Abstract
Two mechanisms have been proposed to account for the difficulty in recognizing faces of other racial groups (the other-race effect; ORE): perceptual expertise and social cognitive factors. Focusing on the social cognitive factors alone, we manipulated in-group and out-group memberships based on two social categories (nationality and university affiliation), and controlled for perceptual expertise by testing Caucasian participants with Caucasian faces only. Using event-related potentials (ERPs) and focusing on the N170, a brain electrical component sensitive to faces, we provide for the first time strong support for the social cognitive influence on face processing within 200 ms. After participants learned the social categories, the N170 latency differentiated between double in-group and double out-group faces, taking longer to process the latter. In comparison, without group memberships, there was no difference in N170 latency among the faces. These results are consistent with recent findings of behavioral and imaging research, providing further support for the social cognitive model and its potential for understanding ORE.
Keywords: other-race effect, social cognition, face inversion effect, ERP, N170
INTRODUCTION
The difficulty people often have recognizing faces of another race compared with those of their own race is captured by the impression that ‘they all look alike’. This ‘other-race effect’ (ORE) is considered one of the most reliable findings in face recognition research and has significant implications in such legal settings as eyewitness testimony (Meissner and Brigham, 2001).
A number of theories have been proposed to account for the ORE. Broadly, these theories are of two types. The first type relies on perceptual explanations (Valentine, 1991) and assumes an inadequate development of perceptual expertise when processing other-race faces. In contrast, social-cognitive models (Levin, 2000) do not assume a difference between own- and other-race faces in perceptual processing, but rather suggest that the difficulty in recognizing other-race faces is a result of reduced attention and lack of motivation to individuate other-race members.
Among the first to investigate the neural correlates of ORE, Golby et al. (2001) found in their fMRI study that the brain region specialized for face processing (fusiform face area; FFA) showed greater responses to own-race than to other-race faces. In addition, behavioral ORE correlated with the changes in brain signals to own- vs other-race faces in the left fusiform and in the right parahippocampal and hippocampal areas. Besides the overall response magnitude of FFA, the neural response patterns across the ventral temporal regions also differentiate own-race from other-race faces reliably (Natu et al., 2011). Demonstrating the neural substrate of the ORE, these results however did not differentiate between perceptual expertise and social cognitive factors (e.g. attention allocation) as the primary mechanism. Recently, Van Bavel et al. (2008, 2011) manipulated social group memberships by assigning own- and other-race faces to either an in-group (one’s own team) or an out-group (a competing team). Despite this arbitrary manipulation, they found that in-group faces, compared with out-group faces, elicited a greater fMRI response in FFA. In contrast, race did not affect FFA; neither did race modulate the group membership effect on FFA. Therefore, by demonstrating this race-independent group membership effect on face processing, Van Bavel et al. have provided some neural support for the social-cognitive explanations of ORE.
Event-related potentials (ERPs) can provide better temporal resolution for neural events than fMRI. Past research has identified several components that are particularly relevant for face processing: the N170 (Bentin et al., 1996), the P2 and the N250 (Schweinberger et al., 2002). Functionally, these components may reflect different stages of face recognition (Bruce and Young, 1986; Zheng et al., 2012). Most ORE researchers have focused on the N170 component, although the P2 and the N250 have also been studied (Herrmann et al., 2007; Stahl et al., 2008, 2010; Tanaka and Pierce, 2009).
Although the N170 was not initially found to be sensitive to face race (e.g. Caldara et al., 2003), more recently, a number of researchers have reported that own-race faces elicit an N170 that is smaller (Herrmann et al., 2007; Stahl et al., 2008, 2010; Walker et al., 2008; Balas and Nelson, 2010; Caharel et al., 2011), and peaks earlier than for other-race faces (Stahl et al., 2008, 2010; Wiese et al., 2009; Ofan et al., 2011; but see Balas and Nelson, 2010). The face inversion effect (FIE) on the N170 (i.e. a larger and delayed N170 to inverted faces than to upright faces) (Rossion et al., 1999) also seems to differ between own-race and other-race faces, although the specific results have not been entirely consistent across studies (Gajewski et al., 2008; Vizioli et al., 2010; Caharel et al., 2011). Furthermore, using an adaptation paradigm in which two faces of either the same or different identities were presented in sequence and with single-trial analyses, Vizioli et al. (2010) found that the N170 was sensitive to face identity of own-race faces, but not with other-race faces. The effect of race on the N170 may also vary among individuals, relating to the amount of social contact and individuating experience with other-race members (Walker et al., 2008) and to a person’s automatic racial attitude and controlled responses to prejudice-congruent information (Ofan et al., 2011). Overall, these recent ERP studies suggest that the N170 is sensitive to face-race information, and that the ORE is evident at a neural level within 200 ms of seeing a face. However, because perceptual experience with other-race faces and social cognitive factors (e.g. attention and motivation to individuate other-race members) are usually confounded, it is not clear whether these early N170 effects are due to perceptual or social cognitive factors.
In this study, using only Caucasian faces to control for perceptual expertise, we randomly assigned four face stimuli into four distinct groups based on two social categories: university affiliation and nationality. Given that the research participants were Canadians studying at Brock University, the Canadian Brock face represents a double in-group member; the non-Canadian non-Brock face represents a double out-group member, and the Canadian non-Brock (in-/out-group) and the non-Canadian Brock (out-/in-group) faces as in between. Previous social psychological research on cross-categorization has reported that a person’s liking of a group and perception of the group’s similarity to self decreased gradually from double in-group to double out-group, with mixed groups in the middle (Crisp et al., 2003). By creating a similar change in group membership affiliation through such cross-category manipulation, we investigated whether an effect of social cognition on face processing could occur as early as the N170 component. In addition, the faces were presented in both upright and inverted orientations in the experiment. When faces are inverted, they are processed less holistically (Farah et al., 1995). This manipulation thus allowed us to determine whether social group membership is related to changes in holistic processing, a factor not investigated in the previous imaging studies (Van Bavel et al., 2008, 2011).
