Abstract
Early life stress (ELS) is known to have considerable influence on brain development, mental health and affective functioning. Previous investigations have shown that alexithymia, a prevalent personality trait associated with difficulties experiencing and verbalizing emotions, is particularly related to ELS. The aim of the present study was to investigate how neural correlates of emotional experiences in alexithymia are altered in the presence and absence of ELS. Therefore, 50 healthy individuals with different levels of alexithymia were matched regarding ELS and investigated with respect to neural correlates of audio-visually induced emotional experiences via functional magnetic resonance imaging. The main finding was that ELS modulated hippocampal responses to pleasant (>neutral) stimuli in high-alexithymic individuals, whereas there was no such modulation in low-alexithymic individuals matched for ELS. Behavioral and psychophysiological results followed a similar pattern. When considered independent of ELS, alexithymia was associated with decreased responses in insula (pleasant > neutral) and temporal pole (unpleasant > neutral). Our results show that the influence of ELS on emotional brain responses seems to be modulated by an individual’s degree of alexithymia. Potentially, protective and adverse effects of emotional abilities on brain responses to emotional experiences are discussed.
Keywords: early life stress, alexithymia, hippocampus, fMRI, emotion
INTRODUCTION
Early life stress (ELS) is known to have a considerable influence on affective functioning (Cohen et al., 2006b; Pechtel and Pizzagalli, 2010), personality (McFarlane et al., 2005) and brain development (Kaufman et al., 2000; Coplan et al., 2010). Previous investigations, predominantly in individuals with major depression, reveal altered activation patterns in core emotional structures such as hippocampus, orbitofrontal cortex and amygdala to be associated with ELS (Teicher et al., 2003; Taylor et al., 2006; Pechtel and Pizzagalli, 2010). On a behavioral level, early childhood adversities significantly increase the incidence rates of affective disorders such as depression (Heim and Binder, 2012) and can affect the development of emotional abilities, with alexithymia being one possible outcome (Freyberger, 1977; Kooiman et al., 2004; Lumley et al., 2007). Alexithymia is associated with difficulties in identifying, decoding and communicating one’s own emotional state (Franz et al., 2008). It has also been shown to be a risk factor for various psychological (Taylor et al., 1997; Zackheim, 2007), particularly affective disorders (Conrad et al., 2009; Leweke et al., 2012). On a neural level, alexithymia has been linked to reduced functioning of structures important for emotion processing such as insula (Bird et al., 2010; Reker et al., 2010), amygdala (Li and Sinha, 2006; Kugel et al., 2008), parahippocampal gyrus (Reker et al., 2010) and anterior cingulate cortex (ACC; Berthoz et al., 2002; Leweke et al., 2004; Karlsson et al., 2008; Heinzel et al., 2010) in response to visual emotional stimuli.
A number of behavioral studies have been conducted to investigate the relationship between ELS and alexithymia to better understand their effects on affective functioning. The majority of studies on the interaction of ELS and alexithymia were completed in clinical samples in which alexithymia is a common phenomenon, mostly in participants with major depression (Honkalampi et al., 2004; Wingenfeld et al., 2011), but also in substance-dependent patients (Evren et al., 2009), in patients with post-traumatic stress disorder (PTSD; Zahradnik et al., 2009), in patients with Borderline personality disorder (Zlotnik et al., 2001) and in a mixed population of psychiatric patients (Weber et al., 2008). As Frewen et al. (2008) state in their meta-analysis of alexithymia in PTSD, the association of ELS and alexithymia might be represented best by a developmental model. According to this approach, attachment relationships provide a basis for the development of emotional awareness and expression during childhood. This basis can be disturbed by early traumatic experiences, such as abuse and neglect, facilitating the development of alexithymic tendencies. Recently, our group investigated a healthy, community-dwelling population (Aust et al., 2013), showing that alexithymia is significantly related to the experience of emotional adversities during childhood. In addition, high-alexithymic individuals with a history of early emotional trauma showed specific patterns in experiencing emotions. Nevertheless, this interaction of alexithymia and ELS has only been described on a behavioral level so far. Given the influence of ELS on brain development and affective functioning as previously shown in clinical samples (Heim and Nemeroff, 2001; Hsu et al., 2010), the absence of studies exploring neural correlates of emotional experiences in consideration of both alexithymia and ELS is surprising.
Thus, the aim of the present study was to investigate how neural correlates of emotional experiences in alexithymia are altered in the presence and absence of ELS. Using functional magnetic resonance imaging (fMRI), we investigated neural correlates of emotional experiences in healthy individuals with high and low levels of alexithymia matched with regard to ELS. Since previous neuroimaging studies conducted in alexithymic populations exclusively used visual stimulus material, we enhanced our fMRI design by presenting music simultaneously with human emotional faces to induce pleasant and unpleasant emotional experiences. Previous studies indicate that music can significantly increase the emotional potential of visual stimuli (Baumgartner et al., 2006; Eldar et al., 2007). In addition to the strong behavioral effects of music, its processing involves multiple structures of the ‘emotion network’ such as insula, amygdala, hippocampus, orbitofrontal cortex and ACC (Blood et al., 1999; Eldar et al., 2007; Mitterschiffthaler et al., 2007; Juslin and Västfjäll, 2008; Koelsch, 2010), which have previously been discussed in the context of alexithymia or ELS.
In accordance with previous research, we hypothesized that high degrees of alexithymia would be related to reduced activations in limbic structures such as insula or ACC. Based on previous behavioral results on the conjoint influence of ELS and alexithymia on experiencing emotions as described above, we hypothesized that early childhood adversities would be related to a specific neural signature in high-alexithymic individuals that differs from neural correlates separately associated with alexithymia or ELS, reflecting a specific interaction of alexithymia and ELS on a neural level.
MATERIALS AND METHODS
Participants
Fifty healthy right-handed German native volunteers with an age range between 22 and 55 years were investigated regarding alexithymia and ELS as well as behavioral and neural responses to pleasant and unpleasant emotional stimuli.
Procedure
The process of sample recruitment is described in detail elsewhere (Aust et al., 2013). Twenty-five high-alexithymic (h-ALEX) and 25 low-alexithymic (l-ALEX) individuals from the healthy community-dwelling sample took part in the study. To investigate the specific role of ELS with respect to alexithymia, the two groups were matched regarding their experiences of ELS as measured via questionnaire and interview (see ‘Measures’ section). Therefore, we were able to test how ELS influences behavioral and neural correlates of emotional experiences in the presence and absence of alexithymia.
At a previous step of recruitment, diagnostic interviews (Mini-International Neuropsychiatric Interview; Sheehan et al., 1998) had been conducted by a trained clinical psychologist. Participants with any current or lifetime psychiatric disorder, current intake of psychoactive medication, substance abuse, severe physical diseases or complaints were excluded from the study. Further criteria leading to exclusion were any neurological disorders, head trauma or surgery, ferromagnetic pacemakers or implants and claustrophobia. As depression seems to be particularly related to both ELS (Heim and Binder, 2012) and alexithymia (Honkalampi et al., 2004), we controlled for depressive mood on the day of MRI assessment (see ‘Measures’ section). The study protocol was approved by the local ethics committee and carried out in accordance with the declaration of Helsinki.
