Committee (Parma).
Stimuli
Video-clips were presented to the participants showing two actors (a male and a female) performing a series of different actions, once by the male actor and once by the female. Half of the videos represented actions where the interaction between the two actors was not mediated by an object (e.g. executing a stop gesture; Figure 1A); half of the videos represented actions where the interaction between the two actors occurred using an object (e.g. moving a bottle towards the other actor; Figure 1B). Each actor executed eight different actions (with object: move a bottle towards the other actor, give a packet of crackers, pass a ball, hand a mug—see Supplementary Figure S2; without object: caress, clap hands, stroke the other actor’s backhand, stop gesture—see Supplementary Figure S3).
Fig. 1.
Example of video-clips as viewed by the participants of this study. (A1) Frame representing an action without object in the start position; (A2) frame representing the actress executing a stop gesture; (B1) frame representing an action with object in the start position; (B2) frame representing the actor executing an action with object in the end position (passing a bottle).
In all videos, the actors started from a rest position and returned, after action execution, to the same position (Figure 1, A1 and B1). All actions were performed with two vitality forms: energetic and gentle. After video-recording, using the software Avimeca v.2.3 (see Supplementary Material for a detailed description of the calculation method) we assessed the kinematic and dynamic profiles associated with each vitality form in terms of action velocity, duration, trajectory and, for the actions with object only, the kinetic and potential energy of the object, which give an estimates of the power developed to perform the action. An example of the graphic depiction of each parameter is in Figure 2. A graphic representation of the parameters for all actions is in Supplementary Figures S2 and S3.
Fig. 2.
Kinematic and dynamic profiles associated with one of the actions (passing a bottle) performed by the female actress with the two vitality forms (gentle; energetic). (A) Velocity profiles (y-axes) and duration (x-axes). (B) Trajectories (gentle, green line; energetic, red line). (C) Potential energy (blue line), that is the energy that the actress gave to the object during the lifting phase of the action; kinetic energy (red line), that is the energy that the actress gave to the object to move it with a specific velocity from the start to the end point. (D) Power required to perform the action on the object in an energetic (blue solid line) and gentle (blue dashed line) vitalities. As it can be observed in the graphs, the vitality forms gentle and energetic generally differ from each other on each of the tested parameters.
Using VirtualDubMod software v.1.5, the original videos were cropped to remove the head area. This was done to avoid the vision of the face area that represents a highly attractive social cue, which could have deviated the viewer’s attention from the performed action on which the participants were required to give judgements. Additionally, to focus participants’ attention on the performed actions, the videos were recorded in a dark scenario and the actors wore black shirts to emphasize the forelimb area. Finally, each recorded video was flipped to balance the actors’ placement within the scene across each action type. Each video lasted 3 s. A total of 64 stimuli were produced (8 actions × 2 vitality forms × 2 actors × 2 actors’ placements in the videos).
Paradigm and task
The stimuli were presented to the participants in pairs of consecutive videos, where the observed action (what) and vitality (how) could be the same or change between video-pairs. To counterbalance all what–how possibilities, four different combinations of action-vitality were created: (i) same action—same vitality (SASV); (ii) same action—different vitality (SADV); (iii) different action—same vitality (DASV) and (iv) different action—different vitality (DADV).
All video combinations were presented in two tasks. The what task required the participants to pay attention to the type of action observed in the two consecutive videos and to decide whether the represented action was the same or different regardless of vitality form. The how task required the participants to pay attention to the vitality form and to decide whether the represented vitality was the same or different between the two consecutive videos regardless of the type of action performed. Each video combination was presented 32 times within each task.
The participants lay in the scanner in a dimly lit environment. The stimuli were viewed via digital visors (VisuaSTIM) with a 500 000 px × 0.25 square inch resolution and horizontal eye field of 30°. The digital transmission of the signal to the scanner was via optic fibre. The software E-Prime 2 Professional (Psychology Software Tools, Inc., Pittsburgh, PA, USA, http://www.pstnet.com) was used both for stimuli presentation and the recording of participants’ answers.
In each scanning session (functional run), what task started with the instruction ‘Pay attention to WHAT’. The instruction was written in a blue color and instructed the participants to focus on the type of action. The how task started with the instruction ‘Pay attention to HOW’. The instruction was written in green color and instructed the participants to focus on how the action was performed (the action vitality form).
Each trial started with a colored fixation point (blue for what task and green for how task) positioned at the centre of a black screen for 500 ms. The color of the fixation point corresponded to the color of the instructions provided (described earlier) to help the participants remembering the current task. The fixation point was followed by the presentation of pairs of video-clips. The first video-clip was presented for 3 s followed by a 100 ms fixed interval and by the second video-clip lasting 3 s. The second video was followed by a jittered interval ranging between 2.5 and 4 s (fixation cross), in which, in ∼10% of cases, the participants had to provide an explicit response to the stimuli (catch trials). More specifically, the participants had to indicate, on a response box placed inside the scanner, whether the two consecutive videos were the same or different according to the task type. One-sixth of the experimental trials was characterized by three consecutive videos representing a still image of the actors during rest position before action performance (static control).
All video-pairs were shown in four functional runs. Within each run, the two tasks (what and how) were presented, each, in four independent mini-blocks in a sequential order. Within each mini-block/task, the video-pairs were presented eight times (four with object, four without object) in a randomized order. The stimuli were randomized within each run and balanced across runs so to make an equal number of trial types. In total, the participants viewed 256 experimental video-pairs. Each functional run lasted ∼13 min. The whole study lasted ∼60 min.
Before the scanning session, the participants underwent a training session with different stimuli than those used during scanning to familiarize with the experimental procedure.
fMRI data acquisition
Anatomical T1-weighted and functional T2*-weighted MR images were acquired with a 3 Tesla General Electrics scanner equipped with an eight-channel receiver head-coil. Functional images were acquired using a T2*-weighted gradient-echo, echo-planar (EPI) pulse sequence (acceleration factor asset 2, 37 interleaved transverse slices covering the whole brain, TR = 2100 ms, TE = 30 ms, flip angle = 90°, FOV = 205 × 205 mm2, inter-slice gap = 0.5 mm, slice thickness = 3 mm, in-plane resolution 2.5 × 2.5 × 2.5 mm3). Each scanning sequence comprised 366 sequential volumes. Immediately after the functional scanning a high-resolution inversion recovery prepared T1-weighted anatomical scan (acceleration factor arc 2, 156 sagittal slices, matrix 256 × 256, isotropic resolution 1 × 1 × 1 mm3, TI = 450 ms, TR = 8100 ms, TE = 3.2 ms, flip angle 12°) was acquired for each participants.
