Abstract
Besides food restriction, hyperactivity is considered a key behavioral trait of anorexia nervosa (AN), playing a major role in the pathogenesis and progression of the disorder. However, the underlying neurophysiology remains poorly understood. We used functional magnetic resonance imaging during two affective go/no-go tasks to probe inhibitory control in response to stimuli depicting physical activity vs inactivity and food vs non-food in AN patients compared with 26 healthy athlete and non-athlete controls. We hypothesized that neural correlates of behavioral inhibition are biased by the emotional information of the stimuli in AN patients, leading to a differential neural inhibitory pattern during the two tasks. Indeed, we found reduced response inhibition for food and non-food images in the putamen, while stimuli depicting physical activity resulted in an exaggerated response of the prefrontal cortex (PFC) and cerebellum in AN patients. However, both AN patients and athletes revealed an increased response in the somatosensory cortex to physical activity stimuli. These results suggest that physical activity stimuli might place an increased demand on the inhibitory control system in AN patients. The resulting hyperactivity of the PFC and cerebellum may lead to altered executive function and motor control, sustaining increased physical activity in AN patients.
Keywords: anorexia nervosa, hyperactivity, inhibitory control, fMRI; reward, eating disorder
INTRODUCTION
Anorexia nervosa (AN) is a devastating condition with one of the highest mortality rates among psychiatric disorders (Zipfel et al., 2000), which commonly begins during adolescence in women and is characterized by severe emaciation (APA, 2000). However, the etiology of the disease is still largely unknown and mechanisms that maintain the disorder remain poorly understood. Physical hyperactivity is an ubiquitous symptom of AN and plays a central role in the pathogenesis and progression of the disorder (Davis, 1997), as it is associated with poorer recovery rates, higher rates of relapse and longer periods of hospitalization (Casper and Jasbine, 1996; Strober et al., 1997; Carter et al., 2004). It was recently recommended that hyperactivity should be recognized as a core psychopathology of AN (Hebebrand and Bulik, 2011).
Functional brain imaging (fMRI) studies of patients with AN have identified altered brain functions especially when viewing rewarding stimuli, resulting in reduced activations in mesolimbic reward regions in conjunction with increased prefrontal cortex (PFC) activations (Kaye et al., 2009; Pietrini et al., 2011; Brooks et al., 2012b). Particularly food or body images, which are considered to provoke core symptoms of AN, have been used in a wide range of fMRI studies reporting a prominent increase in PFC activity (Uher et al., 2004; van Kuyck et al., 2009; Brooks et al., 2011).
However when challenging cognitive control, reduced prefrontal activity has been revealed in AN patients compared with healthy controls (Zastrow et al., 2009; Lock et al., 2011; Oberndorfer et al., 2011). Zastrow et al. (2009) reported decreased activity in limbic, anterior cingulate and prefrontal regions during a target detection task in AN patients, indicating a possible interaction between limbic and frontal networks to exert inhibitory control. Furthermore, using a validated inhibition task, it has been shown that recovered AN patients need less medial PFC activation as inhibitory load increased compared with healthy controls (Oberndorfer et al., 2011). Therefore, it has been assumed that AN patients require less inhibitory resources to maintain behavioral performance. Since these studies used a general inhibition task without symptom-provoking stimuli, no conclusion can be drawn if this altered inhibition is biased by stimulus category.
In this study, we used affective go/no-go tasks during fMRI that implemented ‘symptom-provoking’ stimuli to characterize the neural correlates of response inhibition in AN. This is to our knowledge the first study to investigate response inhibition to stimuli depicting physical activity vs inactivity and food vs non-food in AN patients. The fMRI paradigm isolated brain regions associated with withholding a prepotent response to food and physical activity-related stimuli. We hypothesized that neural correlates of behavioral inhibition are biased by the emotional information of the stimuli in AN patients, leading to a differential neural inhibitory pattern in the two affective go/no-go tasks. We evaluated the inhibitory response of AN patients compared with two healthy controls groups, displaying different levels of physical activity: healthy non-athletes (HC) and healthy athletes (HCA). We predicted a similar inhibitory response between AN and athletes in sensorimotor brain regions.
METHODS
Participants
Twelve female individuals with AN (mean age = 23.3 ± 4.7 years) were recruited from the inpatient, daypatient and outpatient service programs of the Department of Psychosomatic Medicine and Psychotherapy at the University Hospital of Tübingen. We used the Eating Disorder Examination (EDE) to diagnose eating disorder and the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (Fairburn and Cooper, 1993; Wittchen et al., 1997) to diagnose comorbid Axis I disorders in patients. Patients were excluded from the study for the following reasons: body mass index (BMI) <12 kg/m2, intake of neuroleptics or benzodiacepines, a primary obsessive–compulsive or affective disorder, psychosis, bipolar disorder and substance abuse or addiction according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV). Of the 12 individuals with AN, 8 were identified to belong to the restrictive subtype and 4 to the binge/purging subtype. One patient was on psychoactive medication [Trevilor selective serotonin-noradrenaline-reuptake-inhibitor].
