Abstract
The present fMRI study investigated whether placebo treatment can change disgust feelings. Disgust-prone women underwent a retest design where they were presented with disgusting, fear-eliciting and neutral pictures once with and once without a placebo (inert pill presented with the suggestion that it can reduce disgust symptoms). The placebo provoked a strong decrease of experienced disgust, which was accompanied by reduced insula activation. Exploratory psychophysiological interaction analyses revealed decreased connectivity in a network consisting of the insula, the amygdala, the anterior cingulate cortex and the orbitofrontal cortex. Moreover, the placebo increased amygdala–DMPFC coactivation. Our findings suggest that placebo use can modulate a specific affective state and might be an option as a first therapy step for clinical samples characterized by excessive and difficult-to-control disgust feelings.
Keywords: placebo, disgust, fMRI, insula, prefrontal cortex, affective pictures
INTRODUCTION
During the last decade, there have been a growing number of neuroimaging investigations on the topic of emotion regulation. Most often cognitive reappraisal strategies for the reduction of negative feelings such as fear, sadness and disgust have been studied (e.g. Ochsner et al., 2004; Wager et al., 2008; Hermann et al., 2009; Urry et al., 2009). The participants were asked to reevaluate the meaning of an emotion elicitor (e.g. it is not real), which increased activation in prefrontal cognitive control regions and decreased amygdala activity.
Voluntary engagement in reappraisal is generally beneficial when the experienced emotions are of moderate intensity. However, when someone has very strong negative feelings, e.g. in the context of certain mental disorders, this is usually accompanied by deficient cognitive capacities to properly control these emotional reactions. For example, patients suffering from washing compulsions or borderline personality disorder are afflicted by very intense and difficult-to-control disgust feelings when they are exposed to disorder-relevant stimuli (e.g. Schienle et al., 2003; Schienle et al., 2013). Surprisingly, few studies have attempted to directly address disgust in the context of therapy. Some authors even state that there is emerging evidence that disgust is resistant to conventional treatment, such as exposure therapy (e.g. McKay and Olatunji, 2009).
Therefore, it seems promising to look for alternative intervention strategies in order to attenuate excessive disgust reactions. Such alternative approaches may bypass voluntary cognitive control mechanisms and may use implicit approaches, such as placebo treatment.
The most commonly studied placebo effect in neuroimaging investigations is placebo analgesia (e.g. Petrovic et al., 2002; Wager et al., 2004, 2011; Lieberman et al., 2004; Zubieta et al., 2006). These studies demonstrated that the expectation and experience of placebo-associated pain relief was mediated by prefrontal cortex areas, such as the dorsolateral prefrontal cortex (DLPFC), the orbitofrontal cortex (OFC), the dorsomedial prefrontal cortex (DMPFC) and the anterior cingulate cortex (ACC).
The influence of a placebo on emotional processing has hardly been investigated (Mayberg et al., 2002; Petrovic et al., 2005). Petrovic et al. (2005) presented their subjects with unpleasant pictures after they had been treated with an anxiolytic drug. The medication markedly reduced the unpleasantness ratings. In a subsequent trial, a placebo was given with the instruction that the treatment would be repeated. The placebo responders displayed increased activity in the ACC, the orbitofrontal and ventromedial prefrontal cortex.
In the present fMRI study, we investigated whether a ‘disgust placebo’ (an inert pill which was presented to the recipient with the instruction that the substance efficiently reduces disgust symptoms) is able to change the affective experience as well as the brain activation during visual disgust elicitation. We expected that in the placebo condition, participants would report less intense disgust feelings and would show reduced activation of the insula, which is crucial for disgust processing (e.g. Phillips et al., 1997). Moreover, we investigated insula connectivity with other brain regions via psychophysiological interactions (PPIs).
MATERIALS AND METHODS
Participants
Thirty-four right-handed, healthy women (mean age = 23.9 years, s.d. = 4.0) participated in this study. They were recruited via announcements at the campus. All participants had a high school diploma (91% were students). The sample had been restricted to females as there are significant sex differences in disgust proneness (Schienle et al., 2002a). The participants had answered the Questionnaire for the Assessment of Disgust Proneness (QADP; Schienle et al., 2002a). This self-report measure consists of 37 items that have to be judged on 5-point scales (0 = ‘not disgusting’, 4 = ‘very disgusting’), e.g. ‘you smell vomit’. The Cronbach’s alpha of the total scale was 0.90 in the construction sample and 0.88 in the present sample. As an inclusion criterion for the study, the participants were required to have at least average disgust proneness. The mean QADP score was mean = 2.72 (s.d. = 0.47) and differed significantly from the original sample of the questionnaire construction (n = 310 women; mean = 2.28; s.d. = 0.52; t(342) = 4.5; P < 0.001). All participants were free from mental disorders, medication and somatic problems as assured by the Brief Symptom Inventory (Derogatis, 1993). Written informed consent was obtained from all subjects. The study was conducted in accordance with the Declaration of Helsinki and was reviewed by the ethics committee of the Medical University of Graz. None of the women had previously participated in a drug study.