METHODS
Participants
Fifteen Caucasian female undergraduate students (mean age = 20.9 ± 2.0 years) participated in the current ERP study for either a research credit or monetary compensation. All participants were Canadians and were studying at Brock University at the time of testing. They were right-handed with normal or corrected-to-normal vision. There was no report of neurological disorders, psychiatric history or attentional problems. The experimental procedures were approved by Brock University Research Ethics Board.
Stimuli
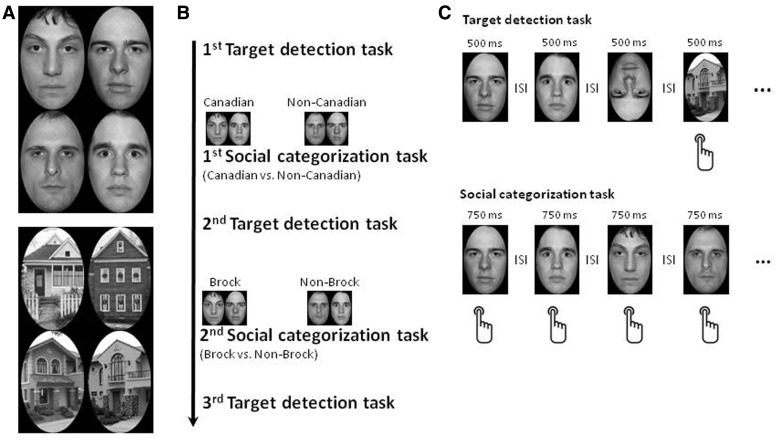
The stimuli were in black and white and consisted of four Caucasian male faces with a neutral expression selected from the NimStim face set (Tottenham et al., 2009) and four houses used as non-face stimuli (Figure 1A). A black background with an oval shape (8.7 cm in width × 14.4 cm in height) cut-out in the middle was placed on top of each stimulus. The positions of the face stimuli were adjusted so that their eyes were approximately at the same horizontal level. There were two experimental conditions, a target detection task and a social categorization task, each performed multiple times. For the target detection task, the faces and the houses were presented in upright and inverted orientations. For the social categorization task, only upright faces were presented. For both tasks, participants viewed the visual stimuli from a distance of 100 cm, subtending a visual angle of 2.49° (horizontally) by 4.12° (vertically).
Fig. 1.
Experimental stimuli (A) and the procedure (B, C). Participants performed a target detection task and a social categorization task multiple times. For the target detection task (C), faces and houses were presented in both upright and inverted orientations; participants were asked to press a button whenever they saw a house without regard to its orientation. For the social categorization task (C), only upright faces were presented. Based on the social category (either nationality or university affiliation) learned at the beginning of the task, participants on each trial categorized the face stimulus by pressing one of two buttons.
Procedure
Throughout the experiment, participants performed the target detection task three times interwoven with the social categorization task performed twice (Figure 1B).
The target detection task (Figure 1C) had 400 trials in total. On each trial, a face or a house in either an upright or an inverted orientation was presented for 500 ms in the center of a computer screen, followed by a randomly selected interstimulus interval (ISI) of 450, 500 or 550 ms. There were 80 trials (40 upright; 40 inverted) for each face, and 20 trials (10 upright; 10 inverted) for each house model. The order of presentation was randomized. Participants were instructed to press a button whenever they saw a house without regard to orientation.
For the social categorization task, the four face stimuli were first shown simultaneously to participants, who were then informed that two faces were from one social category while the other two faces were not, indicated by the labels above the faces (Figure 1B). After participants reported that they had memorized the group memberships, they started the social categorization task (Figure 1C), in which an upright face was presented on each trial for 750 ms followed by a variable ISI of 450, 500 or 550 ms; there were 160 trials (40 trials for each face) presented randomly, and participants had to indicate the group membership for each face stimulus by pressing one of the two buttons. No feedback was given concerning their accuracy. The order in which the two social categorization tasks were performed based on either nationality or university affiliation was counter-balanced across participants. The assignment of nationality and university affiliation to the face stimuli was orthogonal so that each face was associated with a distinct group. Stimuli were rotated through social categories across participants.
Finally, at the end of the study, to examine whether participants had learned the social categories successfully, we asked participants to identify the two social categories associated with each face.
ERP recording and analysis
The EEG was recorded from an elasticized net (Electrical Geodesics, Inc.) with 128 silver chloride-plated electrodes, referenced to the vertex (Cz), and amplified by Net Amps 200 (band-pass filter 0.01–100 Hz; digitized sampling rate 500 Hz; impedance less than 50 kΩ). Eye movements and blinks were monitored by electrodes placed below and beside each eye. The EEG data were segmented into epochs of 1000 ms including a baseline of 200 ms prior to stimulus onset. After visual inspection, trials contaminated by movements were manually rejected, and approximately 38 trials (i.e. 95%; range: 94.9–96.3%) remained for each stimulus type; trials with eye artifacts were corrected through the artifact correction method provided by BESA 5.1 software (MEGIS Software GmbH). The 128-channel data were then transformed through spherical spline interpolation to the standard 81 electrode montage according to the expanded 10–10 system.