Before participation, subjects completed an informed consent form and were educated about the course of the experiment. They also rated the music stimuli with respect to familiarity on a four-point scale (from 1 = ‘Never heard it before’ to 4 = ‘I know this song, it is by [name of composer or interpreter]’). As familiarity decreases the arousal potential of an emotional stimulus (Schellenberg et al., 2008) and memory is known to affect the emotional value of music (Jäncke, 2008), we controlled for familiarity and associated memory processes. None of our subjects was a professional musician or knew any of the experimental music stimuli. Participants then completed several test trials to get used to the experimental design outside the MR scanner. The stimuli used in the test trials were not included in the final experiment to prevent familiarity effects. All subjects were reimbursed for participation.
Measures
Assessment of ELS
Early childhood adversities were assessed in retrospect using the Childhood Trauma Questionnaire (CTQ; Bernstein and Fink, 1998) with 28 items that are assigned to the following five subscales: emotional neglect, emotional abuse, physical neglect, physical abuse and sexual abuse. The CTQ has good psychometric qualities in clinical and nonclinical samples (Cronbach’s α > 0.83; Bernstein et al., 1994). On the basis of CTQ total scores, we matched h-ALEX and l-ALEX participants regarding their history of ELS. Individual CTQ scores were reassessed by a 45 min face-to-face interview (Early Trauma Interview; Bremner et al., 2000), which shows good convergent validity with the CTQ (r = 0.72; Wingenfeld et al., 2010).
Assessment of alexithymia
Alexithymia was assessed using the 20-item Toronto Alexithymia Scale (TAS-20; Bagby et al., 1994a) and the more detailed 40-item Bermond–Vorst Alexithymia Questionnaire (BVAQ; Vorst and Bermond, 2001). Both scales showed good psychometric qualities that have been investigated in healthy samples (TAS-20: Cronbach’s α > 0.80; Bagby et al., 1994b; BVAQ: Cronbach’s α > 0.85; Vorst and Bermond, 2001). The TAS-20 includes three cognition-oriented subscales (difficulty describing feelings, difficulty identifying feelings and externally oriented thinking). The BVAQ consists of three cognitive and two affective subscales assessing the individual capacity of identifying, verbalizing and analyzing feelings as well as the capacity of emotionalizing and fantasizing. On both TAS-20 and BVAQ, high scores indicate high levels of alexithymia.
Assessment of depressive symptoms
We used the 21-item Beck Depression Inventory (BDI; Beck et al., 1961) to assess the current degree of depression. The BDI shows a good validity in differentiating between depressed and nondepressed subjects (Richter et al., 1998). The cutoff point to exclude subjects with a clinically relevant depressive episode was 12 (Beck et al., 1988; Rush et al., 2006). The questionnaire has been validated for use in German clinical and nonclinical samples (Kühner et al., 2007).
fMRI
Stimulus material
We used music and human emotional faces to induce pleasant and unpleasant emotional experiences in our participants; neutral stimuli were used as a control condition. For visual stimulation, we used pictures of facial affect of the recently developed database ‘FACES’ (Ebner et al., 2010), including naturalistic emotional faces of young, middle-aged and older women and men that correspond well to the age distribution of our sample. Previous studies report an own-age bias in face recognition (Anastasi and Rhodes, 2006), and face recognition, in turn, was shown to be essential for emotional contagion through others’ facial expressions (Hatfield et al., 2009). The chosen faces expressed joy (pleasant condition), fear (unpleasant condition) or showed a neutral expression (control condition). For auditory stimulation, we used music from different epochs and musical genres to induce pleasant (e.g. classical music, jazz, Irish dances) and unpleasant (music taken from horror movies) emotional experiences. Sequences of random tones were used as neutral control stimuli, carefully matched with each pleasant/unpleasant piece of music with regard to mean pitch, pitch variation, spectral complexity, instrumentation and tempo (beats per minute), because previous studies report an influence of these parameters on emotional responses (Khalfa et al., 2008). The analysis of these parameters was completed using ‘Essentia’, an in-house library for extracting audio and music features from audio files (http://mtg.upf.edu/technologies/essentia). We tested a large set of stimuli in a behavioral prestudy with 30 healthy low-alexithymic (TAS-20 < 45) volunteers who did not participate in the final fMRI study. The purpose was to exclude stimuli eliciting ambiguous emotional reactions and to select a homogeneous set of equally arousing stimuli with the best-possible distance on the valence dimension. Behavioral data reported in the ‘Results’ section show that the stimuli induced the intended emotional states in our study population.
fMRI design
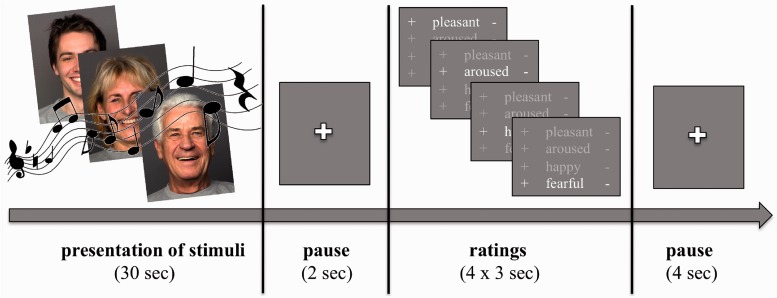
We used a block design with the three conditions ‘pleasant’, ‘unpleasant’ and ‘neutral’. One trial consisted of 30 s of stimulus presentation (one stimulus refers to one piece of music running simultaneously with a sequence of three face pictures, each presented for 10 s) followed by four ratings (3 s each), with a 2 and a 4 s pause before and after the rating period (Figure 1). During these pauses, participants looked at a white fixation cross on a gray screen. Ratings were given according to the current emotional experience evoked by the stimuli and with respect to pleasantness, arousal, joy and fear on six-point scales ranging from ‘very strong’ (=6) to ‘not at all’ (=1). Therefore, a response box that participants operated with their right hand was implemented inside the scanner. The dimensions ‘pleasantness’ and ‘arousal’ were chosen according to Russell’s model of core affect (Russell, 2003). ‘Joy’ and ‘fear’ directly corresponded to the emotions induced by the stimuli. In addition, Galvanic Skin Responses (GSRs) running simultaneously with MR scanning were recorded with two electrodes on the inside of the participant’s left index and middle finger using Brain Vision Recorder Software (Brain Products, Munich, Germany). The total duration per trial was 48 s and the experiment comprised three functional runs with 18 trials each. Total scanning time was 43.2 min.
Fig. 1.
fMRI design to audio-visually induce pleasant (shown in figure) and unpleasant emotional experiences.
Scanning procedure
Subjects were scanned in a 3 T Siemens Magnetom TimTrio system. Structural image acquisition consisted of 176 T1-weighted images with a slice thickness of 1 mm. Blood oxygenation-level-dependent (BOLD) contrast was obtained with an echo planar imaging sequence (TR = 2000 ms, TE = 30 ms, flip angle = 90°, field of view = 192 mm). Thirty-seven axial slices were acquired covering the whole brain (3 mm slice thickness). The orientation of slices was parallel to the AC–PC line. The experiment was programmed using Presentation (NeuroBehavioral Systems, Albany, CA, USA). The audio–visual stimuli were presented using goggles and noise shielded earphones by Visual Stim Digital for fMRI (Resonance Technology Inc., Northridge, CA, USA).
