Abstract
Contemporary learning theories suggest that conditioning is heavily dependent on the processing of prediction errors, which signal a discrepancy between expected and observed outcomes. This line of research provides a framework through which classical theories of placebo effects, expectations and conditioning, can be reconciled. Brain regions related to prediction error processing [anterior cingulate cortex (ACC), orbitofrontal cortex or the nucleus accumbens] overlap with those involved in placebo effects. Here we examined the possibility that the magnitude of objective neurochemical responses to placebo administration would depend on individual expectation-effectiveness comparisons. We show that such comparisons and not expectations per se predict behavioral placebo responses and placebo-induced activation of µ-opioid receptor-mediated neurotransmission in regions relevant to error detection (e.g. ACC). Expectations on the other hand were associated with greater µ-opioid system activation in the dorsolateral prefrontal cortex but not with greater behavioral placebo responses. The results presented aid the elucidation of molecular and neural mechanisms underlying the relationship between expectation-effectiveness associations and the formation of placebo responses, shedding light on the individual differences in learning and decision making. Expectation and outcome comparisons emerge as a cognitive mechanism that beyond reward associations appears to facilitate the formation and sustainability of placebo responses.
Keywords: placebo analgesia, µ-opioid receptor, expectations, prediction error signal and learning
INTRODUCTION
Placebo research theories have been divided on whether placebo effects depend on conscious expectancy (Kirsch, 1985; Price and Barrell, 2000) or learning mechanisms (Ader and Cohen, 1975; Wickramasekera, 1980; Voudouris et al., 1985, 1989); as a consequence, a number of studies have tried to tease expectancy and learning processes apart (for a review, see Stewart-Williams and Podd, 2004). Newer learning theories suggest that conditioning is related to both initial expectancies and association-based plasticity (Atlas and Wager, 2012). Rescorla and Wagner formalized a model of classical conditioning in which learning does not depend on simple contiguity between conditioned and unconditioned stimuli. Instead, conditioning depends on prediction error, which signals discrepancies between expected and observed outcome (Rescorla and Wagner, 1972). This line of work suggests that expectancies underlie most forms of learning (Reiss, 1980). Therefore, prediction error signal theories, as defined by Rescorla and Wagner (1972), would provide a mechanism through which classical theories of placebo analgesia, expectations of clinical improvement and conditioning are reconciled and placebo responses would emerge as a consequence of expectation and outcomes associations. If this was to be the case, the formation and maintenance of placebo effects would represent an instance of expectation and outcome comparisons, and an extension of the mechanisms involved in motivated behavior.
Brain regions involved in the formation of error predictions include the striatum, orbitofrontal cortex and anterior cingulate cortex (Lauwereyns et al., 2002; Samejima et al., 2005; Matsumoto and Hikosaka, 2007; Rushworth et al., 2007). Some of these regions overlap with those in which the endogenous opioid system becomes active during placebo administration in the context of expectations of analgesia (Zubieta et al., 2005; Wager et al., 2007; Scott et al., 2008), suggesting that the endogenous opioid system, in addition to its engagement in analgesia, may also be regulating the function of regions that could potentially be implicated in learning and therefore the sustainability of placebo effects (Pecina et al., 2013).
An extensive literature has related the encoding of expected values and their probabilities to the mesolimbic dopamine (DA) system (Schultz, 1998; Fiorillo et al., 2003; Tobler et al., 2005; Fiorillo et al., 2008). In the context of placebo analgesia, work by our group has previously shown that anticipated placebo effects and the mismatch between anticipated and perceived outcomes are linearly related to the activation of nucleus accumbens (NAc) DA neurotransmission during placebo administration (Scott et al., 2007). Furthermore, the individual neural responses to anticipated monetary rewards tracked the subjects’ expectations of analgesia and deviations from those predictions. Another potential neurotransmitter system that could be involved in the encoding of expectations and their probabilities in placebo responses would be the endogenous opioid system. This system has been classically involved in placebo effects in the context of expectations of analgesia (Kozel et al., 1980; Zubieta et al., 2005; Wager et al., 2007; Scott et al., 2008). Animal models have shown that blocking opioidergic neurotransmission in structures relevant to fear conditioning [amygdala, periaqueductal gray (PAG), rostral ventromedial medulla] inhibits the development of conditioned hypoalgesia (Bellgowan and Helmstetter, 1998; Foo and Helmstetter, 1999). In humans, an fMRI study has shown that blocking opioidergic neurotransmission via naloxone inhibited the development of conditioned hypoalgesia in a conditioning-dependent anticipatory antinociceptive network [rostral anterior cingulate cortex (ACC), PAG and amygdala] leading to more sustained responses to the unconditioned stimulus (Eippert et al., 2008). Given its role in conditioned hypoalgesia, this neurotransmitter system could be a good candidate not simply to exert placebo analgesic effects induced by positive and negative expectations, but also to carry the probabilistic value through which expectations and their comparison with the individual assessment of outcomes relate to the generation of placebo responses.
Here we aimed to examine a potential mechanism through which expectation and outcome comparisons predict placebo responses. Expectation and subjectively reported effectiveness comparisons would appear as a learning mechanism, possibly reconciling classical theories of placebo, expectations and conditioning. To test this hypothesis we used positron emission tomography (PET) and the µ-opioid receptor selective radiotracer [11C]carfentanil during a sustained pain challenge with and without placebo administration. We hypothesized that expectation and outcome comparisons, and not expectations alone, would predict the formation of placebo effects. Further, that neurochemically, µ-opioid neurotransmission in cognitive regions, such as the dorsolateral prefrontal cortex (DLPFC) (Wager et al., 2004; Keltner et al., 2006; Kong et al., 2006), will be relevant for the formation of expectations but not necessarily placebo analgesic effects. If prediction error theories underlie the formation of placebo responses, µ-opioid neurotransmission in regions related to error detection, such as the ACC, the orbitofrontal cortex (OFC) or the NAc, would be associated with reductions in pain ratings during placebo administration.
