Abstract
Prior studies have suggested that positive social interactions are experienced as rewarding. Yet, it is not well understood how social relationships influence neural responses to other persons’ gains. In this study, we investigated neural responses during a gambling task in which healthy participants (N = 31; 18 females) could win or lose money for themselves, their best friend or a disliked other (antagonist). At the moment of receiving outcome, person-related activity was observed in the dorsal medial prefrontal cortex (dmPFC), precuneus and temporal parietal junction (TPJ), showing higher activity for friends and antagonists than for self, and this activity was independent of outcome. The only region showing an interaction between the person-participants played for and outcome was the ventral striatum. Specifically, the striatum was more active following gains than losses for self and friends, whereas for the antagonist this pattern was reversed. Together, these results show that, in a context with social and reward information, social aspects are processed in brain regions associated with social cognition (mPFC, TPJ), and reward aspects are processed in primary reward areas (striatum). Furthermore, there is an interaction of social and reward information in the striatum, such that reward-related activity was dependent on social relationship.
Keywords: reward processing, striatum, social relationships, fMRI
INTRODUCTION
Humans are highly social, forming and maintaining close social relationships is one of the most important life goals. Positive interactions with close others are experienced as rewarding and are linked to happiness (Aknin et al., 2011). Despite the presumed positive and rewarding properties of close social relationships, it is not yet well understood how social relationships influence experience of others’ rewards and the associated neural processes.
A brain region consistently found in studies examining the neural basis of self-relevant reward processing is the ventral striatum (Delgado, 2007). The presumed specificity of the ventral striatum for reward processing is based on studies focusing on reward prediction and receiving rewards in a variety of gambling and risk-taking tasks (for a review, see Haber and Knutson, 2010). These studies consistently show that striatum activation is modulated parametrically by reward magnitude, suggesting that the ventral striatum is highly responsive to self-relevant gain (Delgado et al., 2003). Intriguingly, prior studies have shown that not only primary reinforcers but also interactions with friends activate the striatum (Güroğlu et al., 2008; Izuma et al., 2008), suggesting that interacting with friends has a motivational or rewarding significance. This assumption receives further support from social interaction studies, reporting that cooperation with unknown others results in activation in the striatum (Rilling et al., 2002; van den Bos et al., 2009). This neural response has been interpreted in terms of the possibly primary rewarding aspects of positive social interactions. For example, striatum activation when sharing with other people has been shown to depend on the relative closeness of the other person (Fareri et al., 2012). In this study, sharing outcomes with friends elicited significantly more striatum activation than sharing with a non-close confederate or a computer.
Yet, a growing number of recent novel studies have demonstrated that interactions with others also result in activation in a set of cortical brain areas, also referred to as the ‘social brain network’. More specifically, these neuroimaging studies have revealed a network of brain areas related to mentalizing about other persons’ mental states, making judgments about others and thinking about other persons’ intentions (Frith and Frith, 2012). This network includes, but is not limited to, the temporal parietal junction (TPJ) and cortical midline structures such as the medial prefrontal cortex (mPFC), anterior cingulate cortex (ACC) and precuneus (Blakemore, 2008; Van Overwalle, 2009; Young et al., 2010). For example, Young et al. (2010) previously showed that the TPJ and the precuneus are more active when reading stories about other people’s thoughts compared with reading about physical stories. In addition, Amodio and Frith (2006) showed that the anterior part of mPFC is more active when thinking about others compared to when thinking about self. Finally, Güroğlu et al. (2008) showed that thinking about friends result in activation in the ventral mPFC, more so than when thinking about neutral others. However, it remains to be investigated whether and how TPJ, precuneus and mPFC activation are sensitive to social relationships in a reward context.
Taken together, although previous studies have examined both the neural correlates of self-relevant gain (Knutson et al., 2001) and more general aspects of social interaction (Izuma et al., 2008; Young et al., 2010), very few studies to date have explicitly tried to identify the influence of social relationships on reward processing. Although several studies have used innovative designs to examine how closeness and friendship are related to several types of reward processing (Mobbs et al., 2009; Fareri et al., 2012; Nicolle et al., 2012), it is not yet known whether gains and losses are processed differently for self and others with whom participants have different social relationships. We predict that rewards, in general, are processed in the ventral striatum, and playing for a different person than the self leads to activation in the social brain network (mPFC, precuneus and TPJ). A specific question, within a context where both social relationships and rewards are concerned, is whether ventral striatum responses to rewards are dependent on the beneficiary.
In this study, participants performed a gambling task in which they could win or lose money. To investigate the role of social relationships, we explained that on some trials participants would play for themselves, whereas on other trials, they would play for their best friend. To control for the possibility that neural regions would respond differently simply because participants played for another person (independent of the relationship), we included a third player. For this player, we aimed to create a more distant relationship. In order to make this condition most dissociable from the friend condition, we included a manipulation to make the participants dislike the third player (hereon referred to as ‘antagonist’). The antagonist was created using a social interaction game, based on previous studies showing that prior information about another person is related to reward responses on the neural level (Delgado et al., 2005; de Bruijn et al., 2009). Specifically, the antagonist was manipulated by an unfair game strategy in an Ultimatum Game (UG) played before the start of the task. Previous work has shown that unfair offers in the UG elicit negative emotions (Pillutla and Murnighan, 1996; Sanfey et al., 2003).
The gambling task involved an active choice for heads or tails on each trial, followed by gain or loss on each trial. This task structure was based on prior studies showing that active engagement and perceived control in the task elicits the strongest striatum response (Rao et al., 2008). This created a 2 × 3 design, in which participants could win or lose money for three different persons: themselves, their best friend and an antagonist.