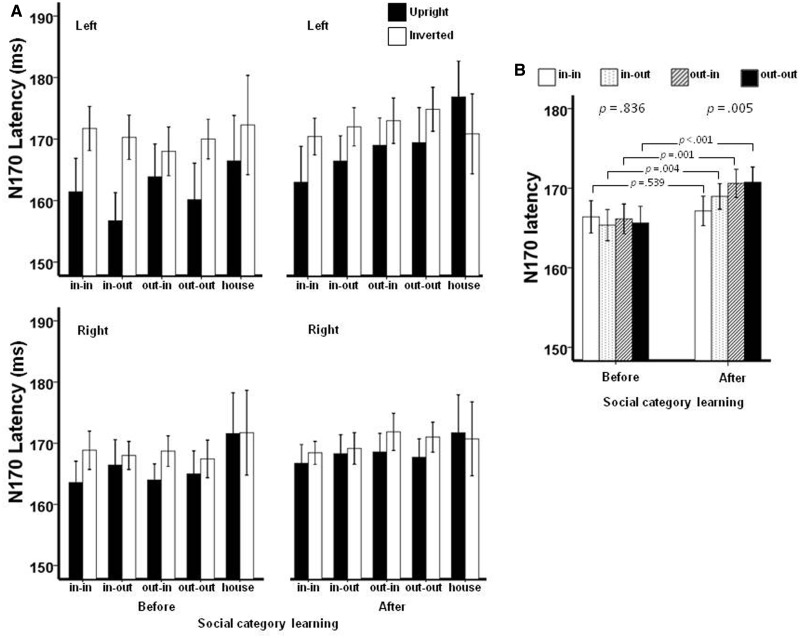
The N170 component (Figure 2) was measured as the maximum peak negativity between 130 and 220 ms poststimulus onset at the left (P5, P7, P9, PO7 and PO9) and at the right (P6, P8, P10, PO8 and PO10) occipito-temporal sites. The use of maximum value from each region, rather than at a single site (e.g. P7 or P8), was to take into account individual differences in the topography of the N170. Repeated measures analysis of variances (ANOVAs) were used to analyze the N170 amplitude and latency. Post hoc comparisons with a Bonferroni procedure were performed to follow up significant main effects. Our analyses were focused on the comparison between the prelearning (first target detection task) and the postlearning (third target detection task) periods after participants had acquired the group memberships of the face stimuli through performing the social categorization task twice. The results would allow us to examine how face-related neural processes (e.g. as they are reflected in the FIE in the N170 amplitude and latency) might be affected by group membership. Because the nationality and university affiliation were mainly used to create changes in group membership affiliation, they were not considered as two separate factors; instead, the four face stimuli were considered together as one factor (group membership) in the ERP analyses.
Fig. 2.
The N170 ERP component to upright and inverted faces and houses at representative sites (P7, P8), before (first target detection task) and after (third target detection task) participants learned the social categories associated with the face stimuli.
RESULTS
Behavioral manipulation check
During the experiment, participants learned the social categories of the face stimuli successfully. The overall response accuracy was 90.1% for the first and 86.9% for the second social categorization task (see Supplementary Material available online for more detailed behavioral results); at the end of the experiment, all participants were able to correctly identify the two social categories associated with each face.
For the target detection task, the average response accuracy for detecting the houses was 98.8% before (i.e. the first target detection task) and 95.4% after (i.e. the third target detection task) participants learned the social categories of the faces (see Supplementary Material available online for more detailed behavioral results). One participant however performed poorly in both occasions, with response accuracies ∼3 s.d. below the group average, and so was excluded from subsequent analyses.1
N170 latency
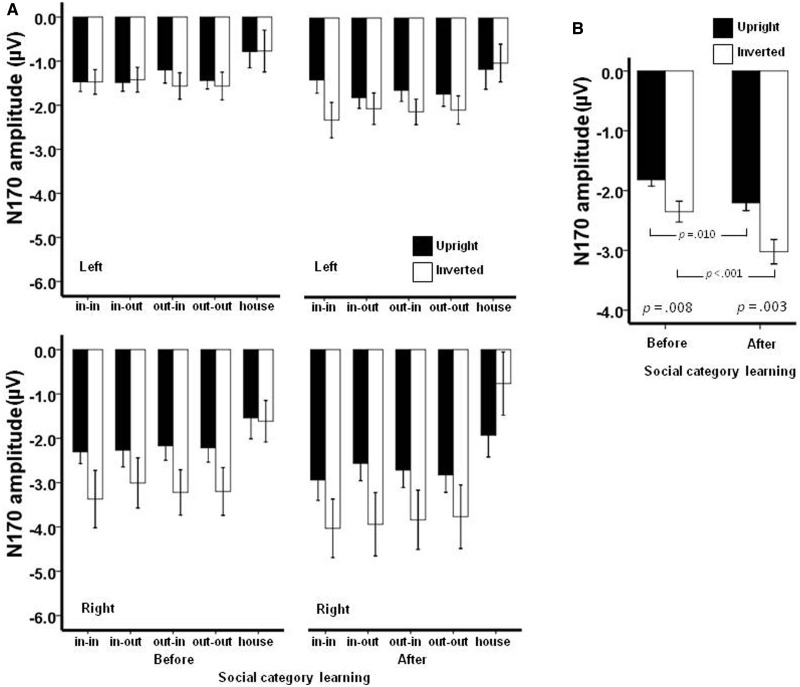
We compared the N170 latencies in a 2 (learning:first vs third target detection task) × 4 (group membership) × 2 (face orientation) × 2 (hemisphere) repeated measures ANOVA. We found that after the acquisition of social categories, the N170 latency was overall longer for the third target detection task compared with the first target detection task, F(1,13) = 17.7, P = 0.001, 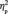 = 0.577 (Figure 3A). Furthermore, there was an interaction between learning and group membership, F(3,39) = 5.4, P = 0.003,
= 0.577 (Figure 3A). Furthermore, there was an interaction between learning and group membership, F(3,39) = 5.4, P = 0.003, 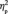 = 0.295. The double in-group face (Canadian Brock) did not change in N170 latency between the first and the third target detection tasks (0.75 ms), F(1,13) < 1.0, P = 0.539; however, the N170 latency increased for the other three types of faces after social category learning, particularly for the double out-group face: 3.6 ms for in-/out-group face (Canadian non-Brock) , F(1,13) = 12.2, P = 0.004,
= 0.295. The double in-group face (Canadian Brock) did not change in N170 latency between the first and the third target detection tasks (0.75 ms), F(1,13) < 1.0, P = 0.539; however, the N170 latency increased for the other three types of faces after social category learning, particularly for the double out-group face: 3.6 ms for in-/out-group face (Canadian non-Brock) , F(1,13) = 12.2, P = 0.004, 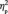 = 0.484; 4.5 ms for out-/in-group face (non-Canadian Brock), F(1,13) = 16.6, P = 0.001,
= 0.484; 4.5 ms for out-/in-group face (non-Canadian Brock), F(1,13) = 16.6, P = 0.001, 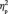 = 0.561; 5.1 ms for double out-group face (non-Canadian non-Brock), F(1,13) = 23.9, P < 0.001,
= 0.561; 5.1 ms for double out-group face (non-Canadian non-Brock), F(1,13) = 23.9, P < 0.001, 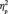 = 0.648 (Figure 3B). When the first and the third target detection task were examined separately, we found that the N170 latency differed among the faces after participants learned their social categories, F(3,39) = 4.9, P = 0.005,