Twenty-six age-matched healthy female participants of normal weight were recruited through local advertisement for two healthy control groups. One control group consisted of healthy endurance athletes (HCA, 12 subjects; mean age = 24.1 ± 3.2 years), required to perform competitively exercise in an endurance sport of at least 5 h a week for at least 1 year. The other control group consisted of healthy non-athletes, only included when performing casual physical exercise (HC, 14 subjects; mean age = 24.6 ± 2.9 years). As assessed by the SCID-I, the healthy female subjects had no history of an eating disorder or any other psychiatric, serious medical or neurological diseases and were not on any psychoactive medication. All participants had normal or corrected-to-normal vision. Written informed consent was obtained from all participants, as approved by the ethics committee of the medical faculty of the University of Tübingen.
Procedure
Participants completed several self-report assessments related to eating disorder symptoms [Eating Disorder Inventory-2 (EDI-2)] (Garner, 1991; Paul and Thiel, 2005), depression (Patient Health Questionnaire Depression Scale) (Löwe et al., 2002), reward sensitivity and behavioral inhibition [behavioral activation/behavioral inhibition system (BAS/BIS)] (Gray, 1970; Strobel et al., 2001), excessive exercise [Commitment to Exercise Scale (CES)] (Davis et al., 1993) and anxiety (State-Trait Anxiety Inventory) (Spielberger et al., 1983). Participants had a standardized breakfast (staff supervised) 1 h before the fMRI measurement, consisting of a bread role with butter, jam or honey and an herbal tea. Prior to breakfast, blood samples were taken to measure leptin levels. In addition, hunger was assessed by a 10 cm visual analog scale ranging from 0 cm (not hungry at all) to 10 cm (strongest feeling of hunger) just before the fMRI measurement. Demographic and clinical characteristics of the patient group and healthy control groups are shown in Table 1.
Table 1.
Participants’ characteristics
| Female AN patients (AN) (n = 12) |
Female non-athletes (HC) (n = 14) |
Female athletes (HCA) (n = 12) |
Analysis |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | Mean | s.d. | Mean | s.d. | Mean | s.d. | F | df | P | Post hoc difference |
| Age (years) | 23.3 | 4.7 | 24.6 | 2.9 | 24.1 | 3.2 | 0.379 | 35 | 0.687 | – |
| Current BMI (kg/m2) | 15.5 | 1.5 | 21.4 | 1.5 | 22.0 | 1.9 | 57.87 | 35 | <0.001 | AN < HCA, HC |
| Leptin (ng/dl) | 0.7 | 0.4 | 5.89 | 3.2 | 4.46 | 3.6 | 10.691 | 34 | <0.001 | AN < HCA, HC |
| Hunger rating (cm) | 0.5 | 0.7 | 0.7 | 0.7 | 1.0 | 1.3 | 1.07 | 35 | 0.352 | – |
| Commitment to exercise scale (CES) | 6.5 | 2.6 | 4.12 | 1.9 | 5.55 | 1.6 | 4.329 | 35 | 0.021 | AN > HC |
| Behavioral activation score (BAS) | 3.1 | 0.4 | 3.18 | 0.4 | 3.24 | 0.2 | 0.455 | 35 | 0.638 | – |
| Behavioral inhibition score (BIS) | 3.5 | 0.5 | 2.98 | 0.4 | 2.83 | 0.5 | 7.247 | 35 | 0.002 | AN > HCA, HC |
| Depression sum score | 11.3 | 4.5 | 1.8 | 1.5 | 1.8 | 1.9 | 44.512 | 35 | <0.001 | AN > HCA, HC |
| State anxiety score | 61.0 | 10.4 | 31.9 | 6.7 | 32.7 | 5.6 | 56.198 | 35 | <0.001 | AN > HCA, HC |
| EDI-2 | 309.8 | 54.68 | 186.57 | 36.92 | 194.08 | 54.68 | 32.127 | 35 | <0.001 | AN > HCA, HC |
| EDEQ | 3.43 | 1.46 | ||||||||
Data are presented as mean ± s.d. P-values for comparison of unadjusted data by ANOVA. AN, anorexia nervosa patient; HN, healthy non-athlete control group; HCA, healthy athlete control group; EDEQ, Eating Disorder Examination Questionnaire; EDE -2, Eating Disorder Inventory.
Stimuli
Examples of food images included different kind of meals, such as salad, meat or soup, desserts, fruits and vegetables (Porubska et al., 2006; Frank et al., 2010; Guthoff et al., 2010). Examples of non-food images included objects that have no association with eating, such as books, cars, money, chair or umbrella. Pictures were selected to be matched for complexity, valence and arousal across categories (Frank et al., 2010). For the physical activity paradigm, 52 images were divided into physically active and inactive stimuli depending on the state of activity of the displayed person, resulting in 26 pairs closely matched for color, brightness and visual complexity. In a pre-experiment, pictures were tested for valence, arousal and, to ensure distinctiveness of stimulus category, the physical strain of each position was estimated.