Analysis
MRI data analysis
MRI data were analyzed using the statistical parametric mapping software package (SPM 8, Wellcome Department of Cognitive Neurology, London, UK) implemented in MATLAB (version 2011a; The MathWorks Inc., Natick, MA, USA). Preprocessing of functional scans included realignment, motion correction, normalization (3 mm3) into MNI space and smoothing using an 8 mm full-width at half-maximum Gaussian kernel. The experimental conditions were modeled with a boxcar function convolved with a hemodynamic response function in the General Linear Model of SPM. T-contrast images were calculated at the individual level regarding valence (e.g. pleasant > neutral). One-sample t-tests were used to evaluate the emotion inducing character of the stimulus material. Two-sample t-tests including covariates were calculated to test for group differences. Linear regression analysis was applied to investigate the influence of ELS in h-ALEX and l-ALEX individuals. A P-value < 0.05 was considered as statistically significant, false discovery rate (FDR) control was applied to correct for multiple comparisons.
Behavioral data analysis
Regarding GSR, we analyzed the amplitude (the difference between the first minimum and the first maximum after stimulus onset) and frequency of peaks after stimulus onset for the duration of 30 s using Brain Vision Analyzer 2 (Brain Products, Munich, Germany). Data from emotion ratings were analyzed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA). In all analyses, a P-value < 0.05 was considered as statistically significant. If necessary, Bonferroni corrections were then used to counteract the problem of multiple comparisons.
RESULTS
Descriptive statistics
The mean age in the sample of 50 healthy volunteers was 34.1 years (s.d. = 9.9). In the h-ALEX (N = 25) group, participants (12 female, 13 male) had a TAS-20 total score above 60, in the low-alexithymic group (l-ALEX; N = 25), participants (12 female, 13 male) had a TAS-20 total score below 45. Since the two groups were matched regarding ELS, there were no differences in terms of CTQ or any of its five subscales. There were also no differences between h- and l-ALEX participants with regard to age, years of education and depression (after applying Bonferroni adjusted alpha-levels; see Table 1). Furthermore, there were no gender differences in either group on all reported measures.
Table 1.
Test of group differences regarding age, years of education, alexithymia, ELS and depression (N = 50)
| h-ALEX (N = 25) | l-ALEX (N = 25) | ||||
|---|---|---|---|---|---|
| Measure | M (s.d.) | M (s.d.) | t | df | P |
| Age | 36.0 (10.4) | 32.7 (9.4) | 1.2 | 48 | 0.238 |
| Years of education | 12.7 (1.2) | 12.5 (1.4) | 0.36 | 48 | 0.718 |
| TAS-20 total score | 66.0 (6.5) | 37.6 (4.6) | 18.1 | 48 | <0.001 |
| BVAQ total score | 131.9 (12.9) | 84.8 (15.0) | 11.8 | 48 | <0.001 |
| CTQ total score | 41.0 (14.6) | 40.2 (15.8) | 0.19 | 48 | 0.845 |
| CTQ emotional neglect | 12.5 (5.6) | 10.9 (5.3) | 1.0 | 48 | 0.302 |
| CTQ emotional abuse | 9.6 (4.7) | 10.0 (5.6) | −0.32 | 48 | 0.751 |
| CTQ physical neglect | 7.5 (3.8) | 6.1 (2.0) | 1.6 | 48 | 0.120 |
| CTQ physical abuse | 6.1 (2.1) | 6.7 (2.2) | −0.96 | 48 | 0.342 |
| CTQ sexual abuse | 5.4 (1.3) | 6.4 (4.3) | −1.1 | 48 | 0.259 |
| BDI | 7.3 (4.8) | 3.9 (3.3) | 2.9 | 48 | 0.006 |
Note: M, mean score; s.d., standard deviation; Bonferroni adjusted alpha-levels of 0.005 per test (0.05/11).
The overall correlation between the two alexithymia measures (TAS-20 and BVAQ total scores) was r = 0.67 (Kendall’s Tau; P < 0.001). Within the h-ALEX group only, ELS was associated with higher alexithymia scores (r = 0.43; P < 0.05; BDI as a covariate), whereas there was no such association within the l-ALEX group. BDI scores did not differ between individuals with and without a history of ELS in neither of the groups.
Subjective rating of emotional experience
To test whether our stimuli induced the intended emotional states in our study population, a one-sample t-test (N = 50) was applied. Stimuli of positive valence were experienced more pleasant (mean = 4.6, s.d. = 0.52) and happier (mean = 4.5, s.d. = 0.67) than unpleasant (mean = 2.4, s.d. = 0.53; mean = 1.9, s.d. = 0.55) or neutral stimuli (mean = 2.7, s.d. = 0.46; mean = 2.3, s.d. = 0.51) (P < 0.001 in each test; BDI included as a covariate). In addition, participants reported to experience more fear in response to stimuli of negative valence (mean = 2.9, s.d. = 1.0) than to stimuli of positive (mean = 1.4, s.d. = 0.41) or neutral valence (mean = 2.3, s.d. = 0.75) (P < 0.001 in each test; BDI included as a covariate). There were no significant differences regarding reported emotional experiences between h-ALEX and l-ALEX participants when controlling for ELS. However, h-ALEX individuals with a history of ELS (mean = 1.57, s.d. = 0.47) reported more fear in response to pleasant (>neutral) stimuli than h-ALEX participants without such a history (mean = 1.27, s.d. = 0.37) (P < 0.05; BDI included as a covariate). Within the l-ALEX group, there were no differences between participants with and without ELS regarding any reported experience of valence, arousal, joy or fear.
Physiological results
GSR recordings were available for 21 subjects (12 h-ALEX and 9 l-ALEX). H-ALEX participants whose GSR data were available for analysis were representative of the whole h-ALEX group with respect to age (P = 0.454), alexithymia (TAS-20: P = 0.595; BVAQ: P = 0.407), ELS (P = 0.846) and depression (P = 0.929). L-ALEX participants whose GSR data were available were also representative of the whole l-ALEX group (age: P = 0.160; alexithymia: TAS-20: P = 0.477, BVAQ: P = 0.217; ELS: P = 0.650; depression: P = 0.860). Before analysis, each GSR data set was assigned to a new ID number by an independent research assistant to impose blinding on the researcher who analyzed GSR data (S.A.).
In response to pleasant and unpleasant (>neutral) stimuli, there were no differences between h-ALEX and l-ALEX individuals with respect to frequency of peaks or GSR amplitude (controlled for depression). In response to pleasant (>neutral stimuli), h-ALEX individuals with a history of ELS showed more peaks per trial (mean = 2.47, s.d. = 1.17) than h-ALEX individuals without such a history (mean = 1.50, s.d. = 0.88) (P < 0.001; BDI included as a covariate), whereas there were no differences regarding the GSR amplitude. Within the l-ALEX group, no differences between participants with and without a history of ELS were found.
fMRI results
We applied one-sample t-tests to clarify that our stimuli elicited emotional brain responses in the sample (N = 50). Brain regions activated in response to pleasant (>neutral) stimuli were the superior temporal gyrus, Heschl’s gyrus, occipital lobe, orbitofrontal cortex, hippocampus, temporal pole, parahippocampal gyrus and ACC (P < 0.05, FDR corrected; see Table 2). In response to unpleasant (>neutral) stimuli, there were stronger activations in the superior temporal gyrus, hippocampus, middle temporal gyrus, orbitofrontal cortex, amygdala and supplemental motor area (P < 0.05, FDR corrected; see Table 3).