Statistical analysis
Data analysis was performed with SPM8 (Statistical Parametric Mapping software; The Wellcome Department of Imaging Neuroscience, London, UK; http://www.fil.ion.ucl.ac.uk) running on MATLAB R2009b (The Mathworks, Inc., Natick, MA, USA). The first four EPI volumes of each functional run were discarded to allow for T1 equilibration effects. For each subject, all volumes were spatially realigned to the first volume of the first functional run and unwarped to correct for between-scan motion. The T1 weighted image was segmented into gray, white and cerebrospinal fluid and spatially normalized to the Montreal Neurological Institute (MNI) space. The spatial transformation derived from this segmentation was then applied to the realigned EPIs for normalization and resampled in 2 × 2 × 2 mm3 voxels using trilinear interpolation in space. All functional volumes were then spatially smoothed with a 6-mm full-width half-maximum isotropic Gaussian kernel for the group analysis.
Data were analysed using a random-effects model (Friston et al., 1999), implemented in a two-level procedure. In the first level, single-subject fMRI responses were entered in three independent general linear models (GLMs) by design matrices modelling the onsets and durations of each trial for each functional run according to specific experimental demands. In particular, the first model (‘task-related’ model) was created to assess and compare the global activation patterns evoked by the tasks what and how modelling, in two separate regressors, all what–how combinations (independently for what and how tasks). The second (‘vitality form’) and third (‘action-type’) models (of the first level) were created purposely for the region of interest (ROI) analyses testing for possible interactions between stimulus-driven and task-related effects on observed activations during the how task. More specifically, in the second model, we regressed the experimental trials as a function of vitality form (gentle vs energetic) entering in two main regressors the what–how pair-combinations having the same vitality, gentle or energetic, for each task. The what–how combinations having different vitalities (SADV, DADV) were modelled separately for the two tasks what and how. In the third model, we regressed the experimental trials as a function of action-type (with object vs without object). Here, we modelled the actions carried out with and without object in two separate regressors for each task (how and what).
In all three first-level models, three additional regressors were entered, modelling the static control images, the instructions and the participants’ responses. All trials representing the video-pairs were modelled as one single epoch lasting 6.1 s. The static controls, instructions and responses were modelled as events having duration 0.
In the second-level analysis (group-analysis), corresponding contrast images from the ‘task-related’ model of the first level were entered for each participant into two independent flexible analysis of variances with sphericity-correction for repeated measures (Friston et al., 2002). In the first model, we compared the pattern of activations within and between tasks (what and how) vs implicit baseline (fixation cross). In the second model, we compared the pattern of activations for each task (what and how) vs explicit baseline (static controls) (PFWE < 0.05 corrected at the cluster or voxel level, cluster size estimated with a voxel-level threshold of P-uncorrected = 0.001).
To test possible stimulus-driven effects on specific activations observed in the how task, ROIs were created on the basis of the functional maps obtained from the group analysis directly comparing how and what tasks (effect of vitality form). Accordingly, a ROI was defined within right dorso-central insula, centring the sphere (radium 5 mm) around the maximum x = 34, y = 12, z = 12 from the contrast ‘how vs what’ using MarsBaR ROI toolbox for SPM (release 0.42). Mean cluster values associated with each vitality form (gentle and energetic) and action-type (with object and without object) were then calculated for each subject on the basis of contrast images from the ‘vitality-form’ and ‘action-type’ models of the first level (described earlier). Signal change for each subject was extracted using REX (http://web.mit.edu/swg/rex).
For all the analyses, the location of the activation foci was determined in the stereotaxic space of MNI coordinates system. Those cerebral regions for which maps are provided were also localized with reference to cytoarchitectonical probabilistic maps of the human brain, using the SPM-Anatomy toolbox v.1.7 (Eickhoff et al., 2005).
Testing for task-complexity: behavioural analysis
Our contrast of interest, how vs what, although producing activations specifically related to vitality forms, could have also reflected some effects associated with task demand. To test this possibility, we carried out a further analysis, based on the responses given by the participants during the scanning sessions when presented with the catch trials, i.e. those trials in which the participants were required to give an explicit response to two consecutive videos presented in a trial, indicating if they were the same or different in terms of vitality form (how task) or action type (what task; see ‘Materials and Methods’ section). Sixteen responses were recorded for each task for each participant.
The dependent variable was the percent of correct responses (‘hits’). Depending on the type of data, both non-parametric (Siegel and Castellan, 1988; Hollander and Wolfe, 1999), and parametric statistical procedures were applied. The data were obtained for 16 participants. Three participants were excluded from analyses because of technical problems reported during response recording in at least one functional run.
RESULTS
Overall effect of ‘what’ and ‘how’ tasks
The observation of all video-clips, pooling together the activations obtained during what and how tasks, vs implicit baseline (fixation cross) showed signal increase in visual occipito-temporal areas, hippocampus, posterior parietal lobe, SMA, inferior frontal gyrus and cerebellum bilaterally. Additional activations were observed in the left hemisphere in the ventral and dorsal premotor cortex and in the insula (see Table 1 for coordinates and statistical values).
Table 1.