Material
The participants were shown a total of 45 affective pictures representing the three categories: Disgust (e.g. dirty toilet, rotten corpse and maggots), Fear (e.g. shark, a man attacking a woman with a knife) and Neutral (e.g. household objects and geometric figures). We used pictures from the International Affective Picture System (IAPS; Lang et al., 2008) and from a collection of disgust pictures (Schienle et al., 2002b). Each scene was presented for 4 s, followed by a variable interstimulus interval (range: 3.5–8 s). The presentation sequence of the stimuli was randomly chosen and repeated once (30 events per condition).
Procedure
All 34 subjects underwent two fMRI sessions where they passively viewed the picture set with disgusting, fear-eliciting and neutral scenes. The sessions were separated by 1 week.
In one session (the placebo condition), the participants received a placebo pill (a 1 cm long silica-filled capsule) prior to the presentation of the pictures. They were told that the pill contains the pulverized bark of the angostura tree (galipea officinalis) which can be found in South America. The native Indians have used this herbal medicine for a long time to treat digestive problems (nausea and diarrhea) and fever. Further, they were informed that a previous investigation using this dietary supplement (without fMRI) had already demonstrated that angostura effectively reduces disgust symptoms, and that the positive effect occurs ∼15 min after the application. Thus, the cover story suggested a clinical trial of a dietary supplement. The study was conducted at the Medical University of Graz (Department of Neuroradiology). The experimenter as well as the fMRI staff wore white coats during the conduction of the study in order to enhance the credibility of the cover story.
Subsequent to the fMRI recording, the subjects were presented with three sheets of paper depicting the 15 pictures representing an affective category (Disgust, Fear and Neutral). They were asked to rate the intensity of elicited fear and disgust for each category by means of 9-point Likert scales (1 = little; 9 = very intense). Mean judgments were obtained for each of the three picture categories.
In the other session (no-placebo condition), the participants received no capsule and viewed the same pictures. The sequence of the pictures within one session as well as the sequence of the two sessions (placebo, no-placebo) was random. At the end of the investigation, the participants were asked whether they were convinced that they had received angostura or a placebo (yes/no).
fMRI
Data were collected using a 3T scanner (Siemens Trio, Erlangen, Germany). A total of 385 volumes were acquired using a modified echoplanar imaging protocol (number of slices: 35, descending, tilted −25° from the AC–PC line; flip angle = 90°; slice thickness: 3 mm; 1 mm gap; matrix: 64 × 64; TE = 30 ms; TR = 2300 ms; FoV: 192; in-plane resolution = 3 × 3 mm). Analyses were conducted using SPM8 (Wellcome Center for Neuroimaging, University College London, UK). Three volumes from the beginning of the time series were discarded to account for saturation effects. Data were slice-time corrected, realigned including unwarping and coregistration, normalized to MNI space (2 mm isotropic voxel) and smoothed with an 8 mm isotropic Gaussian kernel. Individual conditions were modeled using the canonical hemodynamic response function. Each condition (Disgust/Fear/Neutral) was modeled with a duration of 4 s including the six movement parameters from the realignment step. Data were high pass filtered (128 s). Temporal sphericity was controlled by an AR(1) process with consecutive prewhitening of the data. T-contrasts were created for Disgust–Neutral, Fear–Neutral, Disgust–Fear, Fear–Disgust, for both the placebo condition and the no-placebo condition. Resulting contrast images were submitted to random effects analyses for voxel intensities (one-sample t-tests). Then, we compared the emotion conditions with and without application of the placebo (e.g. Disgust–Neutral with placebo vs Disgust–Neutral without placebo).