Table 2.
Brain regions activated in response to pleasant > neutral stimuli (N = 50)
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Brain regions | x | y | z | Z-score | voxels |
| Superior temporal gyrus | −48 | −16 | 4 | >8 | 1406 |
| Heschl’s gyrus | 54 | −10 | 7 | >8 | 1373 |
| Occipital lobe/cuneus | 12 | −91 | 19 | >8 | 782 |
| Orbitofrontal cortex | −3 | 56 | −11 | 7.40 | 410 |
| Hippocampus | 27 | −13 | −17 | 6.04 | 142 |
| Temporal pole | 38 | 5 | −20 | 4.72 | 75 |
| Parahippocampal gyrus | 18 | −4 | −17 | 3.48 | 42 |
| ACC (BA24) | −3 | 32 | −8 | 3.67 | 85 |
| h-ALEX < l-ALEX (controlled for ELS and depression) | |||||
| Insula | 39 | 2 | 10 | 3.26 | 17 |
Note: Height and extend threshold: P < 0.05, FDR corrected on peak level and k = 10 voxels.
Table 3.
Brain regions activated in response to unpleasant > neutral stimuli (N = 50)
| MNI coordinates |
|||||
|---|---|---|---|---|---|
| Brain regions | x | y | z | Z-score | voxels |
| Superior temporal gyrus | 57 | −10 | 4 | >8 | 1340 |
| Hippocampus | 21 | −7 | −14 | >8 | 57 |
| Middle temporal gyrus | −63 | −10 | −17 | 5.65 | 367 |
| Orbitofrontal cortex | −3 | 32 | −11 | 6.43 | 409 |
| Amygdala | 24 | −7 | −14 | 6.04 | 30 |
| Supplemental motor area | 3 | −25 | 61 | 5.09 | 23 |
| h-ALEX < l-ALEX (controlled for ELS and depression) | |||||
| Temporal pole | −54 | 11 | −5 | 2.34 | 13 |
Note: Height and extend threshold: P < 0.05, FDR corrected on peak level and k = 10 voxels.
To assess the influence of alexithymia on emotional brain responses, two-sample t-tests were performed with CTQ and BDI scores as covariates. Regarding pleasant (>neutral) stimuli, h-ALEX showed decreased responses in the right insula as compared to l-ALEX, whereas in response to unpleasant (>neutral) stimuli, h-ALEX showed decreased responses in the left temporal pole (Tables 2 and 3). Increased brain responses in h-ALEX as compared with l-ALEX individuals were found in neither contrast.
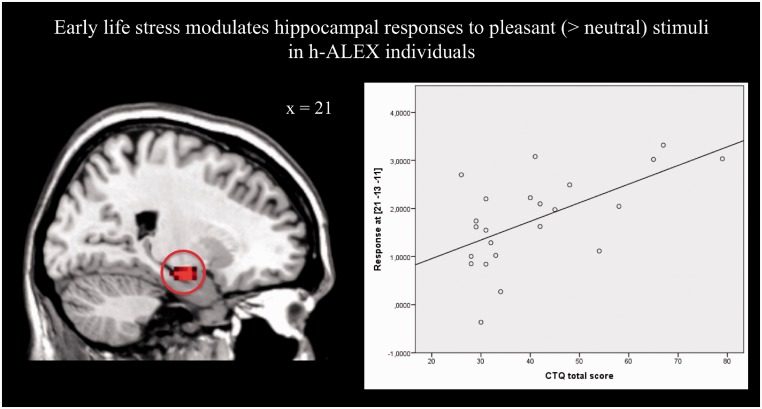
To investigate the specific influence of ELS on alexithymic brain responses, linear regression analysis was performed in the h-ALEX group (with BDI as a covariate). The analysis showed that 36% of the variance in hippocampal responses to pleasant (>neutral) stimuli was explained by ELS as measured by CTQ total score [β = 0.60, R2 = 0.36, F (1,24) = 11.69, P < 0.05, FDR corrected; see Figure 2]. To test whether this effect of ELS was specific for h-ALEX populations, we applied the same analysis in the l-ALEX group and did not find any significant voxels modulated by ELS. For brain responses to unpleasant (>neutral) stimuli, ELS did not explain any variance in either group (also tested at P < 0.05, FDR corrected).
Fig. 2.
Height and extend threshold: P < 0.05, FDR corrected on peak-level and k = 10 voxels.
DISCUSSION
The aim of the present study was to investigate how neural correlates of emotional experiences in alexithymia are altered in the presence and absence of ELS. To our knowledge, this is the first functional MRI study on emotional experiences to account for both ELS and alexithymia in healthy individuals. ELS explained 36% of the variance in hippocampal responses to pleasant (>neutral) emotional stimuli in h-ALEX, but not in l-ALEX individuals. A separate analysis of the effect of alexithymia revealed distinct alterations of limbic responses when controlling for ELS.
Our main finding is that ELS modulates hippocampal responses to pleasant emotional stimuli in h-ALEX individuals, whereas there was no such modulation in closely matched l-ALEX individuals with similar levels of experienced stress during childhood. Corroborating this finding, h-ALEX individuals with a history of ELS showed more peaks per trial in their GSR and report stronger fear responses to pleasant emotional stimuli than h-ALEX individuals without such a history. There is converging evidence that ELS can affect hippocampal development (Schore, 2001): several preclinical studies support the idea that early exposure to elevated levels of the stress-related corticotropin releasing hormone leads to a degeneration of hippocampal neurons (Brunson et al., 2001) or has a direct neurotoxic effect on them (DeBellis et al., 2001; Chen et al., 2012). Associations between reduced hippocampal volume and early stress exposure have been reported in different clinical, mostly depressed study populations (Vythilingam et al., 2002; Kitayama et al., 2005; Kronmüller et al., 2008), suggesting an initially regular hippocampus with subsequent abnormal volumetric development after trauma exposure (Woon and Hedges, 2008). In healthy study populations, however, results on ELS and the hippocampus are less clear (Heim and Binder, 2012). Cohen et al. did not find any significant associations between reduced hippocampal volume and ELS in healthy participants (Cohen et al., 2006a) and at least two studies report significant associations of a hippocampal volume decrease and the experience of early emotional neglect (Edmiston et al., 2011) or ELS in general (Dannlowski et al., 2012) in healthy populations. To our knowledge, examinations of the neural effects of ELS in healthy individuals extending those structural findings to a functional level do not exist.