EXPERIMENTAL PROCEDURES
Subjects
Forty-eight right-handed non-smoking subjects (21 males and 27 females) ranging in age from 19 to 38 years (mean ± 1 s.d.: 26.15 ± 4.9 years) were recruited via advertisement. In addition to completing physical and neurological examinations, study participants underwent screening using the non-patient version of the Structured Clinical Interview for DSM-IV. Participants had no history of or current medical, neurological or psychiatric illnesses, including substance abuse, or dependence, and had alcohol intake of less than five drinks per week. Written informed consent was obtained in all cases. All of the procedures used were approved by the University of Michigan Investigational Review Board for Human Subject Use and the Radioactive Drug Research Committee.
Experimental design
The experimental design, in the absence or presence of placebo, consisted of a control, non-painful condition when pain is potentially expected but not received (0.9% isotonic saline, 5–25 min after start of scanning) and a painful condition (5% hypertonic saline, 45–65 min after start of scanning), infused in the masseter muscle. Volunteers were told that these two conditions would take place, but not their order, allowing for expectation of pain during the non-painful control condition. In the pain condition, a steady state of moderate muscle pain was maintained for 20 min. In this model of sustained deep somatic pain, the intensity of the painful stimulus is standardized across subjects, as described in detail previously (Zhang et al., 1993; Stohler and Kowalski, 1999). Briefly, volunteers are asked to rate pain intensity every 15 s from 0 (no pain) to 100 (most intense pain imaginable) using an electronic 0–100 visual analog scale (VAS) placed in front of the scanner gantry during both control and pain conditions. A suitable infusion rate for the maintenance of pain over time (i.e. VAS of 50) was then estimated by comparing the subject’s response to the mean response of 65 subjects of the same age range exposed to the same bolus. From that point on, the adaptive controller depended on feedback from the subjects. The subject ratings of pain intensity, acquired every 15 s, were fed back to the computer via an analog-digital board, which then changed the infusion rate to maintain pain at similar levels over time. The individual infusion profiles generated during the pain challenges were then repeated for the studies with placebo administration (Scott et al., 2008), maintaining the painful stimulus the same across both, the pain and the pain + placebo condition.
During the placebo condition, subjects were given the following instructions before administration of the placebo: ‘We are studying the effect of a pain relief medication. This medication is thought to have analgesic effects through the activation of natural brain systems that suppress pain’. The placebo condition consisted of the introduction of 1 mL of 0.9% isotonic saline into one of the intravenous ports every 4 min, with the volunteer being made aware that was being the case, starting 2 min before the pain challenges, and lasting for 15 s each time. Subjects were aware that the study drug was to be administered because they were alerted by a computer-generated human voice recording, followed by a second-by-second count of the infusion timing (15 s). Each subject underwent four pain challenges, two of them with placebo administration, as previously described (Scott et al., 2008), and the order of each pair of pain and pain + placebo studies was randomized, but only the results of two of the sets are reported here, those associated with [11C]carfentanil PET scanning.
Immediately after the pain and the pain + placebo challenges subjects completed the McGill Pain Questionnaire (MPQ) (Melzack and Torgerson, 1971). These measures, together with the 0–100 VAS pain intensity ratings acquired every 15 s in the absence and presence of placebo was used as the primary endpoint for the assessment of placebo responses.
For the specific study of prediction error, subjects were asked to estimate the expected analgesia before the introduction of the placebo by answering the question: ‘From 0 to 100 how effective do you think the treatment will be?’ After the pain challenges, they were asked to subjectively estimate the efficacy of the placebo using a VAS ranging from 0 (no analgesic effect) to 100 (maximum analgesia) by answering the question: and ‘From 0 to 100 how effective was the treatment?’
Neuroimaging methods
As previously described (Scott et al., 2008), during the experimental condition two 90 min PET studies per subject were acquired (HR+ scanner; Siemens, Knoxville, TN) in three-dimensional mode (reconstructed full-width/half-maximum resolution, approximately 5.5 mm in plane and 5.0 mm axially), with the septa retracted and scatter correction. Participants were positioned in the PET scanner gantry, and two intravenous (antecubital) lines were placed. A light forehead restraint was used to eliminate intrascan head movement. [11C]carfentanil was synthesized at high specific activity by the reaction of [11C]methyl iodide and a normethyl precursor as previously described (Jewett, 2001). 15 ± 1 mCi were administered per scan session (≤0.03 µg/ml cold mass). Fifty percent of the radiotracer doses were administered as an initial bolus and the remaining 50% by continuous infusion for the remainder of the study to more rapidly achieve steady-state levels. For each study, 21 sets of dynamic scans were acquired with an increasing duration (four 30 s frames, three 1 min frames, two 2.5 min frames, eight 5 min frames and four 10 min frames). Images were reconstructed using iterative algorithms (brain mode; Fourier rebinning algorithm with ordered-subsets expectation maximization, four iterations, and 16 subsets; no smoothing) into a 128 × 128-pixel matrix in a 28.8-cm diameter field of view. Attenuation correction was performed through a 6 min transmission scan (Ge68 source) obtained before the PET study and with iterative reconstruction of the blank/transmission data, followed by segmentation of the attenuation image. Small head motions during PET were corrected by an automated computer algorithm for each subject before analysis, and the images were coregistered with the same software (Minoshima et al., 1993). Time points were then decay corrected during reconstruction of the PET data. Image data were then transformed on a voxel-by-voxel basis into two sets of parametric maps, a tracer transport measure (K1 ratio) and a receptor-related measure (non-displaceable binding potential BPND). To avoid the need for arterial blood sampling, these measures were calculated using a modified Logan graphical analysis (Logan et al., 1996) and the occipital cortex (an area devoid of μ-opioid receptors) as a reference region. Using the bolus-continuous infusion protocol described above, the slope of the Logan plot becomes linear 5–7 min post-tracer administration and is proportional to the receptor concentration divided by its affinity for the radiotracer [(f2Bmax/Kd) +1]; f2Bmax/Kd is termed non-displaceable binding potential (BPND) (Innis et al., 2007) or receptor availability in vivo. Bmax is the receptor concentration and Kd, the receptor-ligand dissociation constant. The term f2 refers to the concentration of free radiotracer in the extracellular fluid and is considered to represent a constant and very small value. Reductions in the in vivo availability of receptors after an acute challenge (i.e., placebo administration during an experimental pain challenge) are thought to reflect processes, such as competition between radiotracer and endogenous ligand, associated with neurotransmitter release (Narendran and Martinez, 2008).