First, we expected a main effect of reward processing located in the striatum. Second, we expected that playing for friends and antagonists would result in activation in the social brain network (mPFC, precuneus and TPJ) compared with playing for self. Third, we expected an interaction effect of social relationship and reward processing. Based on previous research, which showed that striatum activation parametrically follows value of outcome (Delgado, 2007), we hypothesized that reward value of outcome would differ for different beneficiaries, such that winning for self would result in a higher neural response in the striatum compared with winning for friends, and this pattern was expected to be least prominent or possibly even reversed for antagonists.
MATERIALS AND METHODS
Participants
Participants were 34 right-handed adults. Three subjects were excluded, one due to attention deficit disorder (ADD) diagnosis and two due to excessive head movement (more than 3 mm in any direction). Data for 31 healthy adults (18 females) meanage = 20.9 years, s.d.age = 1.95, ranged 18–26 years were used in the analyses. Participants were recruited through local advertisements. Approximation of IQ was determined by two subscales, similarities and block design, of the Wechsler Intelligence Scale for Adults (Wechsler, 1997). Estimated IQ for all participants fell within the average to high-average range (mean = 113.39, s.d. = 9.07). This study was approved by the university medical ethical committee. Informed consent was obtained from all participants prior to the scan session. All participants were screened on MRI contra indications before the scanning session. Participants received €60 for participation in a larger set of studies.
Experimental design
Before the start of the experiment, participants were asked to make an UG offer to another participant in the study. They were explained that they could offer any number of coins out of 10 coins to another person in the experiment. The other person would at the next sessions have the opportunity to accept or reject the offer. If the other person accepted the offer, then the money would be divided as proposed, but if the other person rejected the offer, they would both receive nothing. The offers made by the participants ranged from two to seven with a median of five. In total, 22 participants offered five coins to the other player, 4 participants offered four coins, 2 participants offered six coins and 3 participants made a choice of two, three or seven coins.
Participants were then told that they also received an UG offer from a prior participant of the study. Participants could accept or reject this offer. The offer participants received was the same for all participants, namely an unfair offer of 1 coin out of 10. In total, 26 participants rejected the unfair offer and 4 accepted the offer. Data from 1 additional participant were missing due to technical problems. All the analyses reported below were conducted for the full sample of 31 participants, and separately for the 26 participants who rejected the unfair offer. These analyses did not result in significantly different activation patterns. Therefore, below we report the results from the data set with 31 participants.
Prior to the scan, participants were asked for the name of their best friend. While lying in the scanner, participants performed a gambling task in which they could win or lose money, see below for a task description.
The fMRI task
The task comprised two event-related runs, both lasting ∼7 min. In total, 90 trials were presented: 30 for self, 30 for the best friend and 30 for the antagonist. The amount of coins that could be won or lost on each trial was varied to keep participants engaged in the task. Three variations were included: trials in which five coins could be won or two coins could be lost, trials on which three coins could be won or three coins could be lost and trials on which two coins could be won or five coins could be lost. Since the different trial types were not our primary interest, we averaged across these conditions to have a sufficient number of trials for each participant.
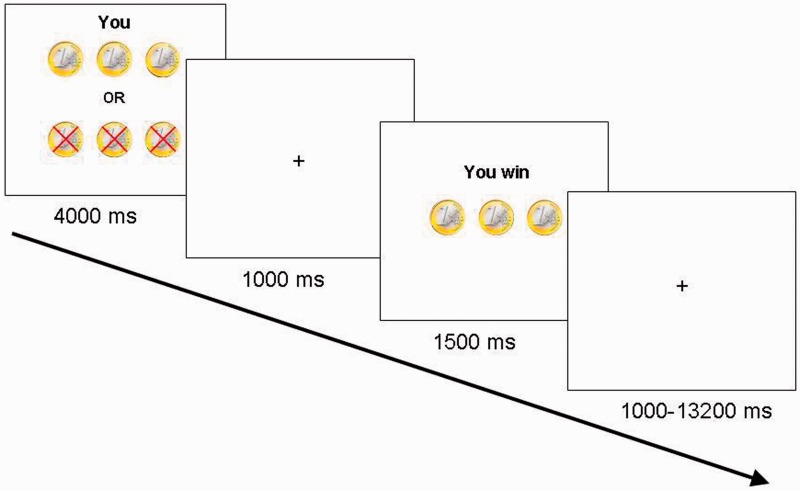
Each trial started with a 4000 ms presentation of the stimulus on which the name of the player they were playing for (i.e. either ‘you’, ‘name of the best friend’ or ‘name of the antagonist’), and the coins at stake were presented. The choice to play for heads or tails was made within this time interval, by pressing the right index finger for heads and the right middle finger for tails. After the 4000 ms stimulus presentation screen, there was a fixed delay of 1000 ms during which a blank screen was presented, which was then followed by the outcome screen that displayed gain or loss. The outcome screen was presented for 1500 ms. The trial ended with a variable jitter of 1000–13 200 ms (average 2298 ms) (Figure 1). Trial sequence and timing were optimized using OptSeq (Dale, 1999); http://surfer.nmr.mgh.harvard.edu/optseq/). Participants were explained that only one of the players, that is, self, friend or antagonist, received the total amount of money won for that person during the task and that the computer selected the winners. In reality, at the end of the experiment, in 50% of the cases participants received the gain for themselves, and in 50% of the cases their best friend received the gain. In all cases, the payment was a €5, gift card, in addition to the initial endowment.