fMRI task
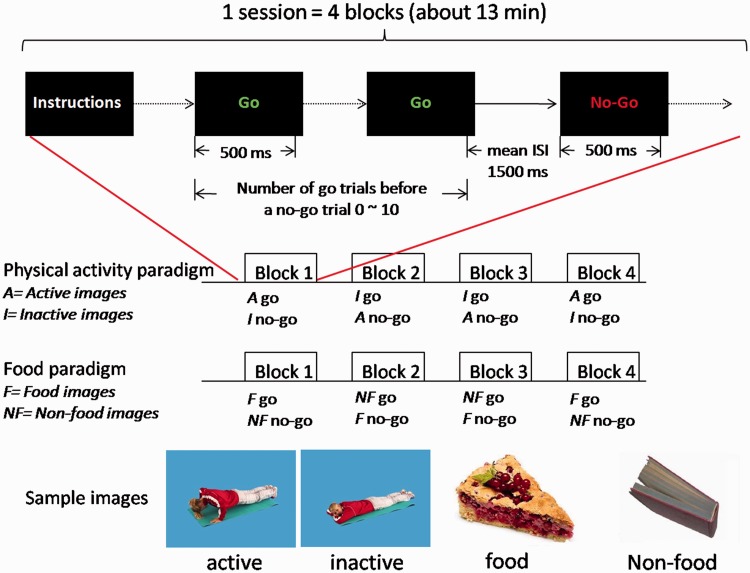
The affective go/no-go paradigm (Schulz et al., 2007; Batterink et al., 2010) was designed to examine inhibition of responses to physical activity vs inactivity and food vs non-food stimuli. The experiment consisted of two sessions each lasting 13 min. In one session (food/non-food paradigm), food and non-food stimuli were used, resulting in four blocks with the following trials: (i) go food/no-go non-food and (ii) go non-food/no-go food. In the other session (physical activity paradigm), physically active and inactive stimuli were used, resulting in four blocks with the following trials: (i) go active /no-go inactive and (ii) go inactive /no-go active. The affective go/no-go tasks required subjects to respond as quickly as possible with a button press to ‘go’ stimuli and withhold responses to ‘no-go’ stimuli. Each session consisted of four blocks, with 100 trials (go trial, 75% occurrence; no-go trial, 25% occurrence). Sessions and blocks appeared in a pseudo-randomized order. Stimuli were presented for 500 ms. The interstimulus interval was on average 1500 ms. Before a no-go trial appeared, 0–10 go trials were presented. At the beginning and end of each block, a 30 s fixation period was added followed by instructions for the next block. Before the imaging session, participants were familiarized with the fMRI environment and paradigm through a practice session with additional stimulus material. Figure 1 gives an overview of the study protocol used.
Fig. 1.
Study protocol of fMRI go/no-go task. The experiment consisted of two sessions, a physical activity and food/non-food paradigm. Each session consisted of four blocks, with 100 trials (go trial, 75% occurrence; no-go trial, 25% occurrence). For the physical activity paradigm, participants were either instructed to respond to active or inactive images. For the food/non-food paradigm, participants were either instructed to respond to food or non-food images.
fMRI acquisition
Whole-brain fMRI data were obtained by using a 3.0 T scanner (Siemens Tim Trio, Erlangen, Germany). For the two go/no-go sessions, functional data were collected by using echo-planar imaging sequence: TR = 2 s, TE = 30 ms, FOV = 210 mm2, matrix 64 × 64, flip angle 90°, voxel size 3 × 3 × 3 mm3, slice thickness 3 mm, and the images were acquired in an interleaved order. Each brain volume comprised 30 axial slices and each functional run contained 386 image volumes, resulting in a total scan time of 12.56 min. In addition, high-resolution T1 weighted anatomical images (MPRage: 176 slices, matrix: 256 × 240 × 192, 1 × 1 × 1 mm3) of the brain were obtained.
Voxel-based morphometry
The T1 weighted images were processed and examined using the VBM8 toolbox with default parameters (http://dbm.neuro.uni-jena.de/vbm.html) implemented in the SPM8 software (Wellcome Department of Imaging Neuroscience Group, London, UK; http://www.fil.ion.ucl.ac.uk./spm). Total GM, WM CSF and total intracranial volumes were extracted. The modulated volumes were smoothed with a Gaussian kernel of 10 mm full width at half maximum (FWHM). Whole-brain tissue volume data were examined by means of one-way analysis of variance (ANOVA) in SPSS (P < 0.05). Voxel-wise GM differences between AN patients and healthy control groups were examined by means of one-way ANOVA with group as the between subject factor in SPM8. Results were thresholded at P < 0.05 family-wise error corrected (FWE).
Data analysis
fMRI data
Event-related analysis of the fMRI data was performed with SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Standard preprocessing including realignment, coregistration to the anatomical T1 weighted image, normalization into MNI space (3 mm isotrop voxel size) and Gaussian special smoothing (FWHM: 10 mm) was performed. fMRI data were highpass (cut-off period 128 s) filtered, and global AR(1) auto correlation correction was performed.
For each subject, a first-level analysis was applied for the food/non-food and physical activity paradigm. The following condition types were included for the food/non-food paradigm: correct go trials food, correct go trials non-food, correct no-go trials food, correct no-go trials non-food and all incorrect trials. The following condition types were included for the physical activity paradigm: correct go trials active, correct go trials inactive, correct no-go trials active, correct no-go trials inactive and all incorrect trials. For each condition, a separate regressor was modeled using a canonical hemodynamic response function. The movement parameters were modeled as confounds. To examine inhibition, ‘no-go vs go’ contrast images were calculated for each subject for the food and non-food condition in the food/non-food paradigm and for the active and inactive condition in the physical activity paradigm.
The individual contrast images were entered into separate second-level analysis for the food/non-food and physical activity paradigm using a full factorial design with the between-subject factor group (AN vs HC vs HCA) and within-subject factor stimuli (food vs non-food or active vs inactive) and using total intracranial volume as a confounding covariate. Contrasts for group, stimuli and for group-by-stimulus interaction were created. A threshold of P < 0.05 FWE-corrected for multiple comparisons, at cluster level, was considered as statistical significant.