= 0.648 (Figure 3B). When the first and the third target detection task were examined separately, we found that the N170 latency differed among the faces after participants learned their social categories, F(3,39) = 4.9, P = 0.005, 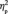 = 0.275, but not before, F(3,39) < 1.0, P = 0.836 (Figure 3B). Post hoc comparisons suggested that after social category learning, the N170 latency was longer for the double out-group face than for the double in-group face (P = 0.029); the N170 latencies for in-/out- and out-/in-group faces were intermediate and did not statistically differ from the N170 latencies for either the double out-group or the double in-group faces (Ps > 0.10).
= 0.275, but not before, F(3,39) < 1.0, P = 0.836 (Figure 3B). Post hoc comparisons suggested that after social category learning, the N170 latency was longer for the double out-group face than for the double in-group face (P = 0.029); the N170 latencies for in-/out- and out-/in-group faces were intermediate and did not statistically differ from the N170 latencies for either the double out-group or the double in-group faces (Ps > 0.10).
Fig. 3.
The N170 latencies (A) for each category of face before and after social category learning, and for houses. The graphs in (B) show that after participants learned the social categories of the face stimuli, the overall N170 latency increased, driven by the out-group-related faces. After social category learning, the N170 latency was shorter for the double in-group face and longer for the double out-group face; in contrast, before social category learning, there were no differences in N170 latencies among the face stimuli. Legend: in–in (double in-group; Canadian Brock); in–out (in/out-group; Canadian non-Brock); out–in (out-/in-group; non-Canadian Brock); out–out (double out-group; non-Canadian non-Brock). Error bars represent the s.e.m.
In addition, for the N170 latency, there was also a marginal interaction between learning and face orientation, F(1,13) = 4.0, P = 0.068. The FIE on the N170 latency (i.e. that the N170 is delayed for the inverted compared with the upright faces) appeared to be larger for the first (6.5 ms), F(1,13) = 12.2, P = 0.004, 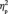 = 0.485, than for the third (3.9 ms) target detection task, F(1,13) = 5.7, P = 0.033,
= 0.485, than for the third (3.9 ms) target detection task, F(1,13) = 5.7, P = 0.033, 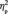 = 0.304. Face orientation did not interact with group membership in either the first or the third target detection task [F(3,39) < 1.0, P = 0.494; F(3,39) < 1.0, P = 0.812]; neither was there a three-way interaction among learning, group membership and face orientation, F(3,39) < 1.0, P = 0.533.
= 0.304. Face orientation did not interact with group membership in either the first or the third target detection task [F(3,39) < 1.0, P = 0.494; F(3,39) < 1.0, P = 0.812]; neither was there a three-way interaction among learning, group membership and face orientation, F(3,39) < 1.0, P = 0.533.
Comparing faces with houses, the N170 latency did not differ between the two for either the first, F(1,13) < 1.0, P = 0.491, or the third target detection task, F(1,13) < 1.0, P = 0.567 (Figure 3A).
N170 amplitude
A similar 2 (learning) × 4 (group membership) × 2 (face orientation) × 2 (hemisphere) repeated measures ANOVA was performed on the N170 amplitude. The N170 was larger on the right than on the left in both conditions [F(1,13) = 9.4, P = 0.009, 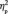 = 0.421; F(1,13) = 7.5, P = 0.017,
= 0.421; F(1,13) = 7.5, P = 0.017, 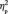 = 0.366] and was larger after social category learning, F(1,13) = 21.3, P < 0.001,
= 0.366] and was larger after social category learning, F(1,13) = 21.3, P < 0.001, 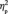 = 0.622 (Figure 4A). The face stimuli however did not differ from each other in the N170 amplitude either before, F(3,39) < 1.0, P = 0.756, or after participants learned their group memberships, F(3,39) < 1.0, P = 0.894. Neither did group membership interact with face orientation [F(3,39) =1.1, P = 0.361; F(3,39) < 1.0, P = 0.477] or with hemisphere [F(3,39) < 1.0, P = 0.743; F(3,39) < 1.0, P = 0.572] in either condition.
= 0.622 (Figure 4A). The face stimuli however did not differ from each other in the N170 amplitude either before, F(3,39) < 1.0, P = 0.756, or after participants learned their group memberships, F(3,39) < 1.0, P = 0.894. Neither did group membership interact with face orientation [F(3,39) =1.1, P = 0.361; F(3,39) < 1.0, P = 0.477] or with hemisphere [F(3,39) < 1.0, P = 0.743; F(3,39) < 1.0, P = 0.572] in either condition.