Anatomical MRI studies were acquired before PET on a 3 T scanner (General Electric, Milwaukee, WI). Acquisition sequences were axial spoiled gradient recall inverse recovery prepared magnetic resonance [echo time, 3.4 ms; repetition time, 10.5 ms; inversion time, 200 ms; flip angle, 25°; number of excitations, 1; using 124 contiguous images, 1.5 mm thickness]. The K1 and BPND images for each experimental period and the MRIs were coregistered to each other and to the International Consortium for Brain Mapping stereotactic atlas orientation (Meyer et al., 1997).
Data analysis
In order to create a measure of expectations and counterfactual comparisons and to avoid exclusively correlative analyses, subjects were assigned to a Low (≤50) or High (>50) Expectations or Effectiveness group based on their answers to the questions: from 0 to 100 how effective do you think the medication will be? (Expectations, prior to scan) and from 0 to 100 was the medication effective? (Subjective assessment of effectiveness, after scan); 19 subjects were classified as having High Expectations and 29 as having Low Expectations. When the two variables were combined, these resulted in two groups: a High Expectation/Low Effectiveness group (n = 7), Low Expectations/Low Effectiveness group (n = 19), High Expectations/High Effectiveness group (n = 13) and Low Expectations/High Effectiveness group (n = 9). This categorical approach was preferred to the subtraction of expectation and subjective assessment, classically know as prediction error signal (Rescorla and Wagner, 1972). The latter would include under the same value very different behavioral responses (e.g. subjects with expectations of 100 and effectiveness of 100 will have the same prediction error signal as subjects with expectations of 0 and effectiveness of 0).
A mixed effects analysis of variance was applied on a voxel-by-voxel basis with four within-subject conditions (pain ± placebo, and their corresponding baselines) and the Expectation or Expectation/Effectiveness group as the between subjects factor in separate analysis. A main interaction effect was tested between the Expectation or Expectation/Effectiveness groups and the within-subject conditions on the opioid activation maps. Activation maps were deemed significant at P < 0.05, and K > 10 after false discovery rate correction for multiple comparisons. For a priori hypothesized regions associated with expectations (DLPFC), predictions of outcomes (OFC, ACC and NAc) and learning (amygdala and hippocampus) a P < 0.001 and K > 10 was considered significant. These data were extracted for examination of the presence of outliers, graphing and the calculation of percent changes in BPND. Using SPSS statistical software (version 19.0; SPSS Inc, Chicago, IL), these values were used to plot the data and perform correlation analyses and to confirm the voxel-by-voxel results for all analyses. Planned analyses included correlations between expectation vs subjective observed outcome comparisons and placebo-induced effects on neurotransmission and psychophysical ratings as measured by the MPQ and the average of momentary pain intensity ratings (VAS) acquired every 15 s during the study.
Mediation analysis
We used the SPSS 20.0 macro Mediate.sbs (http://afhayes.com/spss-sas-and-mplus-macros-and-code.html) to estimate the path coefficients and the size of the indirect effect of the multiple mediator model X (the categorical variable ‘expectation-outcome comparison group’) on Y (the continuous variable ‘changes in VAS/MPQ scores after placebo administration’) through Z (the continuous variable ‘expectation-outcome comparison group effect on regional µ-opioid activation during placebo administration’). µ-Opioid system activation values for each region were modeled simultaneously.
RESULTS
Effects of expectations on placebo analgesia
The variable levels of expectations were normally distributed and had a mean of 49.6 ± 27 (s.d.) with values ranging from 0 to 100. When testing for the effects of Expectations on psychophysical placebo effects, the analysis of covariance model showed no effect of Expectations (Low vs High) on placebo-associated reductions in pain ratings, as measured by the change (Δ) in the average VAS intensity acquired every 15 s (Δ Low = 5.8 ± 15.5, Δ High = 5.9 ± 14, F = 0.001, P = 0.97) or the MPQ Total score (Δ Low = 2.1 ± 8,7, Δ High = 1.2 ± 8.6, F = 0.10, P = 0.75) after placebo administration. Moreover, expectations of pain relief were not correlated with placebo-induced decreases in pain ratings during placebo administrations: (Δ VAS: r = 0.04, P = 0.8; Δ MPQ: r = −0.08, P = 0.5).
Effects of expectation group on placebo-induced Δ in µ-opioid BPND
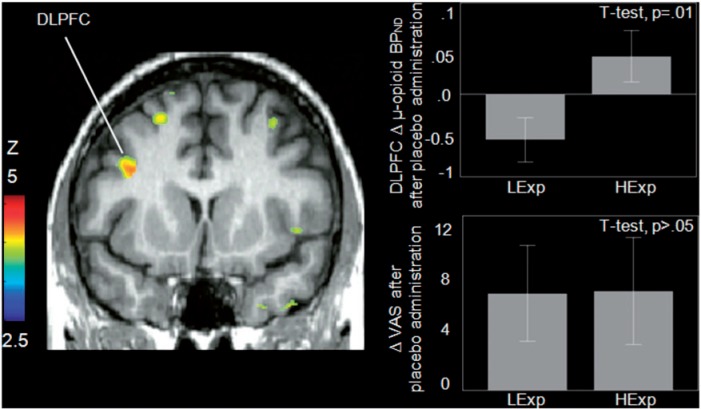
The brain voxel-by-voxel analysis revealed an effect of expectation group (High > Low Expectations) on the Δ in µ-opioid BPND after placebo administration in the left DLPFC (a priori hypothesized regions, P < 0.001 uncorr., K > 10): [(x, y, z, MNI coordinates) −34, 10, 28, 127 mm3, Z = 3.63] (Figure 1). µ-Opioid release in the DLPFC was not significantly correlated with decreases in pain ratings after placebo administration.