Based on evidence from previous fMRI studies on disgust (e.g. Schienle et al., 2002b; Schäfer et al., 2009) and placebo effects (e.g. Wager et al., 2004; Petrovic et al., 2005), we defined the following regions of interest (ROIs) which were taken from the Harvard-Oxford Cortical and Subcortical Structural Atlas (Center for Morphometric Analysis, MGH-East, Boston, MA, USA). The ROIs were constructed with the WFU PickAtlas (WFU Pickatlas v2.4; Wake Forest University School of Medicine): amygdala, insula, OFC, DMPFC, DLPFC and ACC. Statistical maps were thresholded with an uncorrected P of 0.001 and at least five contiguous voxels. Results for exploratory voxel intensity tests were considered significant when P corrected for family-wise error (FWE) was <0.05; the significance level when testing specific hypotheses was additionally corrected for the number of comparisons (= number of ROIs).
We tested the following specific hypotheses:
The viewing of disgust pictures without placebo application (No-placebo: Disgust–Neutral) leads to activation of the bilateral insula, amygdala and OFC (Bonferroni-corrected significance cutoff: 0.05/6 = 0.0083).
The viewing of disgust pictures with placebo application (Placebo: Disgust–Neutral) leads to activation of cognitive control areas including the bilateral DMPFC, DLPFC and ACC (Bonferroni-corrected significance cutoff: 0.05/6 = 0.0083).
Relative to the no-placebo condition, the placebo reduces bilateral insula activation for the contrast Disgust–Neutral (Bonferroni-corrected significance cutoff: 0.05/2 = 0.025).
Relative to the no-placebo condition, the placebo increases activation of the bilateral DMPFC, DLPFC and ACC for the contrast Disgust–Neutral (Bonferroni-corrected significance cutoff: 0.05/6 = 0.0083).
To investigate placebo-specific functional coupling between the insula (seed) and the selected ROIs, we conducted PPI analyses (Gitelman et al., 2003) for each subject. PPIs assess the extent to which an experimental factor modulates the connectivity of one brain region with others, in terms of condition-specific covariation in residuals. Given specific seed regions (left/right insula), PPI identifies voxels that covary differentially with the seed region as a function of an experimental factor. For each participant, a PPI analysis was performed by setting up a design matrix containing three columns of variables: the first regressor, the physiological variable, represented the time series of activity taken from the seed region by taking the first eigenvariate of the corresponding mask. The second regressor, the psychological variable, represented the condition type (e.g. the contrast Disgust–Neutral for the placebo vs no-placebo condition). The PPI variable (PPI term) represented the third regressor, which was computed as the element by element product of the deconvolved extracted time series of the selected seed region and a vector coding for the effect of task. Additionally, the six movement parameters from the realignment step were included in the model. Subject-specific interaction contrast images were then entered into a random-effects analysis [thresholded at P < 0.05, corrected for multiple comparisons (FWE)] in order to compare connectivity in the placebo and no-placebo condition (contrasts: Disgust–Neutral, Fear–Neutral).
RESULTS
Self-reports
We computed paired t-tests (with Bonferroni correction) in order to compare the intensity of experienced disgust and fear between the placebo and the no-placebo condition across the three picture conditions (Table 1). For both the disgust and the fear pictures, the affective ratings were lower when the placebo pill had been administered compared with the no-placebo condition. The comparison of the difference scores (no-placebo minus placebo) between the picture conditions indicated that the reduction of experienced disgust for the disgust pictures was bigger than any other change in affective ratings (all P < 0.001).
Table 1.
Affective picture ratings (means and standard deviations)
| Pictures | No-placebo, M (s.d.) | Placebo, M (s.d.) | Difference | T33(P) |
|---|---|---|---|---|
| Experienced disgust | ||||
| Disgust | 6.53 (1.71) | 2.50 (1.38) | −4.03 | 11.70 (<0.001) |
| Fear | 3.09 (2.01) | 1.82 (1.34) | −1.27 | 3.52 (0.001) |
| Neutral | 1.12 (0.48) | 1.00 (0.01) | −0.12 | 1.44 (0.160) |
| Experienced fear | ||||
| Disgust | 3.26 (2.02) | 1.71 (0.94) | −1.56 | 4.93 (<0.001) |
| Fear | 6.12 (1.97) | 4.09 (1.91) | −2.03 | 4.75 (<0.001) |
| Neutral | 1.09 (0.38) | 1.06 (0.24) | −0.03 | 0.44 (0.661) |
Bold text: statistically significant differences.
The manipulation check at the end of the investigation showed that 100% of the participants were convinced that they had received angostura.
fMRI
We first conducted an exploratory analysis in order to investigate whether the brain activation for a specific contrast (e.g. Disgust–Neutral) differed between the participants who had received the placebo in the first or the second session. There were no statistically significant sequence effects.