Fig. 4.
The N170 amplitudes (A) for each category of face before and after social category learning, and for houses. The graphs in (B) show that after participants learned the social categories of the face stimuli, the overall N170 amplitude increased, and the FIE was significantly enhanced. Legend: in–in (double in-group; Canadian Brock); in–out (in-/out-group; Canadian non-Brock); out–in (out-/in-group; non-Canadian Brock); out–out (double out-group; non-Canadian non-Brock). Error bars represent the s.e.m.
When the FIE on the N170 amplitude (i.e. a larger N170 to inverted than to upright faces) was examined for the first and for the third target detection task, it was found in both conditions [F(1,13) = 9.7, P = 0.008, 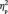 = 0.427, for the first target detection task; F(1,13) = 13.0, P = 0.003,
= 0.427, for the first target detection task; F(1,13) = 13.0, P = 0.003, 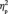 = 0.500, for the third target detection task]. This inversion effect was, however, greater after the acquisition (−0.82 µV) than before the acquisition (−0.53 µV) of the social categories (Figure 4B), indicated by an interaction between learning and face orientation, F(1,13) = 6.3, P = 0.026,
= 0.500, for the third target detection task]. This inversion effect was, however, greater after the acquisition (−0.82 µV) than before the acquisition (−0.53 µV) of the social categories (Figure 4B), indicated by an interaction between learning and face orientation, F(1,13) = 6.3, P = 0.026, 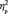 = 0.328. This effect of a larger N170 inversion effect after social category learning was similar across the face stimuli, F(3,39) = 1.1, P = 0.352, and did not differ between the left and the right electrode sites, F(1,13) < 1.0, P = 0.560.
= 0.328. This effect of a larger N170 inversion effect after social category learning was similar across the face stimuli, F(3,39) = 1.1, P = 0.352, and did not differ between the left and the right electrode sites, F(1,13) < 1.0, P = 0.560.
Comparing faces with houses, the N170 amplitude was larger for faces than for houses in both the first, F(1,13) = 4.6, P = 0.052, 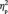 = 0.261, and the third target detection task, F(1,13) = 24.2, P < .001,
= 0.261, and the third target detection task, F(1,13) = 24.2, P < .001, 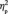 = 0.651 (Figure 4A), as would be expected considering the sensitivity of the N170 amplitude to faces (Bentin et al., 1996).
= 0.651 (Figure 4A), as would be expected considering the sensitivity of the N170 amplitude to faces (Bentin et al., 1996).
DISCUSSION
Two prominent theories have been proposed to account for the mechanisms underlying people’s difficulty in recognizing other-race faces, one with a perceptual explanation (Valentine, 1991) and the other focusing on social-cognitive factors (Levin, 2000). In most studies on the ORE, the perceptual expertise and the social cognitive factors are confounded. In this study, using the face-sensitive N170 ERP component, we focused on the social cognitive factors alone and controlled for perceptual expertise by testing only Caucasian participants with Caucasian faces. We found that in- and out-group memberships could influence the early neural correlates of face processing. To our knowledge, this is also the first demonstration of a social-cognitive influence on face processing in the N170 component. Our results are consistent with the general conclusion drawn from recent behavioral (Bernstein et al., 2007) and imaging studies (Van Bavel et al., 2008, 2011) when in-group and out-group memberships were similarly manipulated. Collectively, our results and these others have implications for understanding the ORE, suggesting the importance of taking social-cognitive processes into account when considering the ORE.
It is generally accepted that the N170 reflects neural processes associated with structural encoding of faces (Eimer, 2000). After participants learned the social categories, the N170 became delayed. While this result alone might be interpreted as a general effect of increased attention or perceptual familiarity as a result of participants viewing the same face stimuli after the learning phase, the interaction between social category learning and the specific group membership of a face suggests that this learning effect on the N170 latency is related to the processing of social information. After social category learning, the N170 latency did not change for the double in-group face, but was delayed for the other three types of faces, especially for the double out-group face.
The reasons for the slight, but reliable, increase in N170 latency for faces once they become associated with out-group status must remain speculative at this point, but we suggest two possibilities, one focusing on affective factors and the other on social information load. The first makes use of the finding that visual information is routed subcortically in the colliculo-pulvinar-amygdala pathway and that the amygdala can differentiate individual faces (Quiroga et al., 2005). The amygdala then feeds back enough information to the extrastriate visual cortex to influence early ERP components. We have invoked this model elsewhere to account for altered early visual ERP components, including a longer N170 latency to angry faces (Jetha et al., 2012). In this study, learning that a particular face is of someone representing an out-group, is equivalent to associating the person with the negative affective tag of an outsider, with an automatically perceived status of a negative bias (Hewstone et al., 2002). This subtle but automatic negative response might provide feedback to the visual cortex (Vuilleumier et al., 2004). This would complicate and thereby delay the full structural encoding of the face, even if by only a few milliseconds. Because the amygdala network reacts primarily to negative information, there would not be a similar slowing of the N170 for in-group stimuli.
A second possibility is to consider the attribution of out-group status to be one of increased social information load. This model assumes that the participants when presented the faces in the initial task (first block of the target detection task) assume that the faces represent in-group members, i.e. the default is to assume that the faces are of individuals similar to the ones they would meet normally, attending the same university and of the same nationality. With the learning of more information concerning an individual as not conforming to this default category, the processing of their face involves the additional social information tag, and this slightly slows the N170 process.