Fig. 1.
Effect of expectation classification (Low vs High) on µ-opioid system activation. Left: expectation group effects (High > Low) on Δ in µ-opioid BPND after placebo administration (P < 0.001, k > 10 voxels) were found in the DLPFC. Upper Right: regions effect of expectation group on Δ in µ-opioid BPND after placebo administration in the DLPFC; lower right: no significant effect of expectations group on Δ VAS ratings after placebo administration was found.
We found no significant effect of expectation group on opioid system activation during the pain challenge (control minus pain condition).
Effects of expectation and outcome comparison group on placebo analgesia
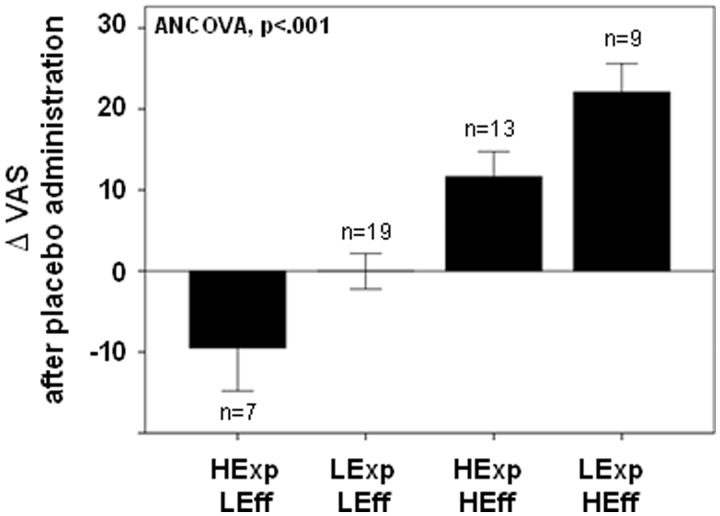
When testing for the effects of Expectation and Outcome comparison group on placebo-induced analgesic responses, a significant effect was found on the Δ in the average VAS intensity ratings over the 20 min challenge (F = 14.3; P < 0.001) and in the MPQ Total scores (F = 6.7; P = 0.001). Subjects showing a positive prediction error signal (Low Expectations and High Effectiveness ratings) had the greater placebo response (Δ VAS: 22 ± 10; Δ MPQ: 10 ± 7.4), whereas lower placebo responses were observed in subjects showing High Expectations/High Effectiveness (Δ VAS: 10 ± 7.4; Δ MPQ: 3.3 ± 8). Subjects showing a negative prediction error signal (High Expectations and Low Effectiveness) had mean hyperalgesic, ‘nocebo’, responses after placebo administration (Δ VAS: −9 ± 14; Δ MPQ: −5.5 ± 10). No mean effects on pain ratings during placebo administration were observed in volunteers who rated Low Expectations and Low Effectiveness (Δ VAS: 0 ± 9.6; Δ MPQ: −0.4 ± 5.6). Significant differences between groups were observed with post hoc analysis with Bonferroni correction for multiple comparisons (Figure 2, Table 1).
Fig. 2.
Effect of expectations-effectiveness classification on placebo-induced Δ in average VAS intensity ratings acquired over 20 min. Greater placebo effects were observed in those with a positive prediction error signal (Low Expectations and High Effectiveness), whereas Lower placebo effects were observed in those with a negative prediction error signal (High Expectations and Low Effectiveness).
Table 1.
Effects of Expectation (Expect.) − Effectiveness (Effect.) comparisons on MPQ and VAS score changes after placebo administration
| High Expect/ Low Effect (1) | Low Expect/ Low Effect (2) | High Expect/High Effect(3) | Low Expect/High Effect(4) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| mean (s.d) | mean (s.d) | mean (s.d) | mean (s.d) | F | Sig. | Post hoca | Mean diff. (s.e.) | Sig. | ||
| Number of subject | 7 | 19 | 13 | 9 | ||||||
| Expectations | 75.7 ± 14 | 24.2 ± 14.2 | 76.15 ± 13.1 | 43 ± 11.3 | 48.6 | <0.001 | 4 > 1 | −30.2 ± 6.7 | <0.001 | |
| 4 > 2 | 20.2 ± 5.4 | 0.003 | ||||||||
| 4 > 3 | −31.7 ± 5.8 | <0.001 | ||||||||
| 3 > 2 | 51.9 ± 4.8 | <0.001 | ||||||||
| 2 > 1 | −51.5 ± 5.9 | <0.001 | ||||||||
| Subjective effectiveness | 18.5 ± 12.5 | 19.15 ± 14.57 | 75 ± 14.9 | 71.1 ± 11.4 | 61.4 | <0.001 | 4 > 1 | 52.5 ± 6.9 | <0.001 | |
| 4 > 2 | 51.9 ± 6 | |||||||||
| 3 > 1 | 56.4 ± 6.5 | |||||||||
| 3 > 2 | 55.8 ± 5 | |||||||||
| Δ MPQ after placebo | −5.5 ± 10 | −0.4 ± 5.6 | 3.3 ± 8 | 10 ± 7.4 | 6.7 | 0.001 | 4 > 1 | 15.5 ± 3.7 | 0.001 | |
| 4 > 2 | 10.3 ± 3 | 0.007 | ||||||||
| Δ VAS after placebo (0–20 min) | −9 ± 14 | 0 ± 9.6 | 11 ± 11 | 22 ± 10 | 14.3 | <0.001 | 4 > 1 | 31 ± 5 | <0.001 | |
| 4 > 2 | 22 ± 4.4 | <0.001 | ||||||||
| 3 > 1 | 21.1 ± 5.1 | 0.001 | ||||||||
| 3 > 2 | 11.7 ± 4 | 0.028 | ||||||||
aBonferroni correction.