Cerebral activity during the observation of what and how tasks vs implicit baseline; what task vs implicit baseline; how task vs implicit baseline
| Anatomical region | Left |
Right |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z-score | ATB | x | y | z | Z-score | ATB | |
| Task-type vs baseline (PFWE-COR VXL LEVEL = <0.05) | ||||||||||
| What and How vs implicit baseline | ||||||||||
| Calcarine gyrus | −10 | −78 | 10 | 7.55 | 80% Area 17* | 12 | −78 | 14 | 7.07 | 80% Area 18* |
| Middle occipital gyrus | −42 | −82 | 4 | 6.82 | ||||||
| Inferior occipital gyrus | −38 | −80 | 4 | 6.28 | 20% hOC5(V5) | |||||
| Fusiform gyrus | −40 | −72 | 18 | 6.41 | 30% hOV4v (V4) | |||||
| Superior temporal gyrus | −62 | −36 | 14 | 4.83 | 30% IPC | 56 | −38 | 8 | 4.96 | |
| Middle temporal gyrus | −60 | −46 | 0 | 6.05 | 58 | −34 | 2 | 5.05 | ||
| Inferior temporal gyrus | −46 | −64 | −8 | 5.03 | 54 | −68 | −8 | 7.04 | 30% hOC5 (V5) | |
| Superior parietal lobule | 32 | −64 | 54 | 5.72 | 40% SPL (7A) | |||||
| Inferior parietal lobule | −42 | −48 | 52 | 6.55 | 40% IPC* | 42 | −40 | 48 | 6.48 | 40% hIP2 |
| Postcentral gyrus | −40 | −32 | 50 | 5.97 | 60% Area 3b | |||||
| Precentral gyrus | −40 | −6 | 64 | 5.19 | 40% Area 6 | |||||
| SMA | 0 | 12 | 52 | 5.89 | ||||||
| Middle frontal gyrus | −50 | 26 | 34 | 5.81 | 30% Area 45 | 50 | 40 | 24 | 5.33 | |
| Inferior frontal gyrus | −56 | 10 | 12 | 5.48 | 50% Area 44* | 60 | 18 | 30 | 4.98 | 50% Area 44* |
| Insula | −28 | 20 | 2 | 5.05 | ||||||
| Hippocampus | −26 | −28 | 0 | 6.03 | 24 | −26 | −4 | 5.37 | ||
| Cerebellum | −48 | −60 | 24 | 6.12 | 46 | −56 | −30 | 6.38 | 69% Lobule VIIa | |
| What vs implicit baseline | ||||||||||
| Calcarine gyrus | −10 | −78 | 10 | 7.36 | 80% Area 17* | 12 | −78 | 14 | 6.89 | 80% Area 18* |
| Lingual gyrus | −12 | −70 | −8 | 6.43 | 50% hOC3V (V3v)* | 12 | −66 | −4 | 7.10 | 60% Area 18* |
| Middle occipital gyrus | −38 | −80 | 4 | 6.70 | 36 | −92 | 8 | 6.21 | ||
| Inferior occipital gyrus | −48 | −76 | −8 | 6.02 | 10% hOC5(V5) | 42 | −84 | 2 | 5.84 | 40% hOV4v (V4) |
| Fusiform gyrus | −40 | −72 | −18 | 6.30 | 30% hOV4v (V4) | |||||
| Superior temporal gyrus | 56 | −38 | 8 | 4.77 | ||||||
| Middle temporal gyrus | −60 | −46 | 0 | 6.20 | 50 | −68 | 4 | 7.06 | 10% hOC5(V5) | |
| Inferior temporal gyrus | −46 | −64 | −8 | 5.30 | 54 | −68 | −8 | 6.91 | 30% hOC5(V5) | |
| Superior parietal lobule | −32 | −62 | 58 | 5.23 | 80% SPL (7°) | 30 | −66 | 54 | 6.12 | |
| Inferior parietal lobule | −42 | −48 | 52 | 6.68 | 40% IPC* | 34 | −52 | 40 | 5.86 | 40% hIP3* |
| Postcentral gyrus | −38 | −20 | 44 | 5.56 | 80% Area 4p* | |||||
| Precentral gyrus | −46 | 2 | 54 | 6.17 | 40% Area 6 | |||||
| SMA | 0 | 12 | 52 | 6.06 | 20% Area 6 | |||||
| Middle frontal gyrus | −50 | 26 | 34 | 6.06 | 30% Area 45 | 50 | 40 | 26 | 5.38 | |
| Inferior frontal gyrus | −44 | 48 | −12 | 6.16 | ||||||
| Insula | −28 | 20 | 2 | 5.65 | ||||||
| Hippocampus | −26 | −28 | 0 | 5.78 | 24 | −26 | −6 | 5.50 | ||
| Cerebellum | −48 | −60 | −24 | 6.29 | 46 | −56 | −30 | 6.40 | 69 % Lobule VIIa | |
| How vs implicit baseline | ||||||||||
| Calcarine gyrus | −10 | −76 | 8 | 7.46 | 70% Area 17* | 12 | −78 | 14 | 6.94 | 80% Area 18* |
| Lingual gyrus | −12 | −70 | −8 | 6.62 | 50% hOC3V (V3v)* | 12 | −66 | 4 | 7.17 | 60% Area 18* |
| Middle occipital gyrus | −42 | −82 | 4 | 6.68 | 36 | −92 | 8 | 6.36 | ||
| Inferior occipital gyrus | −44 | −72 | 8 | 6.07 | 20% hOC5(V5) | 48 | −82 | −4 | 6.19 | 30% hOV4v (V4) |
| Fusiform gyrus | ||||||||||
| Middle temporal gyrus | −60 | −46 | 0 | 5.58 | 56 | −38 | 6 | 4.88 | ||
| Inferior temporal gyrus | 54 | −68 | −8 | 6.87 | 30% hOC5(V5) | |||||
| Superior parietal lobule | ||||||||||
| Inferior parietal lobule | −42 | −48 | 52 | 6.10 | 40% IPC* | 42 | −38 | 48 | 6.24 | 50% hIP2 |
| Postcentral gyrus | −38 | −20 | 44 | 5.75 | 80% Area 4p* | |||||
| Precentral gyrus | −38 | −18 | 64 | 5.67 | 90% Area 6 | |||||
| SMA | 0 | 10 | 50 | 5.47 | 60% Area 6 | |||||
| Middle frontal gyrus | −50 | 26 | 34 | 5.21 | 30% Area 45 | |||||
| Inferior frontal gyrus | −56 | 10 | 12 | 5.24 | 50% Area 44* | |||||
| Insula | −34 | 14 | 0 | 4.97 | ||||||
| Hippocampus | −26 | −28 | 0 | 5.97 | 24 | −26 | −4 | 5.00 | ||
Local maxima, as shown in Figure 3A and B, are given in MNI standard brain coordinates at voxel-level PFWE <0.05 (ATB: most probable anatomical region in the Anatomy Toolbox 1.7, Eickhoff et al., 2005; asterisks (*) denote assigned areas)
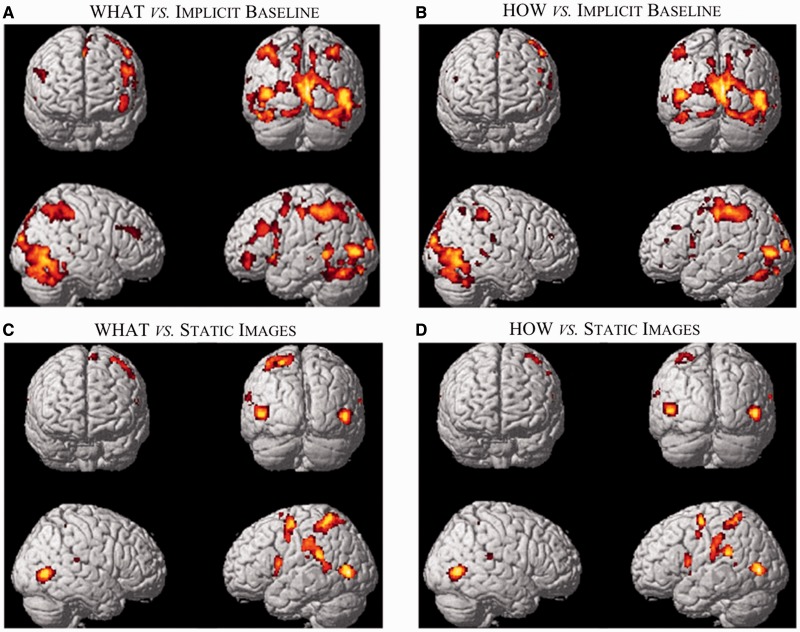
Analyses carried out within each task independently vs implicit baseline revealed for both what and how tasks a similar activation pattern (Figure 3A and B). However, the activations observed for what task (Figure 3A) were more extended compared with those observed for how task (Figure 3B), particularly in the frontal areas (see Table 1 for coordinates and statistical values).