Behavioral data
For each subject, mean reaction times (RTs) for go trials and for no-go trials (that were incorrectly responded to) were calculated. A commission error is a ‘go’ response for no-go trials. Mean rate of commission error was calculated as the total number of failures of inhibition divided by the total number of no-go trials. An omission error is no response in go trials. The mean rate of omission errors was calculated as the total number of failures of response divided by the total number of go trials. Repeated measurement ANOVA with group (AN, HC and HCA) as the between-subject factor and stimuli (food and non-food or active and inactive) as the within-subject factor was used to examine differences in RT, commission and omission error. For all self-reported assessments (Table 1), one-way ANOVA with group as the between-subject factor was used to examine differences between AN, HC and HCA. In case of significant group differences, post hoc tests were calculated (Bonferroni corrected). Pearson correlations (two-sided) were calculated between behavioral measurements and neural response inhibition (P < 0.05).
RESULTS
Group differences in global GM and WM volume
There was a significant group difference in global GM volume (one-way ANOVA, P = 0.039). Post hoc tests, however, only revealed a statistical trend, such that AN (mean = 631.41, s.d. = 51.33) had less total GM volume than HC (mean = 678.64, s.d. = 58.40) (P = 0.07) and HCA (mean = 679.21, s.d. = 39.76) (P = 0.08). There was no difference between HC and HCA in total GM volume (P = 0.1). There was no significant difference in overall WM volume among the three groups (P = 0.417). There were no significant voxel-wise GM differences between AN patients and healthy control groups (P > 0.05, FWE-corrected).
fMRI results
Neural correlates of response inhibition to food and non-food stimuli
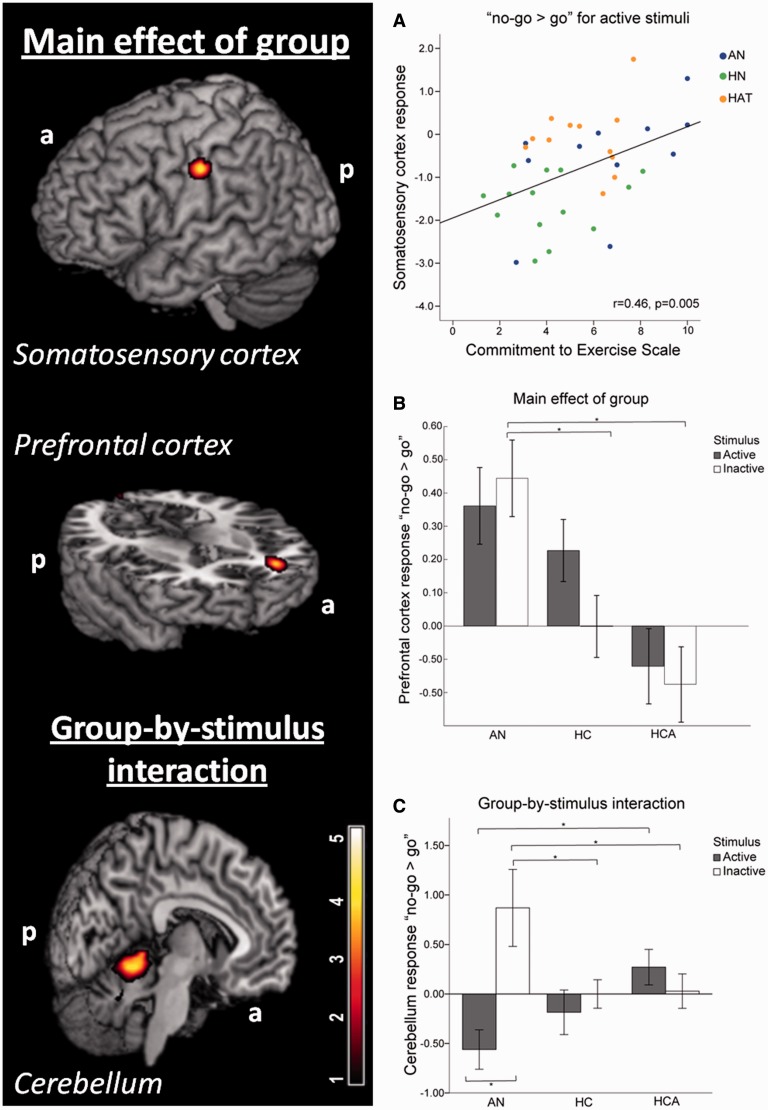
Brain activations during response inhibition (correct no-go minus go trials) across subjects revealed a distributed inhibitory network, with increased activations for no-go minus go trials in the fusiform gyrus, insula, supplementary motor area and regions of the temporal and frontal cortex (Supplementary Table S1). Response inhibition to food compared with non-food stimuli resulted in a higher activation of the insula, middle frontal gyrus, fusiform gyrus and inferior parietal gyrus. Response inhibition to non-food compared with food stimuli, on the other hand, lead to an increased activation of the middle temporal gyrus, posterior cingulum and medial orbital frontal gyrus across subjects (Supplementary Table S1). Significant group differences in response inhibition were found in the right putamen (x: 27, y: 3, z: −6; Table 2). Post hoc analysis revealed reduced response inhibition in AN compared with HCA and HC for food and non-food stimuli (P < 0.05, Bonferroni corrected; Supplementary Table S2). Further correlation analyses revealed a significant negative correlation between the total EDI-2 score and putamen activity during response inhibition for food (r = −0.366; P = 0.024) and non-food trials (r = −0.396, P = 0.014). A positive correlation was also observed between the correct go responses and putamen activity for food (r = 0.345; P = 0.034) and non-food stimuli (r = 0.410; P = 0.011).