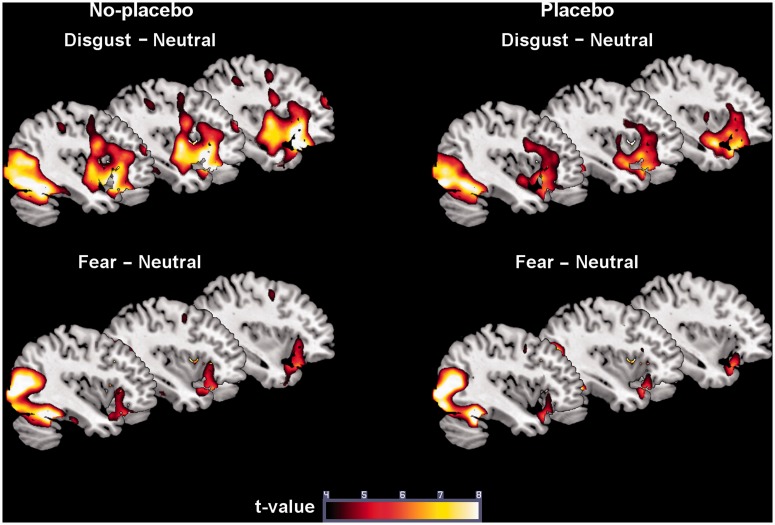
We then looked at the activation in the no-placebo condition for Disgust–Neutral in order to assure that the emotion elicitation had been successful. Significant exploratory effects included activation of visual cortex areas, the right supplementary motor area (SMA), the right OFC, the left ACC and the left amygdala (Figure 1). The ROI tests were significant for the left OFC, the bilateral insula and the right amygdala. The contrast Disgust–Fear showed enhanced activation of the left calcarine fissure, the left postcentral gyrus, the right SMA, the left insula and the left OFC. The exploratory tests for the contrast Fear–Neutral indicated activation of the right amygdala and visual (association) cortex areas (Table 2).
Fig. 1.
Brain activation in the placebo condition and in the no-placebo condition.
Table 2.
Brain activation in the placebo and no-placebo condition
| Region | H | X | Y | Z | T | P(FWE) |
|---|---|---|---|---|---|---|
| No-placebo | ||||||
| Disgust–Neutral | ||||||
| Fusiform gyrus | R | 24 | −80 | 4 | 13.31 | <0.001 |
| Inferior occipital gyrus | L | −18 | −90 | −8 | 13.22 | <0.001 |
| Supramarginal gyrus | L | −62 | −24 | 28 | 7.21 | 0.001 |
| Supramarginal gyrus | R | 68 | −18 | 30 | 6.91 | 0.002 |
| SMA | R | 6 | 6 | 62 | 5.41 | <0.001 |
| OFC | L | −32 | 26 | −18 | 8.82 | <0.001 |
| OFC | R | 30 | 28 | −18 | 10.14 | <0.001 |
| Insula | L | −38 | 18 | −6 | 8.05 | <0.001 |
| Insula | R | 40 | 0 | 2 | 6.62 | <0.001 |
| Amygdala | L | −22 | −2 | −22 | 10.09 | <0.001 |
| Amygdala | R | 22 | −4 | −20 | 8.83 | <0.001 |
| ACC | L | −2 | 8 | 28 | 7.23 | <0.001 |
| Fear–Neutral | ||||||
| Medial occipital gyrus | L | −48 | −80 | 6 | 12.88 | <0.001 |
| Medial temporal gyrus | R | 46 | −62 | 16 | 12.85 | <0.001 |
| Precuneus | L/R | 0 | −56 | 38 | 6.60 | 0.006 |
| Amygdala | R | 22 | −6 | −20 | 7.86 | <0.001 |
| Disgust–Fear | ||||||
| Calcarine fissure | L | −12 | −102 | 0 | 12.67 | <0.001 |
| Postcentral gyrus | L | −62 | −20 | 28 | 8.35 | <0.001 |
| SMA | R | 10 | 8 | 70 | 6.79 | 0.003 |
| OFC | L | −26 | 32 | −14 | 8.97 | <0.001 |
| Insula | L | −40 | −4 | −2 | 9.51 | <0.001 |
| Fear–Disgust | ||||||
| Medial temporal | R | 48 | −60 | 18 | 13.08 | <0.001 |