Fig. 1.
Example of a trial. On trial onset, participants were presented with a screen for 4000 ms indicating for whom they were playing (Self, Friend or Antagonist) and how many coins could be won or lost. During this time, participants chose to play heads or tails by pressing the corresponding button. After a 1000 ms delay, trial outcome was presented for 1500 ms. Participants won when the computer randomly selected the same side of the coin as chosen by the participant.
Procedure
Participants were prepared for the testing session in a quiet laboratory. They were familiarized with the MRI scanner with use of a mock scanner as well as listening to recordings of scanner sounds. After explanation of the task, participants performed six practice trials. At the end of the scanning session, participants rated separately for friend and antagonist: (i) how pleasant they found it when they won or lost for their friend and for the antagonist and (ii) how much they thought the other players deserved to win. Ratings were made on a scale ranging from 1 to 10, with anchors ‘not at all’ and ‘very much’. Only pleasantness (Question 1) was analyzed in this study. No differences were found for Question 2; therefore, this question was not further analyzed.
MRI data acquisition
Scanning was performed on a 3 T Philips Achieva whole-body scanner (Best, The Netherlands) at Leiden University Medical Center, using a standard whole-head coil. The functional scans were acquired using a T2*-weighted echo-planar imaging sequence. The first two volumes were discarded to allow for equilibration of T1 saturation effects [TR = 2.2 s, TE = 30 ms, sequential acquisition, 38 slices of 2.75 mm, field of view (FOV) 220 mm, 80 × 80 matrix, in-plane resolution 2.75 mm]. A high-resolution 3D T1-FFE scan for anatomical reference was obtained (TR = 9.760 ms, TE = 4.59 ms, flip angle = 8°, 140 slices, 0.875 × 0.875 × 1.2 mm3 voxels, FOV = 224 × 168 × 177 mm3). Visual stimuli were displayed onto a screen in the magnet bore and could be seen by the participant via a mirror attached to the head coil. Head movement was restricted by using foam inserts inside the coil.
fMRI data analysis
All data were analyzed with SPM8 (Wellcome Department of Cognitive Neurology, London). Images were corrected for differences in rigid body motion. Structural and functional volumes were spatially normalized to T1 templates. Translational movement parameters never exceeded 1 voxel (<3 mm) in any direction for any participant or scan. The normalization algorithm used a 12-parameter affine transform together with a non-linear transformation involving cosine basis functions and resampled the volumes to 3 mm cubic voxels. Templates were based on the MNI305 stereotaxic space (Cocosco et al., 1997). Functional volumes were spatially smoothed with an 8 mm full width half maximum (FWHM) isotropic Gaussian kernel.
Statistical analyses were performed on individual subjects data using the general linear model in SPM8. Trial and feedback onsets (i.e. outcome processing) were modeled as events of interest. Trials on which the participants failed to respond were modeled separately and excluded from further analyses. The fMRI time series at trial onset were modeled as a series of zero duration events convolved with the hemodynamic response function (HRF) and its temporal derivative, which proved to be the most powerful model to detect differences in neural responses to different social relationships at trial onset. For outcome processing, time series were modeled for the full duration that the outcome was visible on the screen (1500 ms). The trial functions were used as covariates in a general linear model along with a basic set of cosine functions that high-pass filtered the data, and a covariate for session effects. The least-squares parameter estimates of height of the best-fitting canonical HRF for each condition were used in pairwise contrasts. The resulting contrast images (condition vs fixation), computed on a subject-by-subject basis were submitted to group analyses.
At the group level, two ANOVAs were computed. To investigate responses to trial onset, we computed a one-way within-subject ANOVA with three levels (Self, Friend and Antagonist). To investigate responses related to outcome processing, we computed a 3 (Person: Self, Friend, Antagonist) by 2 (Outcome: Gain, Loss) repeated measures ANOVA on feedback onset. Task-related responses were considered significant when they exceeded an uncorrected threshold of P < 0.001 and consisted of at least 10 contiguous voxels, to balance between Type 1 and Type 2 errors (Lieberman and Cunningham, 2009). To correct for multiple comparisons, we used small volume correction for the regions we identified in our a priori hypotheses, that is, striatum, TPJ, precuneus and mPFC. All regions reported with small volume correction survived family wise error (FWE) correction.
We used the MarsBaR toolbox (Brett et al., 2002) (http://marsbar.sourceforge.net/) for SPM8 to perform region of interest (ROI) analyses to further illustrate patterns of activation in the clusters found with whole-brain analyses. ROIs were based on functional activation. Average activation across the ROI was extracted and used to perform further analyses. To examine consistency in neural responses between different conditions, we calculated Pearson’s correlations for striatal responses to all conditions at the moment of outcome processing. Only correlations surviving Bonferroni correction are reported.
RESULTS
Behavioral results
Subjective ratings
To test whether participants subjectively discriminated between the different outcomes for different persons, a repeated measures ANOVA on the subjective ratings of pleasantness was conducted with Person (two levels: Friend and Antagonist) and Outcome (two levels: Gain and Loss) as independent variables. There was a significant main effect of Outcome [F(1,30) = 18.99, P < 0.001] as well as interaction for Person × Outcome [F(1,30) = 72.88, P < 0.001]. Gains for Friend were rated highest (mean = 8.0, s.d. = 1.2), followed by losses for the Antagonist (mean = 6.2, s.d. = 1.9), followed by gains for the Antagonist (mean = 5.0, s.d. = 1.8). The lowest pleasantness ratings were found for losses for Friend (mean = 3.8, s.d. = 2.0). Follow-up paired samples t-tests showed that pleasantness ratings were significantly different from each other [all t’s(30) > 2.36, P’s < 0.025].
fMRI results
The fMRI results are presented in two parts. The analysis of neural responses to trial onset is presented first, followed by the analysis of neural responses to outcome processing.