Table 2.
Brain activations that differed significantly across female adults with AN and age-matched female athlete (HCA) and non-athlete control subjects (HC) during response inhibition (no-go vs go trials) for the food and physical activity paradigm
| Activated region | Side | Brodmann’s area | Number of voxels | Peak location (x, y, z)a | t-Value df = 69 | P-value* |
|---|---|---|---|---|---|---|
| Food/non-food paradigm | ||||||
| Main effect of group | ||||||
| Putamen | Right | 20 | 27, 3, −6 | 4.77 | 0.01 | |
| Physical activity paradigm | ||||||
| Main effect of group | ||||||
| Somatosensory cortex | Left | 3 | 40 | −57, −15, 39 | 4.70 | 0.004b |
| PFC | Left | 10 | 8 | −24, 45, 6 | 3.76 | 0.026b |
| Group-by-stimulus interaction | ||||||
| Cerebellum | Left | 48 | −6, −51, −9 | 3.85 | 0.008 | |
*P < 0.05, FWE-corrected for multiple comparison.
aMontreal Neurological Institute.
bSmall volume corrected.
Neural correlates of response inhibition to physical activity stimuli
Brain activations during response inhibition (correct no-go minus go trials) across subjects revealed a distributed inhibitory network, with increased activations for no-go minus go trials in the insula, supplementary motor area, putamen and regions of the temporal and frontal cortex (Supplementary Table S1). Response inhibition to active compared with inactive stimuli resulted in higher activation of the middle frontal gyrus. Response inhibition to inactive compared with active stimuli revealed increased activation in regions of the occipital and parietal cortices across subjects (Supplementary Table S1). Significant group differences in response inhibition were revealed in the left somatosensory cortex (x: −57, y: −15, z: 39) and left PFC (x: −24, y: 45, z: 6) (Figure 2; Table 2). Post hoc analysis revealed an increased response inhibition in AN and HCA compared with HC for active and inactive stimuli in the somatosensory cortex (P < 0.05, Bonferroni corrected; Supplementary Table S2). This response correlated significantly with the commitment to exercise scale (CES), such that individuals with higher CES showed a stronger response inhibition to active stimuli in the somatosensory cortex (r = 0.46; P = 0.005) (Figure 2, Plot A). Furthermore, a positive correlation was observed between the correct go responses and somatosensory activity for active (r = 0.443; P = 0.006) and marginally significant for inactive physical activity stimuli (r = 0.310; P = 0.062).
Fig. 2.
Differential response inhibition (no-go vs go) during the physical activity paradigm in AN patients compared with HCA and HC control subjects in the somatosensory cortex, PFC and cerebellum. (A) AN and HCA showed increased response inhibition in the somatosensory cortex for active physical activity images compared with HC, which positively correlated with the score on commitment to exercise. (B) AN showed increased response inhibition in the PFC compared with both control groups (HC and HCA) (*P < 0.05). (C) For the response inhibition in the cerebellum a significant group-by-stimulus interaction was found. Post hoc analyses revealed significant between-group and within-group differences for the AN patients (*P < 0.05).
In the PFC, AN patients revealed an increased response inhibition to inactive stimuli in comparison with both healthy control groups (Figure 2, Plot B) (P < 0.05, Bonferroni corrected; Supplementary Table S2). This response showed a significant positive correlation with the total EDI-2 score (r = 0.383; P = 0.018) and correct no-go responses for inactive physical activity stimuli (r = 0.329; P = 0.046). Furthermore, we observed a group-by-stimulus interaction in the left cerebellum (x: −6, y: −51, z: −9; Figure 2, Plot C; Table 2). Post hoc analyses revealed significant between- and within-group differences. Within the AN group, we found a significant difference between the cerebellum response inhibition to active and inactive, resulting in a increased response inhibition to inactive stimuli and in a reduced response inhibition to active stimuli (P = 0.01). Between-group post hoc analyses are summarized in Supplementary Table S3 (P < 0.05, Bonferroni corrected). Furthermore, there was a significant positive correlation between commission error for the inactive stimuli and cerebellum activity (r = 0.356, P = 0.03) and negative correlation between commission error for active stimuli and cerebellum activity (r = 0.323, P = 0.05). And a significant positive correlation was observed between the correct no-go responses and cerebellum activity for the inactive stimuli (r = −0.356; P = 0.031).