| Medial occipital | L | −50 | −72 | 10 | 9.69 | <0.001 |
| Medial temporal | L | −50 | −68 | 22 | 8.85 | <0.001 |
| Precuneus | R | 6 | −60 | 28 | 8.15 | <0.001 |
| Placebo | ||||||
| Disgust–Neutral | ||||||
| Lingual gyrus | R | 20 | −92 | −6 | 14.28 | <0.001 |
| Superior occipital gyrus | L | −18 | −88 | −10 | 13.13 | <0.001 |
| Lingual gyrus | L | −34 | −86 | −14 | 12.93 | <0.001 |
| SMA | R | 12 | 14 | 70 | 10.51 | <0.001 |
| DMPFC | L | −8 | 68 | 20 | 5.08 | 0.003 |
| Fear–Neutral | ||||||
| Medial temporal gyrus | R | 50 | −70 | 8 | 14.04 | <0.001 |
| Medial occipital gyrus | L | −48 | −78 | 6 | 12.61 | <0.001 |
| Disgust–Fear | ||||||
| Lingual gyrus | L | −6 | −76 | 0 | 10.45 | <0.001 |
| Postcentral gyrus | L | −58 | −18 | 22 | 6.80 | 0.004 |
| OFC | L | −26 | 32 | −14 | 6.90 | <0.001 |
| ACC | R | 2 | 4 | 28 | 5.93 | <0.001 |
| Insula | L | −36 | −4 | 12 | 6.39 | <0.001 |
| Insula | R | 38 | −4 | 0 | 6.57 | <0.001 |
| Fear–Disgust | ||||||
| Medial temporal | R | 46 | −64 | 16 | 16.55 | <0.001 |
| Precuneus | R | 4 | −54 | 46 | 10.89 | <0.001 |
| Medial occipital | R | 42 | −70 | 32 | 9.46 | <0.001 |
| Medial temporal | L | −50 | −68 | 16 | 11.13 | <0.001 |
Bold (exploratory analysis); normal (ROI analysis).
The placebo condition (contrast: Disgust–Neutral) provoked activation of visual cortex areas, the right SMA and the left DMPFC (Table 2 and Figure 1). For the contrast Disgust–Fear, we observed activation of the lingual and postcentral gyrus, the left OFC, the right ACC and the left insula. The exploratory tests for the contrasts Fear–Neutral and Fear–Disgust indicated activation of visual (association) cortex regions (Table 2).
We then contrasted the placebo and the no-placebo condition for Disgust–Neutral. Relative to the no-placebo condition, the placebo was associated with reduced activation of the left insula (MNI coordinates x,y,z: −42,6,−2, t(33) = 3.82, P(FWE) = 0.018) (Figure 2). The reduction was marginally significant for the right insula (40,6,2, t(33) = 3.62, P(FWE) = 0.045). For the contrast Disgust–Fear, the decrease in insula activation was also marginally significant (42,2,4, t(33) = 3.32, P(FWE) = 0.038).
Fig. 2.
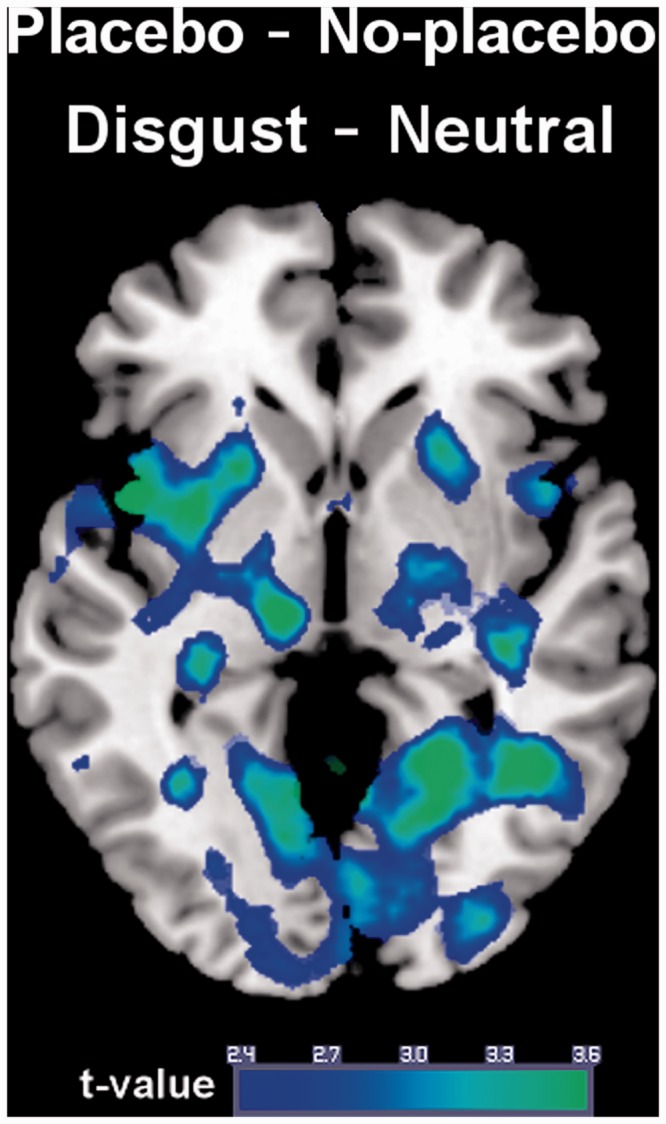
Reduced disgust-related brain activation during placebo administration.