Trial onset
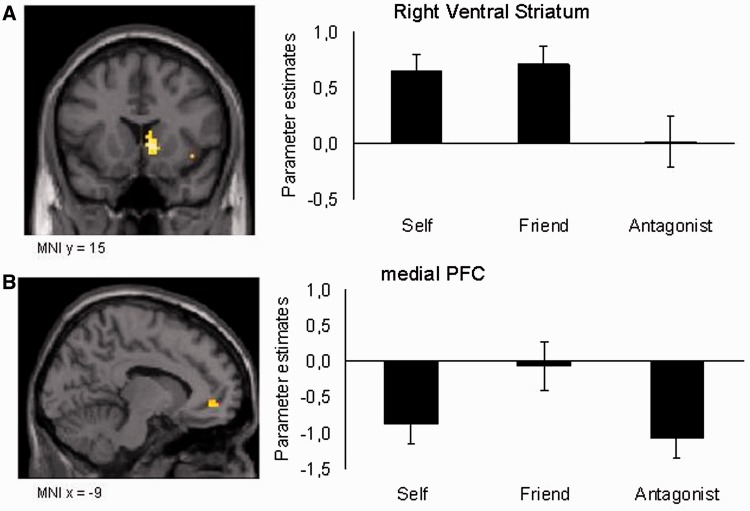
To identify brain regions that respond differently when playing for different persons, a whole-brain one-way ANOVA with Person as within-subject factor (three levels: Self, Friend, Antagonist) was conducted. The ANOVA revealed a main effect of Person located in the right ventral striatum [Montreal Neurological Institute (MNI): 9 15 0, F(2,90) = 14.3; P = 0.002 small volume corrected; Figure 2A] and the mPFC [MNI −12 42 −6, F(2,90) = 9.80, P = 0.006 small volume corrected; Figure 2B; see Table 1 for a complete list of resulting brain regions].
Fig. 2.
An ANOVA for Person, with levels Self, Friend and Antagonist, modeled at stimulus onset resulted in activation in (A) the right ventral striatum (peak voxel MNI 9, 15, 0) and (B) mPFC (peak voxel MNI −12, 42, −6) (P < 0.001, uncorrected for multiple comparisons, >10 contiguous voxels). Post hoc comparisons revealed that the striatum was more active when playing for Self and Friend, whereas the mPFC was selectively active when playing for Friend.
Table 1.
Brain regions identified in the repeated measures ANOVA for Person, with levels Self, Friend and Antagonist modeled at trial onset (P < 0.001, uncorrected for multiple comparisons, >10 contiguous voxels)
| MNI |
||||||
|---|---|---|---|---|---|---|
| Region | R/L | x | y | z | F(2,90) | Voxels |
| Caudate nucleus | R | 9 | 15 | 0 | 14.34 | 163 |
| Insula lobe | R | 42 | 9 | −12 | 11.23 | 31 |
| Dorsolateral prefrontal cortex | R | 42 | 51 | −3 | 11.05 | 11 |
| mPFC | L | −12 | 42 | −6 | 9.80 | 21 |
| Postcentral gyrus | L | −51 | −30 | 51 | 9.97 | 11 |
MNI coordinates of the peak voxel are reported.
To further visualize the different responses in the regions identified by the main effect of Person, ROI analyses were conducted to further investigate person-related activity in the ventral striatum and mPFC. These follow-up ROI analyses showed that the right ventral striatum responses to the Self and the Friend conditions were higher than for the Antagonist condition [respectively t(30) = 3.1, P = 0.004 and t(30) = 4.39, P < 0.001]; activation for Self and Friend did not differ significantly [t(30) = 0.35, ns; see Figure 2A]. The mPFC was relatively more active in the Friend condition than in the Self [t(30) = 3.1, P = 0.004] and Antagonist [t(30) = 3.7, P < 0.001] conditions, whereas Self and Antagonist conditions did not differ [all t’s (30) < 1, ns; see Figure 2B]. To test whether the ventral striatum and the mPFC showed differential patterns of results, we tested the interaction between the two areas and the conditions. The interaction yielded a significant ROI × Person interaction [F(2,60) = 5.0, P = 0.01], indicating a dissociation between mPFC and ventral striatum function, such that mPFC activity was selectively active for friends, whereas ventral striatum activity was found for both self and friends relative to antagonists.
Outcome processing
To investigate outcome-related brain responses, a second ANOVA was conducted. This whole-brain repeated measures ANOVA with within-subject factors Person (three levels: Self, Friend and Antagonist) and Outcome (two levels: Gain and Loss) yielded a main effect for both factors. A small volume correction was applied for predicted regions that did not survive whole-brain false discovery rate (FDR) correction.
The main effect of Person revealed a network comprised of the left TPJ, precuneus and dorsal medial prefrontal cortex (dmPFC; Figure 3A, see Table 2 for MNI coordinates and a full listing of results). Follow-up ROI analyses showed that these three regions were more active during the Friend and Antagonist conditions than during the Self condition (all t’s > 3.2, P’s < 0.003).
Fig. 3.