These two potential models are easily tested with experimental manipulations that compare the effects of social-category information on the N170 with a manipulation of the valence factor. Whatever speculation we have on potential neural mechanisms that should be examined in future research, this interaction between social category learning and group memberships on the N170 latency nevertheless provides strong ERP evidence for the social cognitive influence on the neural processes underlying the early stage of face processing. The slowing effect on the double-out group relative to the double-in group converges with ERP findings on the ORE that showed a delayed N170 for other-race faces than for own-race faces (Stahl et al., 2008, 2010; Wiese et al., 2009; Ofan et al., 2011). It is also important to note the similarity in the magnitude of the N170 latency difference between our study (5 ms between the double in-group and the double out-group faces) and previous studies on the ORE (∼3 ms in Stahl et al., 2008; 4 ms in Stahl et al., 2010; 3 ms in Wiese et al., 2009, between own- and other-race faces). A distinction however is that in this study, we only manipulated the group membership information, and controlled the perceptual expertise by using own-race faces only. The result that group membership alone can affect the N170 latency supports the influence of social-cognitive factors on face processing. Furthermore, because own- and other-race faces can also be classified as in- and out-group members that differ in many social dimensions (e.g. attitude and perception of self-similarity), our results suggest that the N170 latency difference reported previously between own- and other-race faces might be in part, if not entirely, due to the social categorization processes, especially when we consider the similar effect size between our results and the results of studies on ORE.
In addition to the N170 latency effect, we found that the effect of face inversion on the N170 amplitude became larger during the third compared with the first block of the target detection task. Behaviorally, the FIE (i.e. greater difficulty in recognizing faces when they are inverted) is a result of disruption in configural (Freire et al., 2000) and holistic (Farah et al., 1995) processing when faces are inverted; other-race faces, processed less holistically than own-race faces (Tanaka et al., 2004; Michel et al., 2006), show smaller inversion effects (Rhodes et al., 1989). For the N170 amplitude FIE, the specific neural mechanisms are not fully understood, especially as regards the extent to which ‘non-face’ brain areas are involved in the processing of inverted faces. However, finding that the N170 amplitude varies with behavioral performance in a face discrimination task when faces were presented at different orientations (Jacques and Rossion, 2007) suggests that the N170 inversion effect may also relate functionally to a disruption in configural and holistic processes. In light of these previous studies, the greater FIE on the N170 amplitude during the third block of the target detection task after participants learned the group memberships of the faces may suggest that when faces become socially meaningful, they might be processed more holistically. However, we realized that we need to be cautious with this conclusion. Because this larger FIE on the N170 amplitude did not interact further with group memberships and because a non-learning condition was not included in this study, it is as likely that non-social cognitive factors (e.g. stimulus habituation and perceptual familiarity) other than social learning might have accounted for this larger FIE on the N170 amplitude during the third block of the target detection task. Therefore, although it has been shown at a behavioral level that social information (e.g. group memberships) can alter the way in which faces are processed (Cassidy et al., 2011), the question of whether a similar social cognitive influence can also be found at a neural level requires future investigation by carefully controlling for other factors (e.g. perceptual familiarity).
Similarly, because the behavioral studies have demonstrated increased holistic processing when racially ambiguous morphed faces are judged as the same-race (Michel et al., 2007) and more configural processing when the same-race faces are presented as in-group members (Cassidy et al., 2011), a further differentiation in the N170 amplitude FIE might also be expected between the in-group and the out-group faces. However, we did not find this result in our study: the N170 amplitude inversion effect was similar across faces after the acquisition of their group memberships. This discrepancy is possibly due to methodological differences in experimental designs, namely that compared with the large number of face stimuli used by others, there were only four individual faces in this study and participants viewed them many times throughout the experiment. As a result, although the four faces were different in terms of in-group and out-group membership, it is possible that they were all well scrutinized at an individual level. The behavioral results that participants were able to correctly identify the social categories of the face stimuli both during the social categorization task and at the end of the experiment have provided further support for this conclusion. In the behavioral studies, when participants were informed about the ORE and were encouraged to individuate other-race faces, the ORE could be abolished (Hugenberg et al., 2007; Rhodes et al., 2009), suggesting that individuation may change the way in which a face is perceived. Perhaps because of this likelihood that participants perceived the four faces at an individual level regardless of their group memberships, we did not see further differentiation of the N170 amplitude FIE among them. In addition, we think that this may also account for why we did not find a group membership effect on the N170 amplitude after social category learning. It is important to note however that although group memberships might not affect the way in which faces were processed due to the possible individuation in this study, group memberships may still be able to influence the speed of face processing in its very early stages, as indicated by our N170 latency results.
Related to the issue of individuation, our paradigm, using only four faces with which our participants should have become very familiar throughout the experiment, also differs from the paradigms for studying the ORE when large sets of unfamiliar own-race and other-race faces are used. Because of this issue of face familiarity, we may not be able to compare our results directly with the previous findings on ORE. However, we believe that our N170 results, showing that the membership affected the N170 latency even with own-race faces and after participants had become very familiar with the face stimuli, provides even stronger support for the social-cognitive influence on face processing. We would expect that with a large set of unknown own- and other-race faces, the effect of group memberships (such as race) would be stronger considering that the process of social categorization might be engaged to a greater extent for unfamiliar faces than familiar faces. This potential interaction between face familiarity and social categorization should be examined in the future research.
Finally, it is worth noting that we manipulated the group memberships of the face stimuli based on two social categories. We expected that creating group memberships through cross-categorization would provide a greater chance for us to detect the social cognitive influence on neural responses to faces than simply dividing group memberships along a single social category, because previous social psychological research has shown that people’s attitudes and perception of a group’s similarity to self change gradually from double in-group to double out-group (Crisp et al., 2003). Indeed, when we examined the second block of the target detection task after participants had only learned one social category (nationality or university affiliation), we found that neither N170 amplitude (P = 0.931) nor N170 latency (P = 0.758) was affected by group memberships (see Supplementary Material available online for more detailed results about the second block of the target detection task). This is in contrast to the group membership effect found on the N170 latency in the third block of the target detection task after participants learned both social categories of the face stimuli. These results suggest for future research the importance of using cross-categorization in studying the social cognitive influence on face processing, preferably with the social categories with which people may feel strongly affiliated.