Fig. 3.
Signal change during (A) the task what and (B) the task how vs implicit baseline (fixation cross). Signal change during (C) the task what and (D) the task how vs explicit baseline (static controls). The activations (PFWE < 0.05 at vxl level) are rendered into a standard MNI brain template.
A similar activation pattern was obtained when subtracting static controls from observation of the video-clips showing actions (Figure 3C and D; see also Table 2). As expected, there was a clear decrease in the activation of occipito-temporal visual areas, with the exception of those specifically tuned for movement as the mediotemporal area MT. With respect to the analysis vs implicit baseline, an additional activation was found in the inferior parietal lobe extending to superior temporal gyrus. This activation was due to a reduced BOLD signal (with respect to implicit baseline) when observing the static controls. Analyses carried out within each task independently vs static controls revealed a large overlap between activations during what and how tasks. As in the analysis vs implicit baseline, the activations were more widespread during what task than during how task (see Table 2 for coordinates and statistical values).
Table 2.
Cerebral activity during the observation of what and how tasks vs explicit baseline (static controls); what task vs static controls; how task vs static controls
| Anatomical region | Left |
Right |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | Z-score | ATB | x | y | z | Z-score | ATB | |
| Task-type vs baseline (PFWE-COR VXL LEVEL = <0.05) | ||||||||||
| What and How vs explicit baseline | ||||||||||
| Superior temporal gyrus | 68 | −30 | 18 | 5.94 | 80%IPC (PF)* | |||||
| Middle temporal gyrus | −38 | −70 | 4 | 6.93 | 40% hOC5(V5)* | 50 | −72 | 0 | 6.82 | 20% hOC5(V5) |
| Superior parietal lobule | −34 | −50 | 68 | 5.60 | 20% SPL(7PC) | |||||
| Inferior parietal lobule | −38 | −30 | 38 | 6.34 | 30% IPC(PFop)* | |||||
| Supra marginal gyrus | −50 | −30 | 28 | 20% IPC* | ||||||
| Postcentral gyrus | −28 | −42 | 48 | 7.24 | 50% Area 2* | 30 | −42 | 56 | 5.09 | 80% Area2* |
| Precentral gyrus | −50 | −4 | 44 | 5.75 | 60% Area 6 | |||||
| SMA | −4 | 0 | 56 | 6.02 | 80% Area 6 | |||||
| Middle frontal gyrus | −28 | −6 | 58 | 5.67 | 30% Area 6 | |||||
| Inferior frontal gyrus | −54 | 8 | 14 | 6.57 | 40% Area 44* | |||||
| Putamen | −24 | 0 | −2 | 5.35 | ||||||
| What vs explicit baseline | ||||||||||
| Middle temporal gyrus | −34 | −70 | 6 | 6.47 | 10% Area 17 | 48 | −66 | 4 | 5.77 | 40% hOC5(V5)* |
| Precuneus | −12 | −58 | 64 | 5.26 | 60% SPL(7A) | |||||
| Superior parietal lobule | −38 | −46 | 56 | 5.63 | 40% Area 2* | |||||
| Supra marginal gyrus | −50 | −26 | 34 | 5.71 | 50% IPC(PFt)* | |||||
| Postcentral gyrus | −8 | −30 | 70 | 5.18 | 70% Area 4a | |||||
| Precentral gyrus | −34 | −8 | 64 | 5.91 | 20% Area 6 | |||||
| SMA | −8 | 4 | 70 | 5.67 | 70% Area 6 | |||||
| Inferior frontal gyrus | −54 | 10 | 12 | 5.95 | 40% Area 44* | |||||
| How vs explicit baseline | ||||||||||
| Middle temporal gyrus | −34 | −70 | 6 | 5.99 | 10% Area 17 | 48 | −66 | 4 | 5.58 | 40% hOC5(V5)* |
| Superior parietal lobule | −40 | −44 | 56 | 5.15 | 50% Area 2* | |||||
| Inferior parietal lobule | −34 | −32 | 38 | 5.34 | 10% Area 2 | |||||
| Supra marginal gyrus | −58 | −38 | 26 | 5.79 | 40% IPC* | |||||
| Postcentral gyrus | −58 | −20 | 18 | 5.80 | 40% OP1* | |||||
| SMA | −4 | −2 | 56 | 5.73 | 70% Area 6 | |||||
| Superior frontal gyrus | −32 | −8 | 66 | 5.59 | 30% Area 6 | |||||
| Inferior frontal gyrus | −54 | 8 | 12 | 5.31 | 40% Area 44* | |||||
| Insula | −46 | 6 | −2 | 5.18 | ||||||
Local maxima, as shown in Figure 3C and D, are given in MNI standard brain coordinates at voxel-level PFWE < 0.05 (ATB: most probable anatomical region in the Anatomy Toolbox 1.7, Eickhoff et al., 2005; asterisks (*) denote assigned areas)
Contrast between what and how tasks
As shown in Figure 4A, the direct contrast between what vs how tasks produced stronger activations for what task in posterior parietal lobe and premotor cortex extending rostrally to include the caudal part of inferior frontal gyrus, bilaterally. Additional activations were observed in the left hemisphere in the posterior part of inferior temporal gyrus and in the anterior part of inferior frontal gyrus (see also Table 3 for coordinates and statistical values).
Fig. 4.