Effects of expectation and outcome comparison group on Placebo-Induced Δ in µ-opioid BPND
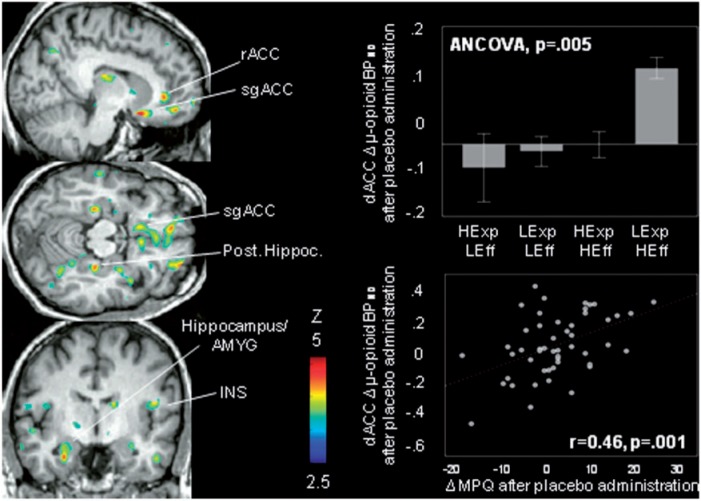
The brain voxel-by-voxel model showed an effect of expectation and outcome comparison group in the following regions (for all a priori hypothesized regions, P < 0.001 uncorr., K > 10): subgenual and rostral ACC [(x, y, z, MNI coordinates) −9, 12, −18, 9066 mm3, Z = 4.02]; dorsal ACC (−15, 33, 31, 257 mm3, Z = 3.57); right OFC (23, 45, −18, 2334 mm3, Z = 3.76), the anterior hippocampus/amygdala bilaterally (left: −28, −8, −27, 931 mm3, Z = 3.76; right: 30, −5, −17, 599 mm3, Z = 3.34) and posterior hippocampus bilaterally (left: −28, −26, −3, 1446 mm3, Z = 4.32; right: 27, −27, −15, 429 mm3, Z = 3.71) (Figure 3). Although not a priori hypothesized to be involved in expectations and outcomes comparisons, we also found an effect in the thalamus (THA) (left: −12, −21, 19, 501 mm3, Z = 3.46) and the insula (INS) bilaterally (left dorsal anterior: −33, 12, −3, 2346 mm3, Z = 3.97; right posterior: 53, −2, 11, 931 mm3, Z = 3.81). Post hoc analyses with Bonferroni correction for multiple comparisons are shown in Table 2.
Fig. 3.
Effect of Expectation − Effectiveness classification on µ-opioid system activation. Left: significant effects of Expectation − Effectiveness comparisons on Δ in µ-opioid BPND during placebo administration were found in (P < 0.001, k > 10 voxels) in the left subgenual, rostral and dorsal anterior cingulate cortex (sgACC, rACC, dACC), the OFC bilaterally and the hippocampus/amygdala (AMYG) bilaterally, the posterior hippocampus bilaterally, the left thalamus and the anterior insula bilaterally (INS). Upper right: effect of expectation-effectiveness comparisons on Δ regional in µ-opioid BPND during placebo administration in the dACC. Lower right: placebo-induced activation of µ-opioid neurotransmission in the dACC was correlated with reductions in pain ratings after placebo administration as measured by the change in MPQ total scores (MPQ: r = 0.4, P = 0.001).
Table 2.
Expectation − Effectiveness comparison group effect on regional opioid system activation during placebo administration
| Whole brain |
Extracted regional peaks |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| High Expect/ Low Effect (1) | Low Expect/ Low Effect(2) | High Expect/ High Effect (3) | Low Expect/ High Effect (4) | ||||||||
| Regional Δ µ-opioid BPND after placebo | H | Coordinates | Z | Mean (S.E.M.) | Mean (S.E.M.) | Mean (S.E.M.) | Mean (S.E.M.) | F | Sig. | Post hoca | Sig. |
| sgACC | L | −9 12 −18 | 4.43 | −0.18 ± 0.05 | −0.01 ± 0.03 | 0.15 ± 0.05 | 0.13 ± 0.08 | 6.6 | 0.001 | 4 > 1 | 0.006 |
| 3 > 1 | 0.002 | ||||||||||
| rACC | L | − 15 39 −2 | 4.38 | −0.08 ± 0.05 | −0.07 ± 0.03 | 0.10 ± 0.04 | 0.12 ± 0.05 | 8.14 | <0.001 | 4 > 1 | 0.025 |
| 4 > 2 | 0.003 | ||||||||||
| 3 > 1 | 0.031 | ||||||||||
| 3 > 2 | 0.003 | ||||||||||
| dACC | L | − 15 33 31 | 3.56 | −.0.06 ± 0.09 | −0.01 ± 0.04 | 0 ± 0.04 | 0.21 ± 0.03 | 5 | 0.005 | 4 > 1 | 0.012 |
| 4 > 2 | 0.008 | ||||||||||
| 4 > 3 | 0.032 | ||||||||||
| OFC | L | − 20 49 3 | 4.1 | 0.005 ± 0.06 | −0.08 ± 0.03 | 0.07 ± 0.03 | 0.09 ± 0.04 | 5 | 0.003 | 4 > 2 | 0.009 |
| 3 > 2 | 0.015 | ||||||||||
| ANT HIPP./AMY | R | 30 −5 −17 | 3.34 | −0.32 ± 0.13 | −0.01 ± 0.04 | 0.15 ± 0.05 | 0.13 ± 0.08 | 6.4 | 0.001 | 4 > 1 | 0.003 |
| 3 > 1 | 0.001 | ||||||||||
| 2 > 1 | 0.023 | ||||||||||
| L | −28 −8 −27 | 3.79 | −.22 ± .04 | −.07 ± .03 | .06 ± .03 | .01 ± .04 | 8.6 | <.001 | 4 > 1 | 0.004 | |
| 3 > 1 | <0.001 | ||||||||||
| 3 > 2 | 0.031 | ||||||||||
| 2 > 1 | 0.05 | ||||||||||
| POST HIPP. | R | 27 −27 −15 | 3.71 | −0.18 ± 0.03 | −0.05 ± 0.01 | 0.04 ± 0.04 | −0.01 ± 0.02 | 9.1 | <0.001 | 4 > 1 | 0.006 |
| 3 > 1 | <0.001 | ||||||||||
| 3 > 2 | 0.035 | ||||||||||
| 2 > 1 | 0.02 | ||||||||||
| L | −28 −26 −3 | 4.32 | −0.03 ± 0.05 | −0.06 ± 0.02 | 0.13 ± 0.04 | 0.04 ± 0.07 | 4.5 | 0.007 | 3 > 2 | 0.005 | |
| THA | L | −12 −21 19 | 3.46 | −0.27 ± 0.1 | −0.14 ± 0.04 | 0.08 ± 0.04 | 0.10 ± 0.07 | 8.9 | <0.001 | 4 > 1 | 0.002 |
| 4 > 2 | 0.014 | ||||||||||
| 3 > 1 | 0.001 | ||||||||||
| 3 > 2 | 0.01 | ||||||||||
| INS | R | 53 −2 11 | 3.81 | −0.14 ± 0.08 | −0.06 ± 0.02 | 0.08 ± 0.02 | −0.01 ± 0.03 | 5.7 | 0.002 | 3 > 1 | 0.003 |
| 3 > 2 | 0.014 | ||||||||||
| L | −33 12 −3 | 3.97 | −0.05 ± 0.06 | −0.06 ± 0.04 | −0.01 ± 0.02 | 0.18 + 0.04 | 6 | 0.002 | 4 > 1 | 0.018 | |
| 4 > 2 | 0.001 | ||||||||||
| 4 > 3 | 0.027 | ||||||||||
aBonferroni correction.