Our data show, for the first time in healthy individuals, that the effects of ELS on emotion-related hippocampal functioning are highly individual and need to be investigated in due consideration of trait variables that potentially moderate emotional functioning such as alexithymia. Still, the question arises how alexithymia modulates the behavioral and neural effects of ELS described above. Interpreting alexithymia as a potentially protective factor could be one possible approach. In h-ALEX individuals, the experience of ELS was positively related with hippocampal responses to pleasant stimuli, whereas no such effect was found for unpleasant stimuli. As a previous study in a sample of PTSD patients showed, changes in hippocampal activity can be a marker for recovery, implicating an involvement of the hippocampus in the process of successful adaptation on a functional level (Dickie et al., 2011). Given that our participants primarily experienced early emotional traumatic events (emotional neglect and abuse; see Table 1) for an extended period of time, alexithymic tendencies, such as not attaching great significance to emotions, could have been developed to adapt to early childhood adversities. Thus, alexithymia might have averted further damage of hippocampal neurons in the face of enduring stress exposure, which is shown by increased responses to pleasant stimuli. In interviews on childhood adversities, many participants reported growing up in emotionally ‘cold and distant’ families with no value attached to emotions, to their expression and understanding. Adapting this attitude to protect oneself from feeling emotionally neglected and abused might have resulted in high-alexithymic features in these individuals—an explanation along the same lines as the developmental model of alexithymia referred to in the ‘Introduction’ section. In addition to alexithymia, further factors might have played a protective role in these individuals. Future studies should investigate the influence of (epi)genetic factors such as hippocampal BDNF expression (Taliaz et al., 2011) and their interaction with alexithymia and early environmental factors to further develop this idea of ‘emotional resilience’ and to reveal its mechanisms on multiple levels.
However, a second, probably more obvious approach could be interpreting high degrees of alexithymia as an additional stressor in individuals who experienced ELS. This might arise from the increased GSR and stronger reported experiences of fear in response to pleasant stimuli in h-ALEX individuals with a history of ELS as compared to h-ALEX individuals without such a history. This interpretation would follow a previous study of our group showing that ELS in h-ALEX individuals is related to a reduced acceptance of one’s own emotions, stronger physical symbolizations of emotions as well as stronger deficits in emotion regulation (Aust et al., 2013). Moreover, in the present study, ELS was positively related to alexithymia in the h-ALEX group, indicating that alexithymic levels rise with increasing levels of ELS. As our h-ALEX participants with a history of ELS showed the highest degrees of alexithymia, the presentation of pleasant stimuli could have evoked stronger experiences of fear as a consequence of participants’ perception of not being emotionally moved by the stimulus material.
Looking at the discrete influence of alexithymia while controlling for ELS, our results show that alexithymia is associated with reduced activations in an ‘emotion network’ of insula and temporal pole in response to pleasant and unpleasant stimuli. The insular cortex plays an important role in mapping visceral states associated with emotional experiences, and thus is a relevant structure for both emotional and bodily awareness (Damasio, 1994; Craig, 2003; Philips et al., 2003; Terasawa et al., 2011). Reduced activity in this region in response to pleasant stimuli might be related to less interoceptive awareness, less mapping of bodily feeling states and to a reduced representation of emotional arousal and feelings, with blunted emotional experiences as a consequence in h-ALEX individuals. Finding reduced insular activations follows previous studies on alexithymia in clinical samples where decreased activations in the insula were linked to reduced emotional awareness (Silani et al., 2008). In addition, we found reduced left temporal pole activity in h-ALEX individuals in response to unpleasant stimuli. The temporal pole is often referred to as a paralimbic region (Mesulam, 2000) and has been described as an important area for the evaluation of emotional states and for accessing interoceptive body information and emotional memory (Koelsch, 2010; Terasawa et al., 2011). Furthermore, the temporal pole is a region integrating current highly processed sensory stimuli and previously experienced emotional states, particularly in the context of facial emotion processing (Olson et al., 2007) and pleasant music (Brown et al., 2004). Lesion studies report a decoupling of high-level perception with visceral emotional experiences in humans with temporal lobectomy (Lipson et al., 2003). Despite its obvious importance in emotional processing, temporal pole activations have mostly been neglected in discussions in favor of apparently more relevant areas such as the nearby amygdala or frontal regions (Olson et al., 2007). We suggest that decreased temporal pole and insula activity could be indicative of an altered accessibility of emotional experiences and reduced emotional awareness in individuals with high-alexithymic features.
Finally, no differences between h-ALEX and l-ALEX participants were found with regard to self-reported emotional responses despite from clear differences in brain regions associated with experiencing emotions. A possible explanation could be that the task to evaluate one’s own emotional responses might have been too difficult for h-ALEX individuals. As a result of experienced difficulties, h-ALEX participants could have done the ratings according to the emotion expressed by the stimuli instead of their own emotional experience.
Limitations
Alexithymia was assessed via self-report; however, it may limit an individual’s capacity to answer items that partly require introspection and the ability to verbally express emotions (Stingl et al., 2008; Vanheule, 2008). Structured interviews can help to overcome these difficulties, but there was no validated German version of the Toronto Structured Interview for Alexithymia (Grabe et al., 2009) available at the time of data acquisition. However, we did not solely rely on the TAS-20 but added the BVAQ, a more detailed, conceptually broader and widely used questionnaire in many studies on alexithymia.
The issue of alexithymia potentially affecting the validity of self-report instruments also applies to the assessment of depressive symptoms via BDI. Although we did not find any significant differences between h-ALEX and l-ALEX individuals with regard to depression, we cannot preclude that alexithymia might have influenced an individual’s perception of depressive symptoms. To minimize any effects of depression on our findings, we (i) conducted clinical interviews to exclude participants with any history or current episode of major depression and (ii) included individual BDI scores as a covariate in all analyses.
In addition, alexithymia itself might have led to an inaccurate interpretation of their childhood environment as negligent and unsupportive. However, the items of the CTQ are carefully worded and based on external behavior (e.g. People in my family called me things like ‘stupid’, ‘lazy’ or ‘ugly’). Furthermore, to overcome possible difficulties of questionnaire assessment and misconstructions due to alexithymia, our subjects ran through a face-to-face interview to validate their individual scores of the CTQ. Participants were explicitly asked for examples of observable behavior to explain their experience of a negligent or abusive environment. In case of lacking consistency or missing examples, subjects were excluded from participation. Furthermore, participants’ potential motivation to minimize difficulties within families was controlled by using the ‘minimization and denial’ subscale of the CTQ to exclude individuals with such tendencies.
When assessing ELS, the problem of recall biases and the nature of reconstructive memory (Hyman and Loftus, 1998) has to be solved. It is usually approached by including medical records in the process of data collection or by interviewing family members. Since emotional traumata mostly lack visible bodily injury, medical records from childhood would have been of limited use. In addition, many participants were not in regular contact with their families, so that family interviews would have been difficult to arrange and probably emotionally draining for our participants. Nevertheless, this limitation should be kept in mind when interpreting the data we presented here.
Regarding fMRI design, inducing emotional experiences in h-ALEX individuals using pictures of facial affect may interfere with difficulties identifying feelings in oneself and others, which is a characteristic feature of alexithymia. However, previous studies have shown that alexithymia is not related to problems in facial emotion recognition (Kessler et al., 2006; Montebarocci et al., 2011). As McDonald and Prkachin (1990) stated in their pilot study on the expression and perception of facial emotion, alexithymia rather includes a deficit of nonverbal expression, which, as our data show, might be generated by a reduced accessibility of emotional experiences. Finally, to overcome potential shortcomings of visually induced emotions in alexithymic populations, we included musical stimuli to increase the emotional stimulation of our fMRI task.