Brain activations resulting from the direct contrast between (A) what vs how task and (B) how vs what task. These activations (PFWE < 0.05 at cluster level) are rendered into a standard MNI brain template. (C) Activation profile within right dorso-central insula (maxima: 34 12 12) in the direct contrast how vs what task. (D) Activation profile in dorso-central insula (maxima: 34 12 12) as a function of vitality form (energetic, gentle) during how task (E) Activation profile in dorso-central insula (maxima: 34 12 12) as a function of action-type (with object, without object) during how task. (F) Percent correct responses (hits) during discrimination of what (is it the same or a different type of action?) and how (is it the same or a different form of vitality?) within each respective task, showing no differences in performance difficulty between tasks (P > 0.05).
Table 3.
Brain activations resulting from the direct contrast between what vs how task and how vs what task
| Anatomical region | Left |
Right |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | Z-score | ATB | x | y | Z | Z-score | ATB | |
| Direct contrast between task-type (PFWE-COR CLUSTER LEVEL = <0.05) | ||||||||||
| What vs How | ||||||||||
| Superior occipital gyrus | 32 | −76 | 42 | 4.49 | ||||||
| Middle occipital gyrus | 36 | −72 | 32 | 3.90 | 20% IPC | |||||
| Inferior temporal gyrus | −56 | −52 | −16 | 3.74 | ||||||
| Superior parietal lobule | −32 | −62 | 58 | 4 | 80% SPL (7A) | 36 | −52 | 56 | 4.69 | 40% SPL (7A) |
| Inferior parietal lobule | −34 | −60 | 48 | 4.60 | 30% hIP3* | |||||
| Angular gyrus | 30 | −64 | 48 | 3.97 | ||||||
| Middle frontal gyrus | 34 | 8 | 40 | 4.52 | ||||||
| Inferior frontal gyrus | −40 | 10 | 24 | 3.98 | 20% Area 44 | 42 | 26 | 28 | 4.17 | |
| Middle orbital gyrus | −42 | 46 | −2 | 4.38 | ||||||
| How vs What | ||||||||||
| Rolandic operculum | 50 | 0 | 8 | 4.55 | 20% OP | |||||
| Putamen | 32 | 8 | 8 | 4.41 | ||||||
| Insula | 34 | 12 | 12 | 4.23 | 20% OP 4 | |||||
Local maxima of activated areas, as shown in Figure 4A and B, are given in MNI standard brain coordinates at cluster-level PFWE < 0.05 (ATB: most probable anatomical region in the Anatomy Toolbox 1.7, Eickhoff et al., 2005; asterisks (*) denote assigned areas).
The opposite contrast (how vs what) revealed a specific activation for how task in the right dorso-central insula (Figure 4B and C; see also Table 3 for coordinates and statistical values).
Testing for task-related and bottom-up stimulus-driven effects: ROI analysis
To assess possible effects exerted by bottom-up stimulus-driven processes on activations observed in the contrast how vs what, we created a ROI centred at dorso-central insula (see ‘Materials and Methods’ section). Task-related and stimulus-driven processes were tested in two independent 2 × 2 GLM analyses, where we assessed separately the effects of vitality form (gentle, energetic) and action-type (with object, without object) on task-related activations (what, how).
The results relative to the effects of task (what, how) and vitality form (gentle, energetic) on insular activation revealed a main effect of task (how > what; F1,18 = 5.66, P = 0.029, partial-η2 = 0.24, δ = 0.62) but no effect of vitality form nor interaction effects between vitality form and task, indicating that insular activation was not modulated by a specific form of vitality (P > 0.05; Figure 4D).
Similarly, the results relative to the effects of task (what, how) and action-type (with object, without object) on insular activation revealed a main effect of task (how > what; F1,18 = 11.55, P = 0.003, partial-η2 = 0.39, δ = 0.9) but no effect of action-type nor interaction effects between action-type and task (P > 0.05; Figure 4E).
Altogether, these results indicate that insular activation is not associated with effects due to bottom–up stimulus-driven processes.
Task-complexity: behavioural analysis
To rule out the possibility that our contrast of interest, how vs what, reflected activations associated with task demand, we assessed the level of complexity in discriminating the what and how of an action within each respective task. To this purpose, we carried out a behavioural analysis based on percent correct responses (hits) given by the participants during the scanning sessions (see ‘Materials and Methods’ section).
The null hypothesis that the percent correct responses for what and how tasks be equal was tested using the related-samples Wilcoxon Signed Rank test (Z = −1.03; P > 0.05), indicating no difference in difficulty between the two tasks.
Additionally, hits were calculated for each what–how combination to assess possible interaction effects of sequence complexity and task-type. To this purpose, we carried out a repeated measure GLM analysis with two levels of task (what and how) and four levels of what–how combination (see ‘Materials and Methods’ section), using the Greenhouse–Geisser correction for sphericity violation (P < 0.05). The results revealed only a main effect of what–how combination (how > what; F3,45 = 4.47, P = 0.02, partial-η2 = 0.23, δ = 0.72; see Supplementary Material and Table S1 for details), showing that difficulties in judging whether pairs of stimuli were same or different did not depend on task type (Figure 4F).
DISCUSSION
The aim of this study was to identify the brain areas underlying the recognition of vitality forms during the observation of actions done by others. Participants were presented with pairs of video-clips showing two actors performing actions towards each other. The same action was carried out with two vitality forms, energetic and gentle. The participants viewed the stimuli in two tasks, what and how, in which they had to decide whether the observed action goal (what) and vitality form (how) were the same or different between two consecutive videos.
Overall, action observation, independently of task (what, how), produced activations, besides visual areas, in posterior parietal lobe bilaterally, left inferior and dorsal premotor cortex and inferior and middle frontal gyrus. Additional activations were found in hippocampus and insula. Although there was a large overlap between the activations observed for the two tasks, cortical activations were more extended during the discrimination of the action-what (e.g. passing a ball) with respect to the action-how (e.g. gently). This cortical activation pattern is similar to that typically described for execution and observation of goal-directed actions (mirror mechanism; see Grafton et al., 1996a; Rizzolatti et al., 1996; Decety et al., 1997; Iacoboni et al., 1999; Buccino et al., 2001; Rizzolatti and Craighero, 2004; Keysers and Fadiga, 2008; Iacoboni, 2009; Caspers et al., 2010; Rizzolatti and Sinigaglia, 2010; Grosbras et al., 2012; Molenberghs et al., 2012).