Behavioral results
We found no group differences for RTs (ms), commission and omission errors (%) (Supplementary Table S3). However, we did observe differences depending on stimulus category across all subjects, resulting in faster RTs for correct food compared with non-food go trials (meanFood = 453.05, s.d. = 49.85; meanNon-Food = 504.29, s.d. = 59.5; P < 0.001) faster RTs for correct active compared with inactive go trials (meanActive = 515.69, s.d. = 56.66; meanInactive = 531.80, s.d. = 55.76; P = 0.006) and faster RTs for incorrect inactive compared with active sport responses (meanActive = 514.59, s.d. = 65.29; meanInactive = 490.68, s.d. = 83.13; P = 0.005). Furthermore, we observed a significant higher commission error for food than non-food stimuli (meanFood = 31.28, s.d. = 16.13; meanNon-Food = 15.73, s.d. = 12.5; P < 0.001), a higher omission error for non-food than food (meanFood = 2.18, s.d. = 3.08; meanNon-Food = 13, s.d. = 11.08; P < 0.001) stimuli and a higher omission error for inactive compared with active stimuli (meanActive = 1.89, s.d. = 2.76; meanInactive = 3.21, s.d. = 3.63; P = 0.035) across all subjects. Mean RTs for correct and incorrect responses (overall stimuli) correlated negatively with BMI in the control groups, such that a higher BMI showed significantly faster RT (P < 0.01). For the physical activity paradigm, we found a significant group-by-stimulus interaction for the commission error (P = 0.047). Post hoc analyses revealed a significant higher commission error for active compared with inactive stimuli in AN patients only (meanActive = 24, s.d. = 12.08; meanInactive = 19, s.d. = 11.27; P = 0.038).
DISCUSSION
This is to our knowledge the first study investigating neural correlates of response inhibition to stimuli depicting physical activity and food in AN patients compared to healthy non-athletes and athlete controls. To probe the inhibitory network, we used affective go/no-go tasks during fMRI. We were able to show that altered inhibitory function in AN depends on stimulus category. For the food/non-food paradigm, we found a hypoactivation in the putamen independent of stimulus category in the AN patients compared with both control groups. In contrast, a hyperactivation in the prefrontal, somatosensory and cerebellum was specifically manifested when AN patients were asked to inhibit prepotent responses during the physical activity paradigm. Group differences in the putamen and PFC significantly correlated with the eating disorder pathology. Due to the intensive exercise in both AN and HCA groups, we predicted a similar response inhibition to images depicting physical activity in sensorimotor brain regions. Indeed both AN patients and athletes showed a hyperactivation in the somatosensory cortex in response to physical activity stimuli.
AN patients seem to require less inhibitory resources to maintain behavioral performance for the affective go/no-go task using food and non-food stimuli. As previously shown, the putamen is prominent in complex go/no-go tasks (Simmonds et al., 2008); furthermore, it is part of the limbic system essential in reward processing. Therefore, the hypoactivity in the putamen could also reflect altered dopamine dysfunction (Rodriguez et al., 2007; Kaye et al., 2009), which could potentially result in altered reward processing, executive control as well as hyperactivity. Indeed, AN patients with a stronger eating disorder pathology (i.e. higher EDI-2 scores) showed a more pronounced putamen decrease.
Interestingly, for the affective go/no-go task using physical activity stimuli, AN patients showed increased activity in regions important for response inhibition especially in the PFC (BA 10) compared to both healthy control groups, which significantly correlated with the eating disorder pathology (EDI-2 scores). This stands in contrast to previous studies showing that AN individuals require less inhibitory resources to maintain behavioral performance resulting in reduced prefrontal activity (Zastrow et al., 2009; Oberndorfer et al., 2011). However, these studies did not use specific physical activity-related stimuli as in this study. We propose that AN patients perceived physical activity stimuli as more rewarding than the control group, placing an increased demand on the inhibitory control system. Therefore, physical activity-related stimuli could lead to an augmented prefrontal inhibitory response. Accordingly, a recent fMRI study found increased activity in the reward system in response to visual stimuli depicting underweight women, challenging the view of general anhedonia in AN (Fladung et al., 2010). Instead, these observations are consistent with theories of starvation dependence in AN, potentially leading to increased reward processing for stimuli related to weight control.
In the somatosensory cortex, we found increased response inhibition for physical activity stimuli in AN patients as well as in our athlete control group compared with the non-athlete control group. Of note, this activity correlated significantly with scores on commitment to exercise indicating that a higher dedication to exercise results in an enhanced sensory processing of physical activity stimuli during inhibitory control (Falconer et al., 2008). The fact that athletes and patients with AN show this neural pattern suggests a possible increased body awareness as a result of extensive exercise. However, AN patients also showed altered prefrontal activity during response inhibition. Together with increased sensory processing, we hypothesize that this could potentially lead to a distorted body size evaluation. However, further studies are needed to evaluate the relationship between somatosensory and PFC activity during response inhibition.
Finally, we found a significant group by stimulus interaction in the cerebellum during the affective go/no-go task using physical activity stimuli, leading to an unexpected differential pattern depending on stimulus category. Only in AN patients, physically inactive stimuli resulted in an increased response inhibition associated activity, whereas physically active stimuli resulted in a reduced activity in the cerebellum. Interestingly, this activation correlated significantly with the respective commission error. Even though all groups showed the same behavioral performance, AN patients revealed higher commission errors for active than inactive stimuli. Since the AN patient group showed hyperactivity, the active stimuli probably had a higher personal salience (i.e. were perceived as more rewarding) leading to the increased error rate. Previous neuroimaging studies have clearly shown that the cerebellum, besides its involvement in motor control, is associated with various higher cognitive processes. Cerebellar activation is related to attention (Bonnet et al., 2009), working memory load (Desmond et al., 1997) and interestingly also in feeding control (Zhu and Wang, 2008; Brooks et al., 2012a). Furthermore, lesions in the cerebellum can lead to impairment of executive functions and personality changes such as disinhibited behavior (Baldacara et al., 2008). The differential response inhibition in the cerebellum could reflect the general difficulty of the AN group to disinhibit motor response especially to stimuli displaying an ‘active’ person.