(A) Brain regions showing a main effect of Person in the Person × Outcome ANOVA modeled at the onset of feedback presentation (P < 0.001, uncorrected for multiple comparisons, > 10 contiguous voxels). These regions included the left TPJ (MNI −48, −63, 39), precuneus (MNI −3, −60, 33) and the dorsal mPFC (MNI −9, 51, 36). Post hoc comparisons revealed more activation in these regions when receiving outcomes for Friend and Antagonist compared with receiving outcomes for Self, independent of the valence of the outcome. (B) Figure showing an interaction effect of Person × Outcome modeled at the onset of feedback presentation in the bilateral ventral striatum (MNI 15, 24, 0 and −12, 21, 0). Post hoc comparisons on ROIs derived from this contrast revealed that the ventral striatum was more active when receiving gain compared with loss for Self and for Friend, whereas for the Antagonist, this pattern was reversed, such that losses for the Antagonist resulted in more striatum activation compared with receiving gain.
Table 2.
Brain regions identified for the main effects of Person and Outcome and the interaction effect of Person × Outcome in the repeated measures ANOVA with factors Person, with levels Self, Friend and Antagonist, and Outcome, with levels Gain and Loss, modeled at outcome processing (P < 0.001, uncorrected for multiple comparisons, >10 contiguous voxels)
| MNI |
|||||||
|---|---|---|---|---|---|---|---|
| Region | R/L | x | y | z | F(1,190) | Voxels | FDRa/ FWEb |
| Main effect of Person | |||||||
| Temporo-Parietal junction | L | −45 | −63 | 39 | 18.71 | 112 | a/b |
| Precuneus | L | −3 | −60 | 33 | 14.34 | 175 | b |
| dmPFC | L | −9 | 51 | 36 | 10.15 | 20 | a |
| Main effect of outcome | |||||||
| Middle occipital gyrus | L | −21 | −99 | 9 | 31.28 | 290 | a/b |
| R | 30 | −87 | 21 | 26.57 | 290 | a/b | |
| Caudate nucleus | R | 9 | 15 | 0 | 15.76 | 21 | a |
| Supramarginal gyrus | R | 60 | −30 | 27 | 16.39 | 41 | a |
| Inferior frontal gyrus | R | 51 | 27 | 6 | 15.31 | 22 | a |
| Interaction effect Person × Outcome | |||||||
| Caudate nucleus | R | 15 | 24 | 0 | 14.98 | 123 | a/b |
| L | −12 | 21 | 0 | 10.34 | 29 | ||
| Superior medial gyrus | L | −12 | 66 | 9 | 12.12 | 18 | a |
| Supramarginal gyrus | L | −54 | −24 | 42 | 8.88 | 12 | |
| Superior parietal lobe | L | −24 | −78 | 51 | 8.64 | 13 | |
| ACC | L | −6 | 45 | 0 | 8.33 | 10 | |
MNI coordinates of the peak voxel are reported.
aSurvives FDR correction.
bSurvives FWE correction.
The main effect of Outcome was located in the right ventral striatum (MNI 9 15 0; see Table 2 for a full listing of results for active areas for the main effect of Outcome). This region overlaps with the region identified in the whole-brain contrast on trial onset (Figures 2 and 3). Directionality of the effect was such that winning resulted in relatively more activation than losing.
The whole-brain Person × Outcome interaction resulted in significant activation in the bilateral striatum [MNI 15 24 0; MNI −12 21 0, F(2,180) = 10.34, P = 0.015 small volume corrected; Figure 3B, see Table 2 for a full listing of results for active areas for the interaction effect of Person × Outcome].
Post hoc paired samples t-tests on extracted ROI values based on this contrast showed that for both clusters (left and right striatum), there was significantly more activation for winning relative to losing for Self [all t’s (30) > 3.49, P’s < 0.002] and Friend [all t’s (30) > 3.25, P’s < 0.003], whereas for the Antagonist, losing was associated with higher striatum activation than winning [right striatum: t(30) = −2.44, P = 0.021 and marginally significant in the left striatum: t(30) = −2.03, P = 0.051], see Figure 3B. Outcomes for Self and Friend were not significantly different, neither for winning [all t’s (30) < 1, ns] nor for losing [all t’s (30) < 1.3, ns]. However, the pattern for outcomes for the Antagonist was significantly different from the pattern for Self and Friend. Winning for Antagonist differed significantly from winning for Self and Friend [all t’s (30) > 2.2, P < 0.040], and losing for Antagonist was significantly different from losing for Self and Friend [all t’s (30) > 2.5, P < 0.020].