In line with these general results, the contrast between what and how revealed greater activations, for what task, in posterior parietal lobe bilaterally, premotor cortex extending rostrally to include the caudal part of inferior frontal gyrus, and in the rostral most part of inferior frontal gyrus. These data indicate that the analysis of actions aimed at goal recognition requires a more extensive activation of the parieto-frontal circuit subserving this function. It is interesting to note that, as far as the parietal lobe is concerned, its activation was located in a more posterior location than that typically observed in studies investigating hand actions, such as grasping (Grafton et al., 1996b; Rizzolatti et al., 1996; Binkofski et al., 1999; Buccino et al., 2001; Grèzes et al., 2003). A similar activation was found in Calvo-Merino et al. (2005) during observation of complex actions such as classical ballet or capoeira. It is then likely that activation of the posterior part of the parietal lobe observed in our study represented a bias of the viewer towards a more ‘global’ description of the observed action in the attempt to extract its goal-related meaning.
With respect to the contrast how vs what, the results revealed enhanced activation for how task in right dorso-central insular cortex. Note that this activation cannot be ascribed to some effects exerted by task demand, as shown by our behavioural analysis indicating no significant differences between what and how tasks (see ‘Behavioural Results’ section).
The insula is an extremely complex and heterogeneous structure including a posterior granular (sensory part), a central large dysgranular and a small rostro-ventral agranular (motor parts) sector (Mesulam and Mufson, 1982, 1985; Augustine, 1996). A recent neurophysiologial study (Jezzini et al., 2012) showed that, in the monkey, the insula is constituted of the following two major functional subdivisions: (i) a sensorimotor sector, occupying the dorso-central portion of the insula, which appears to be a functional extension of the parietal lobe and (ii) a large anterior and ventral sector consisting of a mosaic of oro-facial motor programs. In the anterior and ventral insula, there is a progressive dorso-ventral shift from motor programs without emotional content to motor programs with such a content.
A similar pattern of functional organization has been recently described in a meta-analysis by Kurth et al. (2010) for the human insula. In this meta-analysis, consisting of large number of fMRI studies, they found four functional distinct regions corresponding to sensory motor (Showers and Lauer, 1961), olfacto-gustatory (Small et al., 1999; Poellinger et al., 2001; Kringelbach et al., 2004; Royet and Plailly, 2004), socio-emotional (Dolan, 2002; Phillips et al., 2003; Iacoboni and Dapretto, 2006) and cognitive networks of the brain (Mayer et al., 2007; Soros et al., 2007). Socio-emotional aspects activate the ventro-rostral part of the insula while all tested functions, except for the sensory-motor function, overlap on its anterior dorsal portion, often found activated by cognitive demands (Chong et al., 2009), stimulus or task complexity (Menon and Uddin, 2010) and stimulus emotional salience (Grosbras and Paus, 2005; Pichon et al., 2009, 2011).
The view that the most anterior sector of the insula is functionally segregated from its dorso-central sector is consistent with our findings supporting Stern’s (1985, 2010) notion that vitality forms do not represent a basic emotional state like anger and fear that, differently from our results, determine a consistent activation of the rostral insula (e.g. Wicker et al., 2003; Gallese et al., 2004; Singer et al., 2004; Grosbras et al., 2005; de Gelder, 2006; Jabbi et al., 2008; Pichon et al., 2009). Instead, our data suggest that vitality forms represent a specific aspect of movement processing, subserved by anatomically and functionally distinct areas from those described for emotion processing.
To exclude the possibility that our dorso-central insular activation was affected by some stimulus properties intrinsically describing the type of vitality form (gentle, energetic) or the type of action (with object, without object), we carried out additional analyses. In particular, the dorso-central insular sector showed no statistical difference with respect to the different types of vitality (energetic or gentle). Of course, we cannot exclude the possibility that, within this insular sector, specific neuronal populations are attuned to one or to the other type of vitality. Likewise, the results of these analyses showed that dorso-central insula responded similarly to actions mediated by the presence or absence of an object. Taken together, these data suggest that processing of low-order stimulus features is not responsible for the activation of the dorso-central insula.
What is then the functional role played by dorso-central insula in the processing of vitality affects? Single neuron studies showed that this sector is endowed with sensorimotor properties (Robinson and Burton, 1980a; Schneider et al., 1993). Anatomically, it is connected with the somatosensory cortex (e.g. Mishkin, 1979; Friedman et al., 1986; Augustine, 1996). Furthermore, unlike the anterior part of the insula, which is linked with the frontal lobe and subcortical emotional centres, this posterior sector is connected with medial temporal areas and, in particular, with the hippocampus and the amygdala (e.g. Friedman et al., 1986). A cue clarifying the functional role of dorso-central insula may come from findings showing that this sector receives information from a specific set of unmyelinated cutaneous fibres. These fibres (CT-afferents; see Löken et al., 2009) are activated when the skin is stroked at a pleasant, caress-like speed and their discharge correlates with the subjective hedonic experience of the caress (Morrison et al., 2011). In Morrison et al. (2011), there was also evidence that dorso-central insula was activated during the observation of other individuals being caressed. CT-like processing being triggered also during others’ observation suggests that this insular sector may serve as a platform for the evaluation of specific interaction patterns between individuals.
The coding of vitality forms during action observation suggests the involvement of somato-motor processing, as well as of visual processing. Unfortunately, the perusal of our activations does not allow us to give a precise localization of such putative visual input, although one might suggest the existence of a cortical visual pathway to the insular cortex. Such input may originate from higher order visual areas, such as the superior temporal sulcus (STS), to which dorsal granular/dysgranual insula is shown to be connected (Mesulam and Mufson, 1982; Seltzer and Pandya, 1991). The proposal of a direct visual path reaching the dorso-central insula is in line with the results by Hadjikhani and Roland (1998), who suggested the involvement of this sector in cross-modal transfer of information. More specifically, dorso-central insula may be a site of interaction between modality-specific areas, namely somatosensory and visual.
In sum, the present data show that recognition of vitality forms involves the activation of a specific sector (the dorso-central sector) of the insula. Given the anatomical connections of this sector with other cortical areas, it appears that vitality form processing involves a pathway different from those mediating both ‘cold’ and emotional actions. This pathway links, via dorso-central insula (see also, Dijkerman and de Haan, 2007), sensorimotor cortical areas with the medial limbic temporal areas, and particularly the hippocampus. Activation of the hippocampus could be functional to storage and retrieval of memories associated with specific forms of vitality. Thus, this sensorimotor–insular–limbic network could provide the specific feeling characterizing vitality forms intrinsic to action processing.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We would like to thank F. Fasano, G. Burani, G. Mantelli, M. Rinaldi and Doc. G. Crisi for their kind technical and administrative support. We are further grateful to Fondazione Cassa di Risparmio di Parma (CARIPARMA) for providing the infrastructure that made this work possible. This research was funded by grants from the European Research Council Grant Cogsystems to G Rizzolatti and by Fondazione BancaMonte Parma.