Limitations
Our study sample was too small to examine differences between restricting vs purging subgroups. Further studies are needed to evaluate differences between these subtypes with respect to hyperactivity.
CONCLUSION
This is the first study evaluating response inhibition in AN in comparison with healthy athletes and non-athletes. Previous studies have shown that AN patients need less inhibitory resources to maintain behavioral performance. Likewise, we found reduced response inhibition in the putamen using a go/no-go task with food and non-food stimuli. In contrast, stimuli depicting physical activity resulted in increased response inhibition in the PFC, somatosensory cortex and cerebellum indicating that AN patients need more inhibitory resources to maintain behavioral performance in response to physical activity stimuli. We postulate that the reduced response inhibition in the putamen may be due to dopamine dysfunction, while physical activity-related stimuli are considered more rewarding, placing an increased demand on the inhibitory control system in AN patients. The resulting hyperactivation of the inhibitory system could potentially alter executive function and motor control.
SUPPLEMENTARY DATA
Supplementary data are available at SCAN online.
Conflict of Interest
None declared.
Supplementary Material
Acknowledgments
We gratefully thank Karoline Pfeiffer and Eva Schäflein for their help with patient recruitment; and Maike Borutta and Katrin Stingel for excellent technical support. This work was funded by a grant from the Center of Nutritional Medicine Tübingen/Hohenheim (grant number: ZEM 24.A II-08).
REFERENCES
- Baldacara L, Borgio JG, Lacerda AL, Jackowski AP. Cerebellum and psychiatric disorders. Revista Brasileira de Psiquiatria. 2008;30:281–9. doi: 10.1590/s1516-44462008000300016. [DOI] [PubMed] [Google Scholar]
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52:1696–703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet MC, Dilharreguy B, Allard M, Deloire MS, Petry KG, Brochet B. Differential cerebellar and cortical involvement according to various attentional load: role of educational level. Human Brain Mapping. 2009;30:1133–43. doi: 10.1002/hbm.20575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, O'Daly O, Uher R, et al. Thinking about eating food activates visual cortex with reduced bilateral cerebellar activation in females with anorexia nervosa: an fMRI study. PLoS One. 2012a;7:e34000. doi: 10.1371/journal.pone.0034000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, O'Daly OG, Uher R, et al. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS One. 2011;6:e22259. doi: 10.1371/journal.pone.0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Rask-Andersen M, Benedict C, Schioth HB. A debate on current eating disorder diagnoses in light of neurobiological findings: is it time for a spectrum model? BMC Psychiatry. 2012b;12:76. doi: 10.1186/1471-244X-12-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter JC, Blackmore E, Sutandar-Pinnock K, Woodside DB. Relapse in anorexia nervosa: a survival analysis. Psychological Medicine. 2004;34:671–9. doi: 10.1017/S0033291703001168. [DOI] [PubMed] [Google Scholar]
- Casper RC, Jasbine LN. An eight-year follow-up: outcome from adolescent compared to adult onset anorexia nervosa. Journal of Youth and Adolescence. 1996;25:499–517. [Google Scholar]
- Davis C. Eating disorders and hyperactivity: a psychobiological perspective. Canadian Journal of Psychiatry. 1997;42:168–75. doi: 10.1177/070674379704200207. [DOI] [PubMed] [Google Scholar]
- Davis C, Brewer H, Ratusny D. Behavioral frequency and psychological commitment: necessary concepts in the study of excessive exercising. Journal of Behavioral Medicine. 1993;16:611–28. doi: 10.1007/BF00844722. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Gabrieli JD, Wagner AD, Ginier BL, Glover GH. Lobular patterns of cerebellar activation in verbal working-memory and finger-tapping tasks as revealed by functional MRI. The Journal of Neuroscience. 1997;17:9675–85. doi: 10.1523/JNEUROSCI.17-24-09675.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z. The eating disorder examination. In: Fairburn CG, Wilson GT, editors. Binge Eating: Nature Assessment and Treatment. 12th edn. New York: Guilford Press; 1993. pp. 317–60. [Google Scholar]
- Falconer E, Bryant R, Felmingham KL, et al. The neural networks of inhibitory control in posttraumatic stress disorder. Journal of Psychiatry and Neuroscience. 2008;33:413–22. [PMC free article] [PubMed] [Google Scholar]
- Fladung AK, Gron G, Grammer K, et al. A neural signature of anorexia nervosa in the ventral striatal reward system. The American Journal of Psychiatry. 2010;167:206–12. doi: 10.1176/appi.ajp.2009.09010071. [DOI] [PubMed] [Google Scholar]
- Frank S, Laharnar N, Kullmann S, et al. Processing of food pictures: influence of hunger, gender and calorie content. Brain Research. 2010;1350:159–66. doi: 10.1016/j.brainres.2010.04.030. [DOI] [PubMed] [Google Scholar]