Correlations
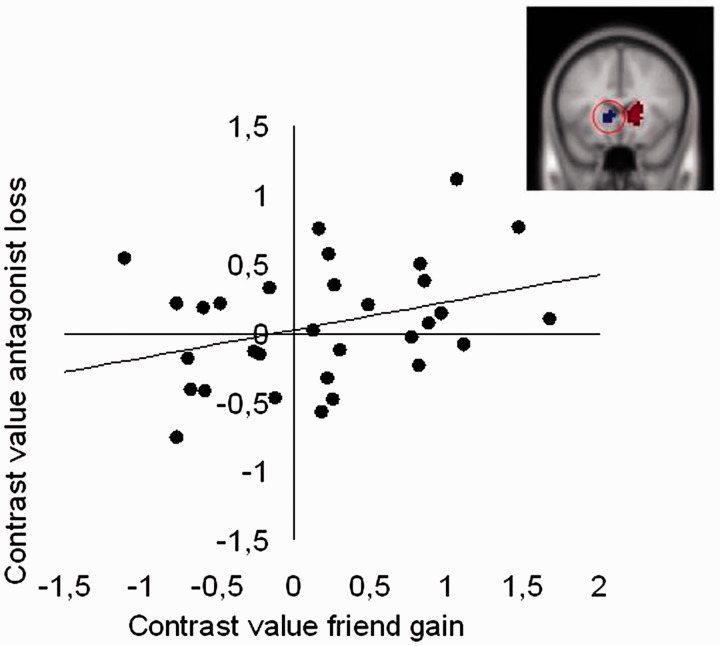
Next, we performed analyses to examine whether there was consistency in neural responses to outcome processing for Self, Friend and Antagonist by correlating neural responses in the ROIs of the ventral striatum identified in the interaction effect of Person × Outcome described above. There were significant correlations between winning for Self and winning for Friend (Left VS: r = 0.52, P = 0.003), and between losing for Self and losing for Friend (Left VS: r = 0.74, P < 0.001, Right VS: r = 0.75, P < 0.001). There was also a significant positive correlation between winning for Friend and losing for Antagonist, such that those individuals who showed the largest ventral striatum response to winning for Friend also showed the largest ventral striatum response to losing for Antagonist (Left VS: r = 0.57, P = 0.001; see Figure 4). There was also a correlation between losing for Self and winning for Friend (Right VS: r = 0.55, P = 0.001), which is difficult to interpret and should be further tested in future studies.
Fig. 4.
Correlation between neural responses to gains for Friend and losses for Antagonist in the right ventral striatum region (MNI 15, 24, 0) (Figure 3).
There were no correlations with the subjective pleasantness ratings that were collected after the scan.
DISCUSSION
In this study, we used fMRI to test how social relationships influence neural processing of rewards that are self-relevant or relevant for others, such as friends and antagonists. We focused on several brain regions that have previously been associated with either one or both of these processes in prior studies, namely the ventral striatum, regions along the cortical midline (mPFC and precuneus) and TPJ. We showed here that the neural response in the ventral striatum to rewards was dependent on the beneficiary, whereas regions involved in thinking about others (mPFC and TPJ) were only responsive to the social relationship (friends and antagonists), independent of reward or loss. Thus, our results show that social relationships influence neural processing of rewards in a social context that involves rewards not only for the self but also for others.
Behavioral ratings of pleasantness of winning
Participants engaged in a gambling task in which they could win or lose money for three different beneficiaries: for themselves, their best friend and an antagonist. Behavioral ratings revealed that winning for a friend was rated higher on pleasantness than winning for an antagonist, whereas losing for a friend was rated as less pleasant than losing for an antagonist. These findings confirm that participants cared about the outcomes for friends, and that the experiment was successful in creating the antagonist based on the interaction in the prior economic exchange game (see Singer et al., 2006, for a similar approach).
Neural responses in the ventral striatum and social brain network at trial onset
At the moment of trial onset, when the participant did not yet know the outcome of the trial, there was a significantly higher neural response in the reward-sensitive ventral striatum when playing for self and friend than when playing for the antagonist. This indicates that anticipation of rewards is modulated by social relationship. Furthermore, at trial onset activation in the mPFC was higher for friends relative to self and antagonist. This region was previously found to be related to self vs other processing (Pfeifer et al., 2007). The current study shows that this neural response depends on the social relationship with the other person, such that it is higher for friends than for unfamiliar others (i.e. antagonists). The next question concerned whether the ventral striatum and mPFC also differed when processing outcomes.
Ventral striatum response to self-relevant and other-relevant rewards
At the moment of outcome processing, an initial comparison of winning for self relative to losing for self resulted in robust activation in bilateral ventral striatum. These results have been reported in numerous other studies (for a review, see Haber and Knutson, 2010) and are consistent with the hypothesis that the ventral striatum is a crucial area for reward representation.
The main question that was addressed in this study was whether a similar neural response would be observed when winning for friends. Indeed, ROI analyses of these regions confirmed that the ventral striatum showed a similar neural response to winning relative to losing for friends. These findings are consistent with prior studies indicating that social interactions with friends are experienced as rewarding (Güroğlu et al., 2008). These results complement previous studies that have shown that interactions with unfamiliar others in various economic games can also be rewarding (Rilling et al., 2002; Fehr and Camerer, 2007; de Bruijn et al., 2009).
In contrast to the neural patterns observed for self-relevant gain and friend-relevant gain, the pattern of neural responses for gain and loss for the antagonist was reversed. Prior studies already showed that bringing individuals in a competition vs cooperation modus results in different responses in the ventral striatum, such that in a cooperation context, individuals show a larger ventral striatum response when an unfamiliar other wins money, whereas in a competition context, individuals show larger ventral striatum response when an unfamiliar other loses money (Delgado et al., 2005; de Bruijn et al., 2009). In the current study, there was a reversal of the neural pattern to reward and loss such that more striatum was observed when losing compared to winning for antagonists. Furthermore, a correlation was found between winning for friend and losing for the antagonist. These findings suggest that losing for an antagonist may be experienced as ‘rewarding’, possibly especially for those individuals who are competitive. These findings fit well with prior studies showing that the ventral striatum is also more active when hurting individuals who have previously treated you unfairly (Singer et al., 2006). Together, these findings provide evidence for the hypothesis that the ventral striatum response to rewards is dependent on the beneficiary.