DMPFC and sgACC, rACC and dACC: subgenual, rostral and dorsal anterior cingulate; OFC: orbitofrontal cortex; Hipp.: hippocampus; AMY: amygdala; INS: insula; THA: thalamus; H: Hemisphere; L: left; R: right; (S.E.M): standard error of the mean.
We found no significant effect of expectation and outcome comparison group on opioid release during the pain challenge (control minus pain condition).
The effects of expectation and outcome comparisons on placebo-induced activation of µ-opioid neurotransmission were indeed correlated with placebo-induced reductions in pain ratings after placebo administration as measured by the average VAS intensity ratings and the change in MPQ total scores: left subgenual ACC (MPQ: r = 0.35, P = 0.01), left rostral ACC (VAS: r = 0.35, P = 0.01; MPQ: r = 0.43, P = 0.002), left dorsal ACC (VAS: r = 0.32, P = 0.02; MPQ: r = 0.47, P = 0.001), right posterior hippocampus (VAS: r = 0.30, P = 0.04), left lateral THA (VAS: r = 0.40, P = 0.005; MPQ: r = 0.47, P = 0.001) and left dorsal anterior INS (VAS: r = 0.32, P = 0.02; MPQ: r = 0.44, P = 0.002).
Mediation analysis
We used a path model (mediation analysis) to test our mechanistic hypothesis that the effect of expectation and subjective effectiveness comparisons on behavioral placebo responses will be mediated by the activation of the µ-opioid system in regions relevant to detection error and learning (OFC, ACC, amygdala and hippocampus). Among the regions where expectation-outcome comparison group showed a significant effect on opioid release during placebo administration, only those that showed a significant correlation with behavioral placebo responses (rostral, subgenual and dorsal ACC, THA, posterior hippocampus and INS) were included in the model. The mediation analysis confirmed that the expectation and outcome comparison group effect on behavioral placebo responses using the total MPQ (as a more comprehensive, integrative assessment of the individual pain experience) is mediated by µ-opioid release in the dACC. The mean indirect effect (coeff. = 12.9) from the bootstrap analysis was significant, with a 95% confidence interval excluding zero (0.08–3.16).
DISCUSSION
The present work suggests that a priori expectations are not sufficient to explain the formation of placebo analgesic responses over a sustained period of time, exemplified here as a 20 min experimental pain model. Using this model, we observed a lack of significant relationships between the level of expectations of pain relief and placebo-associated reductions in pain ratings. Using objective neurochemical measures acquired with PET, individuals with high expectations showed greater µ-opioid system activation in the DLPFC that were not associated with placebo analgesic effects. These findings represent an apparent discrepancy with classical theories where the formation of placebo responses is dependent on the development of positive expectations. Conversely, a learning mechanism defined by the discrepancy between expectations and subjectively rated effectiveness was associated with placebo analgesic responses, and with the activation of regional µ-opioid neurotransmission in a substantial number of regions implicated in opioid-mediated antinociception (Zubieta et al., 2001) (ACC, OFC, amygdala, hippocampus, THA, INS). The largest placebo responses were observed in those with low expectations and high subjective effectiveness (positive prediction error signal) whereas nocebo, hyperalgesic responses, were observed in those reporting high expectations and low reported effectiveness (negative prediction error signal). The magnitude of µ-opioid system activation in regions relevant to error detection was further associated with placebo-induced analgesia, as measured by the changes in momentary pain intensity VAS ratings acquired throughout the study, and the more integrative MPQ ratings acquired at the completion of the pain challenges. Moreover, our data confirmed that the effect of expectation and outcome associations on behavioral placebo responses is mediated by endogenous opioid release and µ-opioid receptor system activation in the dACC.
It has been classically postulated that placebo responses are generated mainly by two distinct mechanisms across clinical conditions, one of which concerns expectations (Kirsch, 1985; Price and Barrell, 2000) and the other one learning via classic Pavlovian conditioning (Ader and Cohen, 1975; Wickramasekera, 1980; Voudouris et al., 1985, 1989). The relationship between these two is still unclear, but it has been the subject of experimental research in recent years. Benedetti et al. (2003) were able to show in both experimental pain and in Parkinson’s disease that expectations were critical for the formation of placebo responses for measures observable or subjectively rated by the volunteers (pain, motor function); however, unconscious processes, such as hormonal secretion, were not affected by expectations alone, but required conditioning. It has also been suggested that expectancies acquired through verbal instructions might also be seen as conditioning stimuli that reactivate earlier stimulus associations (Klinger et al., 2007). Here we show that more than positive expectations per se the discrepancy between actual and predicted pain relief (prediction error signal) plays an important role in the formation of placebo responses suggesting a mechanism that might reconcile previous theories of the placebo effect.