CONCLUSION
Our results indicate that the influence of ELS on hippocampal responses to emotional stimuli is modulated by an individual’s degree of emotional functioning. Personality traits, such as alexithymia, which are associated with a variety of alterations in emotional processes seem to alter emotional brain responses as a function of ELS. We strongly suggest that future studies investigate alexithymia and its neural correlates of emotional processes in consideration of early environmental factors. Whether alexithymia shows protective qualities in individuals with a history of ELS, or whether it is associated with stronger impairments and thus an increased risk for affective disorders as reported in multiple previous studies, are important questions that future studies need to clarify. Since previous research revealed anatomical alterations of the hippocampus in individuals with a history of ELS, we recommend investigating the joint influence of ELS and alexithymia on the hippocampal volume in healthy populations to enlighten the developmental pathways of emotional abilities on multiple levels.
Acknowledgments
We thank our colleagues from the Berlin Alexithymia Research Group for their helpful suggestions, Dr Simone Grimm for her comments on a draft of the manuscript, Corinna Bonhage for her help with the behavioral prestudy, and the team of the Dahlem Institute for Neuroimaging of Emotion for their technical support. This work was supported by the German Research Foundation (DFG, Cluster of Excellence ‘Languages of Emotion’, EXC302). The study sponsor had no influence on study design, on the collection, analysis and interpretation of data, on the writing of the report and on the decision to submit the article for publication.
References
- Anastasi JS, Rhodes MG. Evidence for an own-age bias in face recognition. North American Journal of Psychology. 2006;8(2):237–53. [Google Scholar]
- Aust S, Alkan Härtwig E, Heuser I, Bajbouj M. The role of early emotional neglect in alexithymia. Psychological Trauma: Theory, Research, Practice, and Policy. 2013;5(3):225–32. [Google Scholar]
- Bagby RM, Parker JDA, Taylor GJ. The Twenty-item Toronto Alexithymia Scale—I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research. 1994a;38:23–32. doi: 10.1016/0022-3999(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JDA. The Twenty-Item Toronto Alexithymia Scale—II. Convergent, discriminant, and concurrent validity. Journal of Psychosomatic Research. 1994b;38:33–40. doi: 10.1016/0022-3999(94)90006-x. [DOI] [PubMed] [Google Scholar]
- Baumgartner T, Esslen M, Jäncke L. From emotion perception to emotion experience: emotions evoked by pictures and classical music. International Journal of Psychophysiology. 2006;60(1):34–43. doi: 10.1016/j.ijpsycho.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8(1):77–100. [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An Inventory for Measuring Depression. Archives of General Psychiatry. 1961;4(6):561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bernstein DP, Fink L. Childhood Trauma Questionnaire: A Retrospective Self-report Questionnaire and manual. San Antonio, TX: The Psychological Corporation; 1998. [Google Scholar]
- Bernstein DP, Fink L, Handelsman L, et al. Initial reliability and validity of a new retrospective measure of child abuse and neglect. American Journal of Psychiatry. 1994;151(8):1132–6. doi: 10.1176/ajp.151.8.1132. [DOI] [PubMed] [Google Scholar]
- Berthoz S, Artiges E, Van de Moortele P, et al. Effect of impaired recognition and expression of emotions on frontocingulate cortices: an fMRI Study of men with alexithymia. American Journal of Psychiatry. 2002;159(6):961–7. doi: 10.1176/appi.ajp.159.6.961. [DOI] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T. Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain. 2010;133(5):1515–25. doi: 10.1093/brain/awq060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blood AJ, Zatorre RJ, Bermudez P, Evans AC. Emotional responses to pleasant and unpleasant music correlate with activity in paralimbic brain regions. Nature Neuroscience. 1999;2(4):382–7. doi: 10.1038/7299. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the early trauma inventory. Depression & Anxiety. 2000;12(1):1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Brown S, Martinez MJ, Hodges DA, Fox PT, Parsons LM. The song system of the human brain. Cognitive Brain Research. 2004;20(3):363–75. doi: 10.1016/j.cogbrainres.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Brunson KL, Eghbal-Ahmadi M, Bender R, Chen Y, Baram TZ. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proceedings of the National Academy of Sciences. 2001;98(15):8856–61. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Andres AL, Frotscher M, Baram TZ. Tuning synaptic transmission in the hippocampus by stress: the CRH system. Frontiers in Cellular Neuroscience. 2012;6(13) doi: 10.3389/fncel.2012.00013. April 3 (doi:10.3389/fncel.2012.00013; Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Grieve S, Hoth KF, et al. Early life stress and morphometry of the adult anterior cingulate cortex and caudate nuclei. Biological Psychiatry. 2006a;59(10):975–82. doi: 10.1016/j.biopsych.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Cohen RA, Hitsman B, Paul RH, et al. Early life stress and adult emotional experience: an international perspective. The International Journal of Psychiatry in Medicine. 2006b;36(1):35–52. doi: 10.2190/5R62-9PQY-0NEL-TLPA. [DOI] [PubMed] [Google Scholar]
- Coplan JD, Mathew SJ, Abdallah CG, et al. Early-life stress and neurometabolites of the hippocampus. Brain Research. 2010;1358:191–9. doi: 10.1016/j.brainres.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad R, Wegener I, Imbierowicz K, Liedtke R, Geiser F. Alexithymia, temperament and character as predictors of psychopathology in patients with major depression. Psychiatry Research. 2009;165(1–2):137–44. doi: 10.1016/j.psychres.2007.10.013. [DOI] [PubMed] [Google Scholar]
- Craig AD. Interoception: the sense of the physiological condition of the body. Current Opinion in Neurobiology. 2003;13(4):500–5. doi: 10.1016/s0959-4388(03)00090-4. [DOI] [PubMed] [Google Scholar]
- Damasio A. Decartes’ Error: Emotion, Reason, and the Human Brain. New York: HarperCollins; 1994. [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biological Psychiatry. 2012;71(4):286–93. doi: 10.1016/j.biopsych.2011.10.021. [DOI] [PubMed] [Google Scholar]
- DeBellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biological Psychiatry. 2001;50(4):305–9. doi: 10.1016/s0006-3223(01)01105-2. [DOI] [PubMed] [Google Scholar]
- Dickie EW, Brunet A, Akerib V, Armony JL. Neural correlates of recovery from post-traumatic stress disorder: a longitudinal fMRI investigation of memory encoding. Neuropsychologia. 2011;49(7):1771–8. doi: 10.1016/j.neuropsychologia.2011.02.055. [DOI] [PubMed] [Google Scholar]