The social brain network response to social relationship at the moment of outcome processing
The final question that was addressed was whether playing for friends and antagonists would result in different activation compared with playing for self in the social brain network. The whole-brain analysis on the moment of outcome processing suggested that regions within the social brain network, including the mPFC, precuneus and TPJ, were exclusively activated when receiving outcomes for others, relative to receiving outcomes for self. Prior studies suggested that the TPJ and precuneus are important for mentalizing about others, which was found to be specific to social information and not to increased attention per se (Young et al., 2010). Previously, Güroğlu et al. (2008) contrasted neural activation when approaching personally familiar peers with when approaching personally unfamiliar others (i.e. celebrities) and they found that approaching peers resulted in more activation in TPJ, precuneus and mPFC. However, the striatum and ventral mPFC were specifically engaged during interactions with liked peers (i.e. friends). Thus, the current findings are consistent with prior studies showing that TPJ, precuneus and mPFC are sensitive to social information.
It should be noted that prior studies have reported different results with respect to whether mPFC is more active for self or for others, and this seems to be dependent on the relative location within the mPFC (Denny et al., 2012). In the current study, the activation was more anterior in the outcome processing analyses (MNI −9 51 36), and a recent meta-analysis (Denny et al., 2012) confirmed that this region is important for other-related judgments (see also, Nicolle et al., 2012). One of the important questions for future research is whether the self vs other referential processing distinction in the mPFC is dependent on the timing and specific processing demands of the task.
Limitations
The current study aimed to examine whether social relationships influence neural responses to reward processing in contexts that involve both aspects, that is, outcomes that are relevant for self as well as for others. We show that the ventral striatum activation is outcome as well as beneficiary dependent, whereas the social brain network is exclusively dependent on whether you play for self or others, independent of outcomes. However, there are also several issues that cannot be disentangled in this study and which should be addressed in future research.
First, in the current paradigm, the other players differed not only on valence, liked vs disliked, but also on the level of familiarity, familiar vs unfamiliar. When playing for a friend, previous interactions with the friend and the memories of this person might become activated (Güroğlu et al., 2008), and therefore the emotional response is likely to be stronger for this person than for a person that you only interacted with once. The level of familiarity with the interaction partner might therefore partly explain the difference in striatal activation. For example, a study by Mobbs et al. (2009) showed that similarity to another person might be the critical factor that may explain why striatal activation is more similar for friends than antagonists. A challenge for future studies will be to include real-life antagonists, for instance, determined by peer nominations.
Second, the current paradigm is optimized for investigating brain activity. Participants were unable to avoid risks or in any way influence the outcome of the trials. Possibly, levels of risk taking would be different when playing with own money compared with taking risks with other persons’ money. This level of risk taking might be modulated by the valence toward the other person, decisions for liked others might resemble decisions made with own money more than decisions for a disliked other. Future studies should investigate this using adaptive risk-taking paradigms (Chein et al., 2011).
CONCLUSIONS
To our knowledge, this is currently one of the few studies that directly aimed to investigate the interaction between reward processing and social relationships, by separating the beneficiaries of gains and losses. In prior studies that used economic games, such as a trust game or UG, the rewarding value of earned gains (i.e. money) and social interaction (e.g. reciprocity) were often confounded. That is to say, when the interaction partner reciprocates trust in a trust game, this does not only result in a social reward, but also monetary gain for the participant. Second, in previous studies, social interactions have mostly been investigated with unknown others, whose reputation is established based on few encounters or descriptions of interaction partners (Delgado et al., 2005; Singer et al., 2006), resulting in a less strong social relationships and less ecological validity than interactions with real-life friends. The current study aimed to control for these aspects and we showed based on pleasantness ratings for friends and ventral striatum responses that winning for friends, independent of own outcomes, is as rewarding as winning for self.
Neural responses to rewards during outcome processing could be dissociated from activity in cortical brain regions which have previously been associated with thinking about thoughts and intentions of other, such as the mPFC and TPJ. In the current study, these areas were not dependent on outcome, but only on social context. Only in the striatum, we found an interaction of social and reward information, such that reward-related activity was dependent on social relationship.
This study provides important implications for real-life social interactions, such as observed in the peer context. A prior study in adolescents showed that risk-taking increases when peers are present, and peer presence enhanced striatum activity when taking risks (Chein et al., 2011). Thus, it is likely that reward processing is sensitive to a variety of social contexts. It is well known that there are large individual differences in social status and popularity among peers in adolescence and young adulthood (Crone and Dahl, 2012), which can have large consequences for social well-being and health. This study brings us one step further toward unraveling the mechanisms of this high-stake issue in healthy social development.
Conflict of Interest
None declared.
Acknowledgments
This work was supported by a European Research Council (ERC) starting grant (ERC-2010-StG-263234) awarded to E.A.C. and VENI grants from the Netherlands Science Foundation (NWO) awarded to B.G. (NWO-Veni 451-10-021) and J.S.P (NWO-Veni 451-10-007).