In summary, previous research has suggested that the phenomenon of ORE is likely a result of both perceptual and social-cognitive influences, and needs to be understood with an integrative approach (see Young et al., 2011, for a recent review). Here, focusing on the social cognitive influence alone, we provide neural evidence for the effect of group membership on face processing that could occur within 200 ms after a person sees a face, slowing the processing of out-group faces. Future research is needed to relate these ERP results to individual differences in social attitudes and in social contact with other-race/group members. The malleability of these neural findings should also be examined through a variety of task manipulations using a wide range of face stimuli (e.g. male and female faces of different age and ethnic groups) and participants (e.g. non-Caucasians), and the results should have implications in a broader societal context.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We thank Drs Jane Dywan, Michael Busseri and Gordon Hodson for feedback on and suggestions for the article.
The work was supported by grants from the Natural Sciences and Engineering Research Council of Canada and the Canada Foundation for Innovation (to S.J.S.).
Footnotes
1When the participant was included in the ERP analyses, the results were similar to those reported.
REFERENCES
- Balas B, Nelson CA. The role of face shape and pigmentation in other-race face perception: an electrophysiological study. Neuropsychologia. 2010;48:498–506. doi: 10.1016/j.neuropsychologia.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E, McCarthy G. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–65. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein MJ, Young SG, Hugenberg K. The cross-category effect: mere social categorization is sufficient to elicit an own-group bias in face recognition. Psychological Science. 2007;18:706–12. doi: 10.1111/j.1467-9280.2007.01964.x. [DOI] [PubMed] [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77:305–27. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Caharel S, Montalan B, Fromager E, Bernard C, Lalonde R, Mohamed R. Other-race and inversion effects during the structural encoding stage of face processing in a race categorization task: an event-related brain potential study. International Journal of Psychophysiology. 2011;79:266–71. doi: 10.1016/j.ijpsycho.2010.10.018. [DOI] [PubMed] [Google Scholar]
- Caldara R, Thut G, Servoir P, Michel CM, Bovet P, Renault B. Face versus non-face object perception and the ‘other-race’ effect: a spatio-temporal event-related potential study. Clinical Neurophysiology. 2003;114:515–28. doi: 10.1016/s1388-2457(02)00407-8. [DOI] [PubMed] [Google Scholar]
- Cassidy KD, Quinn KA, Humphreys GW. The influence of ingroup/outgroup categorization on same- and other-race face processing: the moderating role of inter- versus intra-racial context. Journal of Experimental Social Psychology. 2011;47:811–7. [Google Scholar]
- Crisp RJ, Hewstone M, Richards Z, Paolini S. Inclusiveness and crossed categorization: effects on co-joined category evaluations of in-group and out-group primes. British Journal of Social Psychology. 2003;42:25–38. doi: 10.1348/014466603763276108. [DOI] [PubMed] [Google Scholar]
- Eimer M. The face-specific N170 component reflects late stages in the structural encoding of faces. NeuroReport. 2000;11:2319–24. doi: 10.1097/00001756-200007140-00050. [DOI] [PubMed] [Google Scholar]
- Farah MJ, Tanaka JW, Drain HM. What causes the face inversion effect? Journal of Experimental Psychology: Human Perception and Performance. 1995;21:628–34. doi: 10.1037//0096-1523.21.3.628. [DOI] [PubMed] [Google Scholar]
- Freire A, Lee K, Symons LA. The face-inversion effect as a deficit in the encoding of configural information: direct evidence. Perception. 2000;29:159–70. doi: 10.1068/p3012. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Schlegel K, Stoerig P. Effects of human race and face inversion on the N170: a cross-race study. Journal of Psychophysiology. 2008;22:157–65. [Google Scholar]
- Golby AJ, Gabrieli JDE, Chiao JY, Eberhardt JL. Differential responses in the fusiform region to same-race and other-race faces. Nature Neuroscience. 2001;4:845–50. doi: 10.1038/90565. [DOI] [PubMed] [Google Scholar]
- Herrmann M, Schreppel T, Jäger D, Koehler S, Ehlis A, Fallgatter A. The other-race effect for face perception: an event-related potential study. Journal of Neural Transmission. 2007;114:951–57. doi: 10.1007/s00702-007-0624-9. [DOI] [PubMed] [Google Scholar]
- Hewstone M, Rubin M, Willis H. Intergroup bias. Annual Review of Psychology. 2002;53:575–604. doi: 10.1146/annurev.psych.53.100901.135109. [DOI] [PubMed] [Google Scholar]
- Hugenberg K, Miller J, Claypool HM. Categorization and individuation in the cross-race recognition deficit: toward a solution to an insidious problem. Journal of Experimental Social Psychology. 2007;43:334–40. [Google Scholar]
- Jacques C, Rossion B. Early electrophysiological responses to multiple face orientations correlate with individual discrimination performance in humans. NeuroImage. 2007;36:863–76. doi: 10.1016/j.neuroimage.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Jetha MK, Zheng X, Schmidt LA, Segalowitz SJ. Shyness and the first 100 ms of emotional face processing. Social Neuroscience. 2012;7:74–89. doi: 10.1080/17470919.2011.581539. [DOI] [PubMed] [Google Scholar]
- Levin DT. Race as a visual feature: using visual search and perceptual discrimination tasks to understand face categories and the cross-race recognition deficit. Journal of Experimental Psychology: General. 2000;129:559–74. doi: 10.1037//0096-3445.129.4.559. [DOI] [PubMed] [Google Scholar]