- Ebner NC, Riediger M, Lindenberger U. FACES – A database of facial expressions in young, middle-aged and older women and men: development and validation. Behavior Research Methods. 2010;42(1):351–62. doi: 10.3758/BRM.42.1.351. [DOI] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, et al. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Archives of Pediatrics & Adolescent Medicine. 2011;165(12):1069–77. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar E, Ganor O, Admon R, Bleich A, Hendler T. Feeling the real world: limbic response to music depends on related content. Cerebral Cortex. 2007;17(12):2828–40. doi: 10.1093/cercor/bhm011. [DOI] [PubMed] [Google Scholar]
- Evren C, Evren B, Dalbudak E, Oczelik B, Oncu F. Childhood abuse and neglect as a risk factor for alexithymia in adult male substance dependent inpatients. Journal of Psychoactive Drugs. 2009;41(1):85–92. doi: 10.1080/02791072.2009.10400677. [DOI] [PubMed] [Google Scholar]
- Franz M, Popp K, Schaefer R, et al. Alexithymia in the German general population. Social Psychiatry and Psychiatric Epidemiology. 2008;43:54–62. doi: 10.1007/s00127-007-0265-1. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Lanius RA. Meta-analysis of alexithymia in posttraumatic stress disorder. Journal of Traumatic Stress. 2008;21(2):243–6. doi: 10.1002/jts.20320. [DOI] [PubMed] [Google Scholar]
- Freyberger H. Supportive psychotherapeutic techniques in primary and secondary alexithymia. Psychotherapy and Psychosomatics. 1977;28(1–4):337–42. doi: 10.1159/000287080. [DOI] [PubMed] [Google Scholar]
- Grabe HJ, Löbel S, Dittrich D, et al. The German version of the Toronto Structured Interview for Alexithymia: factor structure, reliability, and concurrent validity in a psychiatric patient sample. Comprehensive Psychiatry. 2009;50:424–30. doi: 10.1016/j.comppsych.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Hatfield E, Rapson RL, Le YL. Primitive emotional contagion: Recent research. In: Decety J, Ickes W, editors. The Social Neuroscience of Empathy. Boston, MA: MIT Press; 2009. [Google Scholar]
- Heim C, Binder EB. Current research trends in early life stress and depression: Review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Expermental Neurology. 2012;233(1):102–11. doi: 10.1016/j.expneurol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the Neurobiology of Mood and Anxiety Disorders: preclinical and clinical studies. Biological Psychiatry. 2001;49(12):1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Heinzel A, Schäfer R, Müller H-W, et al. Increased activation of the supragenual anterior cingulate cortex during visual emotional processing in male subjects with high degrees of alexithymia: an event-related fMRI study. Psychotherapy and Psychosomatics. 2010;79(6):363–70. doi: 10.1159/000320121. [DOI] [PubMed] [Google Scholar]
- Honkalampi K, Koivumaa-Honkanen H, Antikainen R, Haatainen K, Hintikka J, Vinamäki H. Relationships among alexithymia, adverse childhood experiences, sociodemographic variables, and actual mood disorder: a 2-year clinical follow-up study of patients with major depressive disorder. Psychosomatics. 2004;45(3):197–204. doi: 10.1176/appi.psy.45.3.197. [DOI] [PubMed] [Google Scholar]
- Hsu DT, Langenecker SA, Kennedy SE, Zubieta J, Heitzeg MM. fMRI BOLD responses to negative stimuli in the prefrontal cortex are dependent on levels of recent negative life stress in major depressive disorder. Psychiatry Research. 2010;183(3):202–8. doi: 10.1016/j.pscychresns.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman IE, Loftus EF. Errors in autobiographical memory. Clinical Psychology Review. 1998;18(8):933–47. doi: 10.1016/s0272-7358(98)00041-5. [DOI] [PubMed] [Google Scholar]
- Jäncke L. Music, memory and emotion. Journal of Biology. 2008;7(6) doi: 10.1186/jbiol82. 21. doi:10.1186/jbiol82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juslin PN, Västfjäll D. Emotional responses to music: the need to consider underlying mechanisms. The Behavioral and Brain Sciences. 2008;31(5):559–75. doi: 10.1017/S0140525X08005293. [DOI] [PubMed] [Google Scholar]
- Karlsson H, Näätänen P, Stenman H. Cortical activation in alexithymia as a response to emotional stimuli. British Journal of Psychiatry. 2008;192(1):32–8. doi: 10.1192/bjp.bp.106.034728. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Plotsky PM, Nemeroff CB, Charney DS. Effects of early adverse experiences on brain structure and function: clinical implications. Biological Psychiatry. 2000;48(8):778–90. doi: 10.1016/s0006-3223(00)00998-7. [DOI] [PubMed] [Google Scholar]
- Kessler H, Schwarze M, Filipic S, Traue HC, von Wietersheim J. Alexithymia and facial emotion recognition in patients with eating disorders. International Journal of Eating Disorders. 2006;39(3):245–51. doi: 10.1002/eat.20228. [DOI] [PubMed] [Google Scholar]
- Khalfa S, Roy M, Rainville P, Dalla Bella S, Peretz I. Role of tempo entrainment in psychophysiological differentiation of happy and sad music? International Journal of Psychophysiology. 2008;68:17–26. doi: 10.1016/j.ijpsycho.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner D. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: a meta-analysis. Journal of Affective Disorders. 2005;88(1):79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Koelsch S. Towards a neural basis of music-evoked emotions. Trends in Cognitive Sciences. 2010;14(3):131–7. doi: 10.1016/j.tics.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Kooiman CG, van Rees Vellinga S, Spinhoven P, Draijer N, Trijsburg RW, Rooijmans HGM. Childhood adversities as risk factors for alexithymia and other aspects of affect dysregulation in adulthood. Psychotherapy and Psychosomatics. 2004;73(2):107–116. doi: 10.1159/000075542. [DOI] [PubMed] [Google Scholar]
- Kronmüller K, Pantel J, Götz B, et al. Life events and hippocampal volume in first-episode major depression. Journal of Affective Disorders. 2008;110(3):241–7. doi: 10.1016/j.jad.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Kugel H, Eichmann M, Dannlowski U, et al. Alexithymic features and automatic amygdala reactivity to facial emotion. Neuroscience Letters. 2008;435(1):40–4. doi: 10.1016/j.neulet.2008.02.005. [DOI] [PubMed] [Google Scholar]
- Kühner C, Bürger C, Keller F, Hautzinger M. Reliabilität und Validität des revidierten Beck-Depressions-Inventars. Befunde aus deutschsprachigen Stichproben. Nervenarzt. 2007;78:651–6. doi: 10.1007/s00115-006-2098-7. [DOI] [PubMed] [Google Scholar]
- Leweke F, Leichsenring F, Kruse J, Hermes S. Is alexithymia associated with specific mental disorders? Psychopathology. 2012;45(1):22–8. doi: 10.1159/000325170. [DOI] [PubMed] [Google Scholar]
- Leweke F, Stark R, Milch W, et al. Neuronale Aktivitätsmuster auf affektinduktive Reize bei Alexithymie. Psychotherapie, Psychosomatik und Medizinische Psychologie. 2004;54(12):437–44. doi: 10.1055/s-2004-828350. [DOI] [PubMed] [Google Scholar]
- Lipson SE, Sacks O, Devinsky O. Selective emotional detachment from family after right temporal lobectomy. Epilepsy & Behavior. 2003;4(3):340–2. doi: 10.1016/s1525-5050(03)00081-7. [DOI] [PubMed] [Google Scholar]