- Garner DM. Eating Disorder Inventory—2: Professional Manual. Odessa, FL: Psychological Assessment Resources; 1991. [Google Scholar]
- Gray JA. The psychophysiological basis of introversion-extraversion. Behaviour Research and Therapy. 1970;8:249–66. doi: 10.1016/0005-7967(70)90069-0. [DOI] [PubMed] [Google Scholar]
- Guthoff M, Grichisch Y, Canova C, et al. Insulin modulates food-related activity in the central nervous system. The Journal of Clinical Endocrinology & Metabolism. 2010;95:748–55. doi: 10.1210/jc.2009-1677. [DOI] [PubMed] [Google Scholar]
- Hebebrand J, Bulik CM. Critical appraisal of the provisional DSM-5 criteria for anorexia nervosa and an alternative proposal. International Journal of Eating Disorders. 2011;44:665–78. doi: 10.1002/eat.20875. [DOI] [PubMed] [Google Scholar]
- Kaye WH, Fudge JL, Paulus M. New insights into symptoms and neurocircuit function of anorexia nervosa. Nature Reviews Neuroscience. 2009;10:573–84. doi: 10.1038/nrn2682. [DOI] [PubMed] [Google Scholar]
- Lock J, Garrett A, Beenhakker J, Reiss AL. Aberrant brain activation during a response inhibition task in adolescent eating disorder subtypes. The American Journal of Psychiatry. 2011;168:55–64. doi: 10.1176/appi.ajp.2010.10010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Löwe B, Spitzer RL, Zipfel S, Herzog W. PHQ-D. Gesundheitsfragebogen für Patienten. Karlsruhe, Germany: Pfitzer; 2002. [Google Scholar]
- Oberndorfer TA, Kaye WH, Simmons AN, Strigo IA, Matthews SC. Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. International Journal of Eating Disorders. 2011;44:1–8. doi: 10.1002/eat.20750. [DOI] [PubMed] [Google Scholar]
- Paul T, Thiel A. EDI-2. Eating Disorder Inventory-2. 2005. Deutsche Version. Göttingen, Germany: Hogrefe. [Google Scholar]
- Pietrini F, Castellini G, Ricca V, Polito C, Pupi A, Faravelli C. Functional neuroimaging in anorexia nervosa: a clinical approach. European Psychiatry. 2011;26:176–82. doi: 10.1016/j.eurpsy.2010.07.011. [DOI] [PubMed] [Google Scholar]
- Porubska K, Veit R, Preissl H, Fritsche A, Birbaumer N. Subjective feeling of appetite modulates brain activity: an fMRI study. Neuroimage. 2006;32:1273–80. doi: 10.1016/j.neuroimage.2006.04.216. [DOI] [PubMed] [Google Scholar]
- Rodriguez S, Mata JL, Lameiras M, Fernandez MC, Vila J. Dyscontrol evoked by erotic and food images in women with bulimia nervosa. European Eating Disorders Review. 2007;15:231–9. doi: 10.1002/erv.724. [DOI] [PubMed] [Google Scholar]
- Schulz KP, Fan J, Magidina O, Marks DJ, Hahn B, Halperin JM. Does the emotional go/no-go task really measure behavioral inhibition? Convergence with measures on a non-emotional analog. Archives of Clinical Neuropsychology. 2007;22:151–60. doi: 10.1016/j.acn.2006.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DJ, Pekar JJ, Mostofsky SH. Meta-analysis of Go/No-go tasks demonstrating that fMRI activation associated with response inhibition is task-dependent. Neuropsychologia. 2008;46:224–32. doi: 10.1016/j.neuropsychologia.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger CD, Gorusch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: 1983. Consulting Psychologists Press. [Google Scholar]
- Strobel A, Beauducel A, Debener S, Brocke B. Einer deutschsprachigen Version des BIS/BAS-Fragebogens von Carver und White. Zeitschrift für Differentielle und Diagnostische Psychologie. 2001;22:216–27. [Google Scholar]
- Strober M, Freeman R, Morrell W. The long-term course of severe anorexia nervosa in adolescents: survival analysis of recovery, relapse, and outcome predictors over 10-15 years in a prospective study. International Journal of Eating Disorders. 1997;22:339–60. doi: 10.1002/(sici)1098-108x(199712)22:4<339::aid-eat1>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Uher R, Murphy T, Brammer MJ, et al. Medial prefrontal cortex activity associated with symptom provocation in eating disorders. The American Journal of Psychiatry. 2004;161:1238–46. doi: 10.1176/appi.ajp.161.7.1238. [DOI] [PubMed] [Google Scholar]
- van Kuyck K, Gerard N, Van Laere K, et al. Towards a neurocircuitry in anorexia nervosa: evidence from functional neuroimaging studies. Journal of Psychiatric Research. 2009;43:1133–45. doi: 10.1016/j.jpsychires.2009.04.005. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Wunderlich U, Gruschwitz S, Zaudig M. SKID-I. Strukturiertes Klinisches Interview für DSM-IV. Achse I: Psychische Störungen. Göttingen, Germany: Hogrefe; 1997. [Google Scholar]
- Zastrow A, Kaiser S, Stippich C, et al. Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. The American Journal of Psychiatry. 2009;166:608–16. doi: 10.1176/appi.ajp.2008.08050775. [DOI] [PubMed] [Google Scholar]
- Zhu JN, Wang JJ. The cerebellum in feeding control: possible function and mechanism. Cellular and Molecular Neurobiology. 2008;28:469–78. doi: 10.1007/s10571-007-9236-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel S, Lowe B, Reas DL, Deter HC, Herzog W. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. Lancet. 2000;355:721–2. doi: 10.1016/S0140-6736(99)05363-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.