References
- Aknin LB, Sandstrom GM, Dunn EW, Norton MI. It's the recipient that counts: spending money on strong social ties leads to greater happiness than spending on weak social ties. PLoS One. 2011;6(2):e17018. doi: 10.1371/journal.pone.0017018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amodio DM, Frith CD. Meeting of minds: the medial frontal cortex and social cognition. Nature Reviews Neuroscience. 2006;7(4):268–77. doi: 10.1038/nrn1884. [DOI] [PubMed] [Google Scholar]
- Blakemore SJ. The social brain in adolescence. Nature Reviews Neuroscience. 2008;9(4):267–77. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton JL, Valabregue R, Poline JB. 2002 Region of interest analysis using an SPM toolbox. Paper presented at the 8th International Conference on Functional Mapping of the Human Brain, Sendai, Japan. [Google Scholar]
- Chein J, Albert D, O'Brien L, Uckert K, Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain's reward circuitry. Development Science. 2011;14(2):F1–10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocosco RA, Kollokian V, Kwan RKS, Evans AC. Brain web: online interface to a 3D MRI simulated brain database. Neuroimage. 1997;5:S452. [Google Scholar]
- Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews Neuroscience. 2012;13(9):636–50. doi: 10.1038/nrn3313. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Human Brain Mapping. 1999;8(2–3):109–14. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruijn ER, de Lange FP, von Cramon DY, Ullsperger M. When errors are rewarding. The Journal of Neuroscience. 2009;29(39):12183–6. doi: 10.1523/JNEUROSCI.1751-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado MR. Reward-related responses in the human striatum. Annals of the New York Academy of Sciences. 2007;1104:70–88. doi: 10.1196/annals.1390.002. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Frank RH, Phelps EA. Perceptions of moral character modulate the neural systems of reward during the trust game. Nature Neuroscience. 2005;8(11):1611–8. doi: 10.1038/nn1575. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Locke HM, Stenger VA, Fiez JA. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective & Behavioral Neuroscience. 2003;3(1):27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Denny BT, Kober H, Wager TD, Ochsner KN. A meta-analysis of functional neuroimaging studies of self- and other judgments reveals a spatial gradient for mentalizing in medial prefrontal cortex. Journal of Cognitive Neuroscience. 2012;24(8):1742–52. doi: 10.1162/jocn_a_00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fareri DS, Niznikiewicz MA, Lee VK, Delgado MR. Social network modulation of reward-related signals. The Journal of Neuroscience. 2012;32(26):9045–52. doi: 10.1523/JNEUROSCI.0610-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr E, Camerer CF. Social neuroeconomics: the neural circuitry of social preferences. Trends in Cognitive Sciences. 2007;11(10):419–27. doi: 10.1016/j.tics.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Frith CD, Frith U. Mechanisms of social cognition. Annual Review of Psychology. 2012;63:287–313. doi: 10.1146/annurev-psych-120710-100449. [DOI] [PubMed] [Google Scholar]
- Güroğlu B, Haselager GJ, van Lieshout CF, Takashima A, Rijpkema M, Fernandez G. Why are friends special? Implementing a social interaction simulation task to probe the neural correlates of friendship. Neuroimage. 2008;39(2):903–10. doi: 10.1016/j.neuroimage.2007.09.007. [DOI] [PubMed] [Google Scholar]
- Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4–26. doi: 10.1038/npp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58(2):284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport. 2001;12(17):3683–7. doi: 10.1097/00001756-200112040-00016. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Cunningham WA. Type I and type II error concerns in fMRI research: re-balancing the scale. Social Cognitive and Affective Neuroscience. 2009;4(4):423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mobbs D, Yu R, Meyer M, et al. A key role for similarity in vicarious reward. Science. 2009;324(5929):900. doi: 10.1126/science.1170539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolle A, Klein-Flugge MC, Hunt LT, Vlaev I, Dolan RJ, Behrens TE. An agent independent axis for executed and modeled choice in medial prefrontal cortex. Neuron. 2012;75(6):1114–21. doi: 10.1016/j.neuron.2012.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, Lieberman MD, Dapretto M. “I know you are but what am I?!": neural bases of self- and social knowledge retrieval in children and adults. Journal of Cognitive Neuroscience. 2007;19(8):1323–37. doi: 10.1162/jocn.2007.19.8.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillutla MM, Murnighan JK. Unfairness, anger, and spite: Emotional rejections of ultimatum offers. Organizational Behavior and Human Decision Processes. 1996;68(3):208–24. [Google Scholar]
- Rao H, Korczykowski M, Pluta J, Hoang A, Detre JA. Neural correlates of voluntary and involuntary risk taking in the human brain: an fMRI Study of the Balloon Analog Risk Task (BART) Neuroimage. 2008;42(2):902–10. doi: 10.1016/j.neuroimage.2008.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rilling J, Gutman D, Zeh T, Pagnoni G, Berns G, Kilts C. A neural basis for social cooperation. Neuron. 2002;35(2):395–405. doi: 10.1016/s0896-6273(02)00755-9. [DOI] [PubMed] [Google Scholar]
- Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300(5626):1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- Singer T, Seymour B, O'Doherty JP, Stephan KE, Dolan RJ, Frith CD. Empathic neural responses are modulated by the perceived fairness of others. Nature. 2006;439(7075):466–9. doi: 10.1038/nature04271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, van Dijk E, Westenberg M, Rombouts SA, Crone EA. What motivates repayment? Neural correlates of reciprocity in the Trust Game. Social Cognitive and Affective Neuroscience. 2009;4(3):294–304. doi: 10.1093/scan/nsp009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Overwalle F. Social cognition and the brain: a meta-analysis. Human Brain Mapping. 2009;30(3):829–58. doi: 10.1002/hbm.20547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Third Edition. Administration and Scoring Manual. San Antonio: The Psychological Corporation; 1997. [Google Scholar]
- Young L, Dodell-Feder D, Saxe R. What gets the attention of the temporo-parietal junction? An fMRI investigation of attention and theory of mind. Neuropsychologia. 2010;48(9):2658–64. doi: 10.1016/j.neuropsychologia.2010.05.012. [DOI] [PubMed] [Google Scholar]