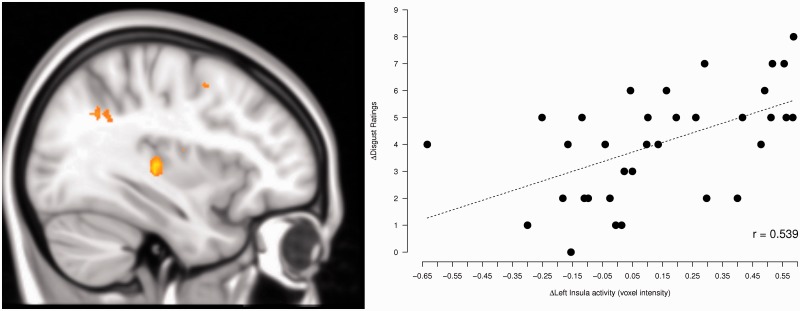
The reduction of disgust ratings (no-placebo minus placebo, Disgust–Neutral) was correlated with the reduction of left insula activation (MNI coordinates: −38,−18,−2, t(32) = 3.62, P(FWE) = 0.03) as computed by a simple regression (Figure 3).
Fig. 3.
The reduction of disgust ratings (no-placebo minus placebo) correlates with the reduction of left insula activation.
There was no significant activation increase in the placebo condition (contrast: placebo–no-placebo) for Disgust–Neutral. However, we observed an effect in the DMPFC [MNI coordinates (x,y,z): 20,60,10; t = 3.47; P = 0.001 (uncorrected)].
The exploratory tests for the Fear–Neutral contrast showed no significant activation changes when comparing both conditions.
The PPI analysis computed for the insula seed (contrasts placebo–no-placebo, no-placebo–placebo, Disgust–Neutral) showed no significant effects. Therefore, we computed exploratory connectivity analyses for all ROIs (contrasts placebo–no-placebo, no-placebo–placebo, Disgust–Neutral, Fear–Neutral). In the placebo condition, there was decreased coupling between the left and right amygdala (seeds) with the insula and the OFC (Table 3). Increased inter-regional coactivation occurred between the DMPFC (seed) and the amygdala. This pattern of coupling was observed only for Disgust–Neutral and not for Fear–Neutral.
Table 3.
Increased coupling between ROIs
| Seed | Region | H | X | Y | Z | T | P(FWE) | |
|---|---|---|---|---|---|---|---|---|
| No-placebo–placebo (Disgust–Neutral) | ||||||||
| Amygdala left | OFC | L | −32 | 16 | −22 | 3.93 | 0.011 | |
| OFC | R | 28 | 16 | −18 | 4.37 | 0.003 | ||
| Insula | L | −32 | 12 | −16 | 3.59 | 0.019 | ||
| Insula | R | 38 | −2 | 10 | 3.86 | 0.009 | ||
| Amygdala | R | 26 | 0 | −28 | 3.46 | 0.013 | ||
| Amygdala right | Insula | L | −34 | 8 | 8 | 3.31 | 0.039 | |
| Placebo–no placebo (Disgust–Neutral) | ||||||||
| DMPFC left | Amygdala | L | −14 | −6 | −16 | 3.29 | 0.018 | |
| DMPFC right | Amygdala | L | −14 | −4 | −18 | 3.10 | 0.026 | |
| Placebo–no-placebo (Fear–Neutral) | ||||||||
| OFC left | Amygdala | L | −16 | −2 | −18 | 3.22 | 0.026 | |
DISCUSSION
We demonstrated that an inert capsule administered with the suggestion that it is able to reduce experienced disgust during visual disgust elicitation was very effective. On average, the self-reported intensity of disgust feelings was more than halved in the placebo condition. Moreover, feelings of fear were also reduced, however to a lesser degree, reflecting the relative specificity of the disgust placebo effect on the self-report level. The changes in affective ratings were accompanied by decreased activation of the left insula.