- Luminet O. Commentary on the paper “Is alexithymia a risk factor for major depression, personality disorder, or alcohol use disorders? A prospective population-based study. Journal of Psychosomatic Research. 2010;68(3):275–7. doi: 10.1016/j.jpsychores.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Lumley MA, Neely LC, Burger AJ. The assessment of alexithymia in medical settings: implications for understanding and treating health problems. Journal of Personality Assessment. 2007;89(3):230–46. doi: 10.1080/00223890701629698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald PW, Prkachin KM. The expression and perception of facial emotion in alexithymia: a pilot study. Psychosomatic Medicine. 1990;52(2):199–210. doi: 10.1097/00006842-199003000-00007. [DOI] [PubMed] [Google Scholar]
- McFarlane A, Clark CR, Bryant RA, et al. The impact of early life stress on psychophysiological, personality and behavioral measures in 740 non-clinical subjects. Journal of Integrative Neuroscience. 2005;4(1):27–40. doi: 10.1142/s0219635205000689. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Paralimbic (mesocortical) areas. In: Mesulam MM, editor. Principles of Behavioral and Cognitive Neurology. New York: Oxford University Press; 2000. pp. 49–54. [Google Scholar]
- Mitterschiffthaler MT, Fu CHY, Dalton JA, Andrew CM, Williams SCR. A functional MRI study of happy and sad affective states induced by classical music. Human Brain Mapping. 2007;28(11):1150–62. doi: 10.1002/hbm.20337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montebarocci O, Surcinelli P, Rossi N, Baldaro B. Alexithymia, verbal ability and emotion recognition. Psychiatric Quarterly. 2011;82(3):245–52. doi: 10.1007/s11126-010-9166-7. [DOI] [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130(7):1718–31. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology. 2010;214(1):55–70. doi: 10.1007/s00213-010-2009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: the neural basis of normal emotion perception. Biological Psychiatry. 2003;54(5):504–14. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Reker M, Ohrmann P, Rauch AV, et al. Individual differences in alexithymia and brain response to masked emotion faces. Cortex. 2010;46(5):658–67. doi: 10.1016/j.cortex.2009.05.008. [DOI] [PubMed] [Google Scholar]
- Richter P, Werner J, Heerlein A, Kraus A, Sauer H. On the validity of the Beck Depression Inventory. Psychopathology. 1998;31(3):160–8. doi: 10.1159/000066239. [DOI] [PubMed] [Google Scholar]
- Rush AJ, Kraemer HC, Sackheim HA, et al. Report by the ACNP task force on response and remission in Major Depressive Disorder. Neuropsychopharmacology. 2006;31(9):1841–53. doi: 10.1038/sj.npp.1301131. [DOI] [PubMed] [Google Scholar]
- Russell JA. Core affect and the psychological construction of emotion. Psychological Review. 2003;110(1):145–72. doi: 10.1037/0033-295x.110.1.145. [DOI] [PubMed] [Google Scholar]
- Schellenberg EG, Peretz I, Vieillard S. Liking for happy- and sad-sounding music: effects of exposure. Cognition and Emotion. 2008;22(2):218–37. [Google Scholar]
- Schore AN. The effects of early relational trauma on right brain development, affect regulation, and infant mental health. Infant Mental Health Journal. 2001;22(1–2):201–69. [Google Scholar]
- Sheehan DV, Lecrubrier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. Journal of Clinical Psychiatry. 1998;59:34–57. [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U. Levels of emotional awareness and autism: An fMRI study. Social Neuroscience. 2008;3(2):97–112. doi: 10.1080/17470910701577020. [DOI] [PubMed] [Google Scholar]
- Stingl M, Bausch S, Walter B, Kagerer S, Leichsenring F, Leweke F. Effects of inpatient psychotherapy on the stability of alexithymia characteristics. Journal of Psychosomatic Research. 2008;65:173–80. doi: 10.1016/j.jpsychores.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Taliaz D, Loya A, Gersner R, Haramati S, Chen A, Zangen A. Resilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factor. Journal of Neuroscience. 2011;31(12):4475–83. doi: 10.1523/JNEUROSCI.5725-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor GJ, Bagby RM, Parker JDA. Disorders of Affect Regulation. Alexithymia in Medical and Psychiatric Illness. Cambridge: Cambridge University Press; 1997. [Google Scholar]
- Taylor SE, Eisenberger NE, Saxbe D, Lehman BJ, Lieberman MD. Neural responses to emotional stimuli are associated with childhood family stress. Biological Psychiatry. 2006;60(3):296–301. doi: 10.1016/j.biopsych.2005.09.027. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Terasawa Y, Fukushima H, Umeda S. How does interceptive awareness interact with the subjective experience of emotion? An fMRI study. Human Brain Mapping. 2011;34(3):598–612. doi: 10.1002/hbm.21458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanheule S. Challenges for alexithymia research: a commentary on “The Construct of Alexithymia: Associations with Defense Mechanisms”. Journal of Clinical Psychology. 2008;64(3):332–7. doi: 10.1002/jclp.20467. [DOI] [PubMed] [Google Scholar]
- Vorst HCM, Bermond B. Validity and reliability of the Bermond-Vorst Alexithymia Questionnaire. Personality and Individual Differences. 2001;30(3):413–34. [Google Scholar]
- Vythilingam MD, Heim C, Newport J, et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. American Journal of Psychiatry. 2002;159(12):2072–80. doi: 10.1176/appi.ajp.159.12.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K, Rockstroh B, Borgelt J, et al. Stress load during childhood affects psychopathology in psychiatric patients. BMC Psychiatry. 2008;8(1):63–73. doi: 10.1186/1471-244X-8-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingenfeld K, Riedesel K, Petrovic Z, et al. Impact of childhood trauma, alexithymia, dissociation, and emotion suppression on emotional Stroop task. Journal of Psychosomatic Research. 2011;70(1):53–8. doi: 10.1016/j.jpsychores.2010.06.003. [DOI] [PubMed] [Google Scholar]
- Wingenfeld K, Spitzer C, Mensebach C, et al. The German version of the Childhood Trauma Questionnaire (CTQ): Preliminary psychometric properties. Psychotherapie, Psychosomatik & Medizinische Psychologie. 2010;60(11):442–50. doi: 10.1055/s-0030-1247564. [DOI] [PubMed] [Google Scholar]
- Woon FL, Hedges DW. Hippocampal and amygdala volumes in children and adults with childhood maltreatment-related posttraumatic stress disorder: a meta-analysis. Hippocampus. 2008;18(8):729–36. doi: 10.1002/hipo.20437. [DOI] [PubMed] [Google Scholar]
- Zackheim L. Alexithymia: The expanding realm of research. Journal of Psychosomatic Research. 2007;63(4):345–7. doi: 10.1016/j.jpsychores.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Zahradnik M, Stewart SH, Marshall GN, Shell TL, Jaycox LH. Anxiety Sensitivity and aspects of alexithymia are independently and uniquely associated with posttraumatic distress. Journal of Traumatic Stress. 2009;22(2):131–8. doi: 10.1002/jts.20397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik C, Jill IJ, Zimmermann M. The relationship between posttraumatic stress disorder, childhood trauma and alexithmia in an outpatient sample. Journal of Traumatic Stress. 2001;14(1):177–88. [Google Scholar]