REFERENCES
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research Reviews. 1996;2:229–44. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- Binkofski F, Buccino G, Posse S, Seitz R, Rizzolatti G, Freund H. A fronto-parietal circuit of object manipulation in man: evidence from an fMRI study. European Journal of Neuroscience. 1999;11:3276–86. doi: 10.1046/j.1460-9568.1999.00753.x. [DOI] [PubMed] [Google Scholar]
- Buccino G, Binkofski F, Fink GR, et al. Action observation activates premotor and parietal areas in a somatotopic manner: an fMRI study. European Journal of Neuroscience. 2001;13:400–4. [PubMed] [Google Scholar]
- Calvo-Merino B, Glaser DE, Grèzes J, Passingham RE, Haggard P. Action observation and acquired motor skills: an fMRI study with expert dancers. Cerebral Cortex. 2005;15:1243–9. doi: 10.1093/cercor/bhi007. [DOI] [PubMed] [Google Scholar]
- Caspers S, Zilles K, Laird AR, Eickhoff SB. ALE meta-analysis of action observation and imitation in the human brain. Neuroimage. 2010;50:1148–67. doi: 10.1016/j.neuroimage.2009.12.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong TTJ, Cunnington R, Williams MA, Mattingley JB. The role of selective attention in maching observed and executed actions. Neuropsychologia. 2009;47:786–95. doi: 10.1016/j.neuropsychologia.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Condon WS, Sander LS. Neonate movement is synchronized with adult speech: interactional participation and language acquisition. Science. 1974;183:99–101. doi: 10.1126/science.183.4120.99. [DOI] [PubMed] [Google Scholar]
- Decety J, Grèzes J, Costes N, et al. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain. 1997;120:1763–77. doi: 10.1093/brain/120.10.1763. [DOI] [PubMed] [Google Scholar]
- de Gelder B. Towards the neurobiology of emotional body language. Nature Reviews Neuroscience. 2006;7(3):242–9. doi: 10.1038/nrn1872. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, de Hann EHF. Somatosensory process subserving perception and action. Behavioral and Brain Sciences. 2007;30:189–239. doi: 10.1017/S0140525X07001392. [DOI] [PubMed] [Google Scholar]
- Dolan RJ. Emotion, cognition, and behavior. Science. 2002;298:1191–4. doi: 10.1126/science.1076358. [DOI] [PubMed] [Google Scholar]
- Eickhoff S, Stephan KE, Mohlberg H, et al. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. Neuroimage. 2005;25(4):1325–35. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- Fabbri-Destro M, Rizzolatti G. Mirror neurons and mirror systems in monkeys and humans. Physiology. 2008;23:171–9. doi: 10.1152/physiol.00004.2008. [DOI] [PubMed] [Google Scholar]
- Flom R, Bahrick LE. The development of infant discrimination of affect in multimodal and unimodal stimulation: the role of intersensory redundancy. Developmental Psychology. 2007;43:238–52. doi: 10.1037/0012-1649.43.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O’Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. The Journal of Comparative Neurology. 1986;252:323–47. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ. How many participants constitute a study? Neuroimage. 1999;10:1–5. doi: 10.1006/nimg.1999.0439. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Glaser DE, Henson RN, Kiebel S, Phillips C, Ashburner J. Classical and Bayesian inference in neuroimaging: applications. Neuroimage. 2002;16:484–512. doi: 10.1006/nimg.2002.1091. [DOI] [PubMed] [Google Scholar]
- Gallese V, Keysers C, Rizzolatti G. A unifying view of the basis of social cognition. Trends in Cognitive Sciences. 2004;8:396–403. doi: 10.1016/j.tics.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Localization of grasp representations in humans by PET: 2. Observation compared with imagination. Experimental Brain Research. 1996a;112:103–11. doi: 10.1007/BF00227183. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Arbib MA, Fadiga L, Rizzolatti G. Functional anatomy of pointing and grasping in human. Cerebral Cortex. 1996b;6(2):226–37. doi: 10.1093/cercor/6.2.226. [DOI] [PubMed] [Google Scholar]
- Grèzes J, Armony JL, Rowe J, Passingham RE. Activations related to ‘mirror’ and ‘canonical’ neurones in the human brain: an fMRI study. Neuroimage. 2003;18:928–37. doi: 10.1016/s1053-8119(03)00042-9. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Paus T. Brain networks involved in viewing angry hands or faces. Cerebral Cortex. 2005;16:1087–96. doi: 10.1093/cercor/bhj050. [DOI] [PubMed] [Google Scholar]
- Grosbras MH, Beaton S, Eickoff SB. Brain regions involved in human movement perception: a quantitative voxel-based meta-analysis. Human Brain Mapping. 2012 doi: 10.1002/hbm.21222. (Epub ahead of print; doi:10.1002/hbm.21222) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjikhani N, Roland PE. Cross-modal transfer of information between the tactile and the visual representations in the human brain: a positron emission tomographic study. The Journal of Neuroscience. 1998;18(3):1072–84. doi: 10.1523/JNEUROSCI.18-03-01072.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander M, Wolfe DA. Nonparametric Statistical Methods. New York: John Wiley; 1999. [Google Scholar]
- Iacoboni M. Imitation, empathy, and mirror neurons. Annual Reviews of Psychology. 2009;60:653–70. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Dapretto M. The mirror neuron system and the consequences of its dysfunction. Nature Reviews Neuroscience. 2006;7:942–51. doi: 10.1038/nrn2024. [DOI] [PubMed] [Google Scholar]
- Iacoboni M, Woods RP, Brass M, Bekkering H, Mazziotta JC, Rizzolatti G. Cortical mechanisms of human imitation. Science. 1999;286:2526–8. doi: 10.1126/science.286.5449.2526. [DOI] [PubMed] [Google Scholar]
- Jabbi M, Bastiaansen J, Keysers C. A common anterior insula representation of disgust observation, experience and imagination shows divergent functional connectivity pathways. PLoS One. 2008;3(8):e2939. doi: 10.1371/journal.pone.0002939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jezzini A, Caruana F, Stoianov I, Gallese V, Rizzolatti G. The functional organization of the insula and of inner perisylvian regions: an intracortical microstimulation study. Proceedings of the National Academy of Sciences of the Unites States of America. 2012;109(25):10077–82. doi: 10.1073/pnas.1200143109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keysers C, Fadiga L. The mirror neuron system: new frontiers. Social Neuroscience. 2008;3:193–8. doi: 10.1080/17470910802408513. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, de Araujo I, Rolls ET. Taste-related activity in the human dorsolateral prefrontal cortex. Neuroimage. 2004;21:781–8. doi: 10.1016/j.neuroimage.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure Function. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löken LS, Wessberg J, Morrison I, McGlone F, Olausson H. Coding of pleasant touch by unmyelinated afferents in humans. Nature Neuroscience. 2009;12:547–8. doi: 10.1038/nn.2312. [DOI] [PubMed] [Google Scholar]