Growing evidence (Wager et al., 2004; Benedetti et al., 2006) highlights the critical role of the PFC in placebo effects during expectation of analgesia. Individual differences in expectancy effects on pain and the magnitude of neural responses to placebo treatment have consistently engaged the DLPFC and the OFC, regions associated with cognitive control and expected value computation (Wager et al., 2004; Zubieta et al., 2005; Keltner et al., 2006; Kong et al., 2006) as well as in cognitive and attention-related elements of the pain experience (Wiech et al., 2008). Consistent with this line of research, we show greater µ-opioid neurotransmission in the DLPFC, but not the OFC, during placebo administration when high expectations were present. The OFC has been also involved with the update of the chosen value (Feierstein et al., 2006), as was confirmed in our study. Furthermore, the OFC has been hypothesized to carry essential information for associative learning regarding the value of expected rewards (Schoenbaum et al., 2003; Takahashi et al., 2011). A large body of literature has involved the ACC in detecting discrepancies between actual and intended outcomes (Amador et al., 2000; Ito et al., 2003; Matsumoto et al., 2007; Sallet et al., 2007), proving a signal to drive learning as contingencies change (Behrens et al., 2007). In particular, dACC is thought to be important linking reward-related information to action (Williams et al., 2004; Sheth et al., 2012), monitoring for conflict between competing responses (Carter et al., 1998; Botvinick et al., 1999; Botvinick et al., 2004; Botvinick, 2007) or detecting the likelihood of error commission (Carter et al., 1998; Brown and Braver, 2005) and outcome surprisingness (Hayden et al., 2011). Here, in addition to confirming the engagement of the ACC in expectation and outcome comparisons during placebo analgesia, we also show that µ-opioid neurotransmission in the dACC mediates the effect of such comparisons on psychophysical placebo responses. An effect of expectation and outcome comparison group was also found in the medial temporal lobe (amygdala–hippocampal complex and parahippocampal gyrus) and the thalamus. Much evidence links the amygdala and its target, the basal forebrain, with surprise-based learning (Reiss et al., 1982; Denis et al., 1983; Reiss and Szyszko, 1983). Moreover, the amygdala plays a role in both acquisition and consolidation of cued-fear associative learning (Reiss, 1973; Tashkin et al., 1977). The hippocampus, which receives reward-related information from the amygdala and OFC (Amaral and Cowan, 1980; Suzuki and Amaral, 1994), is also engaged during expectation and outcome comparisons. This is consistent with the role of this region computing an uncertainty signal which may constitute a fundamental mechanism underlying the role of the hippocampus in a number of functions, including attention-based learning, associative learning, probabilistic classification and binding of stimulus elements (Vanni-Mercier et al., 2009). Finally, the left dorsal anterior insula, ipsilateral to the painful stimulus, showed the greatest opioid release in those having low expectations and high effectiveness. Consistent with these results, the dorsal anterior insula has been found to be functionally connected to a set of regions previously described as a cognitive control network (Dosenbach et al., 2007). In particular, the dorsal anterior insula/dACC network has been involved in decision making: activity in these regions has been shown to increase with the degree of difficulty or uncertainty in a decision (Critchley et al., 2001; Grinband et al., 2006; Thielscher and Pessoa, 2007).
Supporting the role of µ-opioid neurotransmission in learning mechanisms, rodent studies have shown that injection of µ-opioid, but neither δ- nor κ-opioid, antagonists into the amygdala can inhibit the production of conditioned hypoalgesia (Bellgowan and Helmstetter, 1998; Foo and Helmstetter, 1999). In humans, the role of endogenous opioid neurotransmission in fear conditioning has been demonstrated where blocking opioidergic neurotransmission via naloxone inhibited the development of conditioned hypoalgesia, leading to more sustained responses to the unconditioned stimuli. Naloxone also blocked processing in a conditioning-dependent anticipatory antinociceptive network that includes areas with a high density of opioid receptors including rACC, amygdala and PAG. Finally, naloxone led to stronger conditioned responses and more sustained conditioning-dependent activity in the amygdala (Eippert et al., 2008). On the other hand, substantial evidence has directly related the activation of the opioid system to placebo during expectations of analgesia (Kozel et al., 1980; Zubieta et al., 2005; Wager et al., 2007; Scott et al., 2008); however, its role in encoding expectation and outcome comparisons as it relates to placebo responses has not been described so far. Consistently with the role of DA in prediction error signal (Schultz, 1998; Fiorillo et al., 2003; Tobler et al., 2005; Fiorillo et al., 2008), we have previously shown that the introduction of a placebo during pain anticipation was associated with the activation of DA neurotransmission and D2/D3 receptors in the NAc in a manner proportional to the anticipated analgesic effects of the otherwise inert agent as well as with the difference between anticipated and subjectively perceived effectiveness of the placebo. Furthermore, the individual neural responses to anticipated monetary rewards tracked the subjects’ expectations of analgesia and deviations from those predictions (Scott et al., 2007). Here, we extend those results by confirming that, in addition to the role of the DA system in prediction error signal, the µ-opioid system appears to code for the emotional response to positive and negative expectations and their comparison with observed outcomes in the context of placebo administration. The opioid and DA systems are closely related functionally and neuroanatomically (Di Chiara and Imperato, 1988), which might be responsible for the role of these two neurotransmitter systems in prediction error signaling. It could also be hypothesized that whereas the DA system, represented subcortically in the VTA-NAc projections, plays a role in anticipation of placebo analgesia, the opioid system, represented cortically, plays a greater role in expectation and outcome associations; this same system, in subcortical regions such as the amygdala, THA and in the INS, is centrally involved in stress and pain regulation (Zubieta et al., 2001). This prediction error model has been previously described in the context of expected value computation, in which the magnitude of potential gains was represented subcortically by the NAc, whereas correction for their future likelihood occurred cortically in the mPFC (Knutson et al., 2003). Such a model has anatomical feasibility, because tract-tracing studies indicate an ascending spiral of connectivity running from the ventral striatum to the ventral prefrontal cortex and back to more dorsal aspects of the striatum and prefrontal cortex, before terminating in motor pathways (Haber, 2003).