- Meissner CA, Brigham JC. Thirty years of investigating the own-race bias in memory for faces: a meta-analytic review. Psychology, Public Policy, and Law. 2001;7:3–35. [Google Scholar]
- Michel C, Corneille O, Rossion B. Race categorization modulates holistic face encoding. Cognitive Science. 2007;31:911–24. doi: 10.1080/03640210701530805. [DOI] [PubMed] [Google Scholar]
- Michel C, Rossion B, Han J, Chung C-S, Caldara R. Holistic processing is finely tuned for faces of one’s own race. Psychological Science. 2006;17:608–15. doi: 10.1111/j.1467-9280.2006.01752.x. [DOI] [PubMed] [Google Scholar]
- Natu V, Raboy D, O’Toole AJ. Neural correlates of own- and other-race face perception: spatial and temporal response differences. NeuroImage. 2011;54:2547–55. doi: 10.1016/j.neuroimage.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Ofan RH, Rubin N, Amodio DM. Seeing race: N170 responses to race and their relation to automatic racial attitudes and controlled processing. Journal of Cognitive Neuroscience. 2011;23:3153–61. doi: 10.1162/jocn_a_00014. [DOI] [PubMed] [Google Scholar]
- Quiroga RQ, Reddy L, Kreiman G, Koch C, Fried I. Invariant visual representation by single neurons in the human brain. Nature. 2005;435:1102–7. doi: 10.1038/nature03687. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Brake S, Taylor K. Expertise and configural coding in face recognition. British Journal of Psychology. 1989;80:313–31. doi: 10.1111/j.2044-8295.1989.tb02323.x. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Locke V, Ewing L, Evangelist E. Race coding and the other-race effect in face recognition. Perception. 2009;38:232–41. doi: 10.1068/p6110. [DOI] [PubMed] [Google Scholar]
- Rossion B, Delvenne J-F, Debatisse D, et al. Spatio-temporal localization of the face inversion effect: an event-related potentials study. Biological Psychology. 1999;50:173–89. doi: 10.1016/s0301-0511(99)00013-7. [DOI] [PubMed] [Google Scholar]
- Schweinberger SR, Pickering EC, Jentzsch I, Burton AM, Kaufmann JM. Event-related brain potential evidence for a response of inferior temporal cortex to familiar face repetitions. Cognitive Brain Research. 2002;14:398–409. doi: 10.1016/s0926-6410(02)00142-8. [DOI] [PubMed] [Google Scholar]
- Stahl J, Wiese H, Schweinberger SR. Expertise and own-race bias in face processing: an event-related potential study. NeuroReport. 2008;19:583–7. doi: 10.1097/WNR.0b013e3282f97b4d. [DOI] [PubMed] [Google Scholar]
- Stahl J, Wiese H, Schweinberger SR. Learning task affects ERP-correlates of the own-race bias, but not recognition memory performance. Neuropsychologia. 2010;48:2027–40. doi: 10.1016/j.neuropsychologia.2010.03.024. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Kiefer M, Bukach CM. A holistic account of the own-race effect in face recognition: evidence from a cross-cultural study. Cognition. 2004;93:B1–9. doi: 10.1016/j.cognition.2003.09.011. [DOI] [PubMed] [Google Scholar]
- Tanaka JW, Pierce LJ. The neural plasticity of other-race face recognition. Cognitive, Affective, Behavioral Neuroscience. 2009;9:122–31. doi: 10.3758/CABN.9.1.122. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, et al. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Research. 2009;168:242–49. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentine T. A unified account of the effects of distinctiveness, inversion, and race in face recognition. The Quarterly Journal of Experimental Psychology. 1991;43A:161–204. doi: 10.1080/14640749108400966. [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA. The neural substrates of in-group bias: a functional magnetic resonance imaging investigation. Psychological Science. 2008;19:1131–9. doi: 10.1111/j.1467-9280.2008.02214.x. [DOI] [PubMed] [Google Scholar]
- Van Bavel JJ, Packer DJ, Cunningham WA. Modulation of the fusiform face area following minimal exposure to motivationally relevant faces: evidence of in-group enhancement (not out-group disregard) Journal of Cognitive Neuroscience. 2011;23:3343–54. doi: 10.1162/jocn_a_00016. [DOI] [PubMed] [Google Scholar]
- Vizioli L, Foreman K, Rousselet GA, Caldara R. Inverting faces elicits sensitivity to race on the N170 component: a cross-cultural study. Journal of Vision. 2010;10:1–23. doi: 10.1167/10.1.15. [DOI] [PubMed] [Google Scholar]
- Vizioli L, Rousselet GA, Caldara R. Neural repetition suppression to identity is abolished by other-race faces. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20081–6. doi: 10.1073/pnas.1005751107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P, Richardson MP, Armony JL, Driver J, Dolan RJ. Distant influences of amygdala lesion on visual cortical activation during emotional face processing. Nature Neuroscience. 2004;7:1271–8. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- Walker PM, Silvert L, Hewstone M, Nobre AC. Social contact and other-race face processing in the human brain. Social Cognitive and Affective Neuroscience. 2008;3:16–25. doi: 10.1093/scan/nsm035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiese H, Stahl J, Schweinberger SR. Configural processing of other-race faces is delayed but not decreased. Biological Psychology. 2009;81:103–9. doi: 10.1016/j.biopsycho.2009.03.002. [DOI] [PubMed] [Google Scholar]
- Young SG, Hugenberg K, Bernstein MJ, Sacco DF. Perception and motivation in face recognition: a critical review of theories of the cross-race effect. Personality and Social Psychology Review. 2011;16:116–42. doi: 10.1177/1088868311418987. [DOI] [PubMed] [Google Scholar]
- Zheng X, Mondloch CJ, Segalowitz SJ. The timing of individual face recognition in the brain. Neuropsychologia. 2012;50:1451–61. doi: 10.1016/j.neuropsychologia.2012.02.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.