- Mayer JS, Bittner RA, Nikolic D, Bledowski C, Goebel R, Linden DE. Common neural substrates for visual working memory and attention. Neuroimage. 2007;36:441–53. doi: 10.1016/j.neuroimage.2007.03.007. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ. Saliency, switching, attention and control: a network model of insula function. Brain Structure Function. 2010;214(5–6):655–67. doi: 10.1007/s00429-010-0262-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the Old World monkey (III): efferent cortical output and comments on function. Journal of Comparative Neurology. 1982;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. The insula of Reil in man and monkey. Cerebral Cortex. 1985;4:179–226. [Google Scholar]
- Mishkin M. Analogous neural models for tactual and visual learning. Neuropsychologia. 1979;17:139–51. doi: 10.1016/0028-3932(79)90005-8. [DOI] [PubMed] [Google Scholar]
- Molenberghs P, Cunnigton R, Mattingley JB. Brain regions with mirroe properties: a meta-analysis of 125 fMRI studies. Neuroscience and Behavioural Reviews. 2012;36:341–9. doi: 10.1016/j.neubiorev.2011.07.004. [DOI] [PubMed] [Google Scholar]
- Morrison I, Bjsdotter M, Olausson H. Vicariuos responses to social touch in posterior insular cortex are tuned to pleasant caressing speeds. The Journal of Neuroscience. 2011;31(26):9554–62. doi: 10.1523/JNEUROSCI.0397-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel J, Butterworth G, editors. Imitation in Infancy. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54:504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Pichon S, Gelder B, Grezes J. Two different face of threat. Comparing the neural systems for recognizing fear and anger in dynamic body expressions. Neuroimage. 2009;47:1873–83. doi: 10.1016/j.neuroimage.2009.03.084. [DOI] [PubMed] [Google Scholar]
- Pichon S, Gelder B, Grezes J. Threat prompts defensive brain responses independently of attentional control. Cerebral Cortex. 2011;22(2):274–85. doi: 10.1093/cercor/bhr060. [DOI] [PubMed] [Google Scholar]
- Poellinger A, Thomas R, Lio P, et al. Activation and habituation in olfaction—an fMRI study. Neuroimage. 2001;13:547–60. doi: 10.1006/nimg.2000.0713. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighero L. The mirror-neuron system. Annual Review Neuroscience. 2004;27:169–92. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Sinigallia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nature Review Neuroscience. 2010;11:264–74. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fadiga L, Fogassi L, Gallese V. Premotor cortex and the recognition of motor actions. Cognitive Brain Research. 1996;3:131–41. doi: 10.1016/0926-6410(95)00038-0. [DOI] [PubMed] [Google Scholar]
- Robinson CJ, Burton H. Somatotopographic organization in the second somatosensory area of M. fascicularis. The Journal of Comparative Neurology. 1980;192:43–67. doi: 10.1002/cne.901920104. [DOI] [PubMed] [Google Scholar]
- Rochat P. Others in Mind: The Origins of Self-Consciousness. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- Royet JP, Plailly J. Lateralization of olfactory processes. Chemical Senses. 2004;29:731–45. doi: 10.1093/chemse/bjh067. [DOI] [PubMed] [Google Scholar]
- Schneider R, Friedman D, Mishkin M. A modality-specific somatosensory area within the insula of the rhesus monkeys. Brain Research. 1993;621:116–20. doi: 10.1016/0006-8993(93)90305-7. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Post-Rolandic cortical projections of the superior temporal sulcus in the rhesus monkey. The Journal of Comparative Neurology. 1991;312:625–40. doi: 10.1002/cne.903120412. [DOI] [PubMed] [Google Scholar]
- Shai D, Belsky J. When words just won’t do: introducing parental embodied mentalizing. Child Development Perspectives. 2011;5(3):173–80. [Google Scholar]
- Showers MJ, Lauer EW. Somatovisceral motor patterns in the insula. Journal of Comparative Neurology. 1961;117:107–15. doi: 10.1002/cne.901170109. [DOI] [PubMed] [Google Scholar]
- Siegel S, Castellan NJ. Nonparametric Statistics for the Behavioral Sciences. New York: McGraw-Hill; 1988. [Google Scholar]
- Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–62. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- Small DM, Zald DH, Jones-Gotman M, et al. Human cortical gustatory areas: a review of functional neuroimaging data. Neuroreport. 1999;10:7–14. doi: 10.1097/00001756-199901180-00002. [DOI] [PubMed] [Google Scholar]
- Soros P, Marmurek J, Tam F, Baker N, Staines WR, Graham SJ. Functional MRI of working memory and selective attention in vibrotactile frequency discrimination. BMC Neuroscience. 2007;8:48. doi: 10.1186/1471-2202-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern DN. Affect attunement. In: Call JD, Galenson E, Tyson R, editors. Frontiers of Infant Psychiatry. New York: Basic Books; 1984. [Google Scholar]
- Stern DN. The Interpersonal World of the Infant. New York: Basic Books; 1985. [Google Scholar]
- Stern DN. The Present Moment in Psychotherapy and Everyday Life. New York: Norton; 2004. [Google Scholar]
- Stern DN. Forms of Vitality Exploring Dynamic Experience in Psychology, Arts, Psychotherapy, and Development. Oxford: Oxford University press; 2010. [Google Scholar]
- Trevarthen C. The concept and foundations of infant intersubjectivity. In: Braten S, editor. Intersubjective Communication and Emotion in Early Ontogeny. Cambridge: Cambridge University Press; 1998. [Google Scholar]
- Trevarthen C, Aitken KJ. Infant intersubjectivity: research, theory and clinical applications. Journal of Child Psychology and Psychiatry. 2001;42(1):3–48. [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in my insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–64. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.