The insula has been identified as a disgust processor in many brain imaging experiments (e.g. Schienle et al., 2002b). This function is not exclusive as this region is more generally involved in interoceptive processes of physiological conditions, emotional awareness and attention (for a review see Craig, 2009). Especially the subjective evaluation of a body state (e.g. pain) seems to be associated with insula activation. Very consistently decreases of insula activation have been reported in studies on placebo analgesia (e.g. Wager et al., 2004, 2011; Lieberman et al., 2004; Zubieta et al., 2006). Our findings suggest that placebo effects in different domains besides pain have their neural correlate in the insula. Interestingly, during placebo administration, the reduction of insula activation correlated with the reduction of experienced disgust.
Our exploratory connectivity analysis for the no-placebo condition detected increased coupling within a network including the insula, the amygdala and prefrontal regions (ACC and OFC). According to Seeley et al. (2007), these regions form a ‘salience network’ anchored by the ACC and orbito-insular cortices with robust connectivity to subcortical limbic structures (e.g. amygdala). Within this network, the OFC represents the reward value of stimuli. The OFC controls and corrects reward-related and punishment-related behavior, and thus controls emotion (Rolls, 2004). In the study by Seeley et al. (2007) on restingstate functional connectivity, the experienced anxiety prior to the fMRI investigation was able to predict connectivity within this salience network.
The placebo was associated with increased coactivation of the amygdala with DMPFC. Functional brain imaging studies on the cognitive re-interpretation of aversive stimuli to reduce negative affect have consistently demonstrated that this type of reappraisal provoked increased DMPFC activation together with reduced amygdala recruitment. The dampening of amygdala activation is most likely achieved by the inhibitory influence of the mentioned cognitive control area. For example, Banks et al. (2007) conducted a PPI analysis and found that reappraisal was associated with increased amygdala coupling with the DMPFC, the OFC, the DLPFC and the ACC. The degree of amygdala–DMPFC interaction could be used to predict the extent of attenuation of negative affect following reappraisal. Within this experiment, the participants were able to attribute the emotion regulation success to their own person, whereas in our placebo study, it was attributed to the alleged medication. The different attribution styles might have contributed to the partly different connectivity patterns in the reappraisal study by Banks et al. (2007) and our placebo study on affect regulation.
It is interesting to note, that although we observed placebo-related increased DMPFC–amygdala coupling, the increase of localized DMPFC activation in the angostura condition was only marginal. Medial prefrontal cortex regions have been consistently implicated in a wide range of socio-cognitive tasks that require the understanding of mental states of one self and of others. Repeatedly, the DMPFC has been identified as a central region for effortful emotion regulation (Ochsner et al., 2004). It is possible that the degree of effort put into affect modulation influences DMPFC recruitment. Then, it would be understandable that placebo-treatment leads to comparably smaller DMPFC activation because it can be understood as ‘implicit regulation of distress, which occurs without attention, typically outside of awareness and may be less effortful than deliberate self-control’ (Cohen et al., 2013). Future studies are needed where neural correlates of intentional and implicit emotion regulation are directly compared with each other. This will allow to identify overlapping as well as specific brain regions involved in different self-control strategies. Moreover, connectivity patterns can be contrasted with each other. In the current placebo study, the disruption of coactivation between the insula, amygdala and the OFC seemed to be one central mechanism, which might not be relevant for other types of self-control and self-control contexts.
Our results show for the first time that a placebo-coupled expectancy manipulation is sufficient to change affective states and that previous learning experiences are not necessary (Petrovic et al., 2005). Moreover, this is the first placebo study to focus on a specific emotion. Consequently, our study implies that affective processes can be modulated with relative specificity via placebo. This finding gives hope to individuals afflicted with extreme and difficult-to-control disgust feelings. When the participants were informed about the nature of this study, they were surprised as well as pleased to learn that they had dampened their disgust responses by themselves and not by means of ‘angostura’. This unexpected self-control potential might be used as a first step in psychotherapy, especially in those cases where traditional approaches have failed. In this sense, placebo might help to overcome the belief of patients that their disgust regulation deficit is untreatable.
Conflict of Interest
None declared.
Acknowledgments
We would like to thank Gernot Reishofer and Karl Koschutnig for their technical support during fMRI data recording.
REFERENCES
- Banks SJ, Eddy KT, Angstadt M, Pradepp JN, Phan KL. Amygdala–frontal connectivity during emotion regulation. Social Cognitive and Affective Neuroscience. 2007;2:303–12. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JR, Berkman ET, Lieberman MD. Intentional and incidental self-control in ventrolateral PFC. In: Stuss DT, Knight RT, editors. Principles of Frontal Lobe Function. 2nd edn. New York: Oxford University Press; 2013. pp. 417–440. [Google Scholar]
- Craig AD. How do you feel now? The anterior insula and human awareness. Nature Reviews Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory (BSI) Administration Scoring and Procedures Manual. 3rd edn. Minneapolis, MN: National Computer Services; 1993. [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19:200–7. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Hermann A, Schäfer A, Stark R, Walter B, Vaitl D, Schienle A. Emotion regulation in spider phobia, role of the medial prefrontal cortex. Social Cognitive and Affective Neuroscience. 2009;4:257–67. doi: 10.1093/scan/nsp013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS), Affective ratings of pictures and instruction manual. Technical Report A-8. Gainesville, FL: University of Florida; 2008. [Google Scholar]
- Lieberman MD, Jarcho JM, Berman S, et al. The neural correlates of placebo effects, a disruption account. NeuroImage. 2004;22:447–55. doi: 10.1016/j.neuroimage.2004.01.037. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. American Journal of Psychiatry. 2002;159:728–37. doi: 10.1176/appi.ajp.159.5.728. [DOI] [PubMed] [Google Scholar]
- McKay D, Olatunji BO. Disgust and psychopathology next steps in an emergent area of treatment and research. In: Olatunji BO, McKay D, editors. Disgust and its disorders. Washington, DC: American Psychological Association; 2009. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chorpa S, Gabrieli JDE. For better or for worse, neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia, imaging a shared neuronal network. Science. 2002;295:1737–40. doi: 10.1126/science.1067176. [DOI] [PubMed] [Google Scholar]
- Petrovic P, Dietrich T, Fransson P, Andersson J, Carlsson K, Ingvar M. Placebo in emotional processing—induced expectations of anxiety relief activate a generalized modulatory network. Neuron. 2005;46:957–69. doi: 10.1016/j.neuron.2005.05.023. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–8. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Rolls E. The functions of the orbitofrontal cortex. Brain and Cognition. 2004;55:11–29. doi: 10.1016/S0278-2626(03)00277-X. [DOI] [PubMed] [Google Scholar]
- Schäfer A, Leutgeb V, Reishofer G, Ebner F, Schienle A. Propensity and sensitivity measures of fear and disgust are differentially related to emotion-specific brain activation. Neuroscience Letters. 2009;465:262–6. doi: 10.1016/j.neulet.2009.09.030. [DOI] [PubMed] [Google Scholar]
- Schienle A, Walter B, Vaitl D. Ein Fragebogen zur Erfassung der Ekelempfindlichkeit (FEE) Zeitschrift für Klinische Psychologie und Psychotherapie. 2002a;31:110–20. [Google Scholar]
- Schienle A, Stark R, Walter B, et al. The insula is not specifically involved in disgust processing, an fMRI study. NeuroReport. 2002b;13:2023–6. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Schienle A, Walter B, Schäfer A, Stark R, Vaitl D. Disgust sensitivity in psychiatric disorders, a questionnaire study. Journal of Nervous Mental Disease. 2003;191:831–4. doi: 10.1097/01.nmd.0000100928.99910.2d. [DOI] [PubMed] [Google Scholar]
- Schienle A, Haass-Krammer A, Schöggl H, Kapfhammer HP, Ille R. Altered state and trait disgust in borderline personality disorder. Journal of Nervous Mental Disease. 2013;201:105–8. doi: 10.1097/NMD.0b013e31827f64da. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry H, van Reekum CM, Johnstone T, Davidson RJ. Individual differences in some (but not all) medial prefrontal regions reflect cognitive demand while regulating unpleasant emotion. NeuroImage. 2009;47:852–63. doi: 10.1016/j.neuroimage.2009.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303:1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager T, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–50. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Atlas LY, Leotti LA, Rilling JK. Predicting individual differences in placebo analgesia. Contributions of brain activity during anticipation and pain experience. Journal of Neuroscience. 2011;31:439–52. doi: 10.1523/JNEUROSCI.3420-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Yau WY, Scott DJ, Stohler CS. Belief or need? Accounting for individual variations in the neurochemistry of the placebo effect. Brain Behavior and Immunity. 2006;20:15–26. doi: 10.1016/j.bbi.2005.08.006. [DOI] [PubMed] [Google Scholar]