One potential limitation of the study is that the effects of expectations and expectation and outcome comparisons on placebo-induced µ-opioid system activation could be due to differences in endogenous opioid release during pain, which could only be tested in the presence of a baseline scan. However, this is unlikely, given that no significant differences between groups were observed when the pain condition was compared with the control condition (which differed from the pain condition only in the painful stimulus).
The results presented here confirm that whereas expectations of clinical improvement have been suggested as a mechanism for the development of placebo analgesia, comparisons between expected and observed outcomes seem to be a better predictor of a sustained placebo analgesic effect, here observed over a 20 min experimental period. Moreover, we highlight the novel role of the µ-opioid system in the regulation of the placebo prediction error signal in the dACC and aid the elucidation of molecular and neural mechanisms underlying the influence of expectation-effectiveness associations on the formation of placebo analgesic responses. These results provide a mechanism through which classical theories of placebo analgesia (expectation vs conditioning) can be reconciled and shed light on individual differences in reward learning and decision-making processes. Expectation and outcome comparisons then emerge as a cognitive mechanism that beyond reward-associations is likely to facilitate the formation and sustainability of placebo responses over time, an effect frequently observed in the context of clinical trials.
Conflict of Interest
The authors have no interests to disclose that are or might be perceived to be in conflict with the work reported in this study. J.K.Z. received compensation as consultant from Eli Lilly, Johnson & Johnson, Abbot and Merck pharmaceuticals during the 3 years prior to manuscript submission for work unrelated to the content of the manuscript.
Acknowledgments
We would like to acknowledge the contribution of the technologists of the PET Center at the University of Michigan. This work was supported by NIH (NIDA) grants R01 DA 022520 and R01 DA 027494 (J.K.Z.) and the Phil F. Jenkins Foundation.
REFERENCES
- Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosomatic Medicine. 1975;37(4):333–40. doi: 10.1097/00006842-197507000-00007. [DOI] [PubMed] [Google Scholar]
- Amador N, Schlag-Rey M, Schlag J. Reward-predicting and reward-detecting neuronal activity in the primate supplementary eye field. Journal of Neurophysiology. 2000;84(4):2166–70. doi: 10.1152/jn.2000.84.4.2166. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Cowan WM. Subcortical afferents to the hippocampal formation in the monkey. Journal of Comparative Neurology. 1980;189(4):573–91. doi: 10.1002/cne.901890402. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD. How expectations shape pain. Neuroscience Letters. 2012;520(2):140–8. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Behrens TE, Woolrich MW, Walton ME, Rushworth MF. Learning the value of information in an uncertain world. Nature Neuroscience. 2007;10(9):1214–21. doi: 10.1038/nn1954. [DOI] [PubMed] [Google Scholar]
- Bellgowan PS, Helmstetter FJ. The role of mu and kappa opioid receptors within the periaqueductal gray in the expression of conditional hypoalgesia. Brain Research. 1998;791(1–2):83–9. doi: 10.1016/s0006-8993(98)00057-2. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Arduino C, Costa S, et al. Loss of expectation-related mechanisms in Alzheimer's disease makes analgesic therapies less effective. Pain. 2006;121(1–2):133–44. doi: 10.1016/j.pain.2005.12.016. [DOI] [PubMed] [Google Scholar]
- Benedetti F, Pollo A, Lopiano L, Lanotte M, Vighetti S, Rainero I. Conscious expectation and unconscious conditioning in analgesic, motor, and hormonal placebo/nocebo responses. Journal of Neuroscience. 2003;23(10):4315–23. doi: 10.1523/JNEUROSCI.23-10-04315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–81. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM. Conflict monitoring and decision making: reconciling two perspectives on anterior cingulate function. Cognitive Affective & Behavioral Neuroscience. 2007;7(4):356–66. doi: 10.3758/cabn.7.4.356. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–46. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Brown JW, Braver TS. Learned predictions of error likelihood in the anterior cingulate cortex. Science. 2005;307(5712):1118–21. doi: 10.1126/science.1105783. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Dolan RJ. Neural activity in the human brain relating to uncertainty and arousal during anticipation. Neuron. 2001;29(2):537–45. doi: 10.1016/s0896-6273(01)00225-2. [DOI] [PubMed] [Google Scholar]
- Denis L, Nowe P, Reiss S, Declercq G. [Value of urinary cytology and cytology of bladder lavage in the diagnosis and observation of bladder cancer] Acta Neurologica Belgica. 1983;51(1):38–42. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences U S A. 1988;85(14):5274–8. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, et al. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences U S A. 2007;104(26):11073–8. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eippert F, Bingel U, Schoell E, Yacubian J, Buchel C. Blockade of endogenous opioid neurotransmission enhances acquisition of conditioned fear in humans. Journal of Neuroscience. 2008;28(21):5465–72. doi: 10.1523/JNEUROSCI.5336-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierstein CE, Quirk MC, Uchida N, Sosulski DL, Mainen ZF. Representation of spatial goals in rat orbitofrontal cortex. Neuron. 2006;51(4):495–507. doi: 10.1016/j.neuron.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Newsome WT, Schultz W. The temporal precision of reward prediction in dopamine neurons. Nature Neuroscience. 2008;11:966–73. doi: 10.1038/nn.2159. [DOI] [PubMed] [Google Scholar]
- Fiorillo CD, Tobler PN, Schultz W. Discrete coding of reward probability and uncertainty by dopamine neurons. Science. 2003;299(5614):1898–902. doi: 10.1126/science.1077349. [DOI] [PubMed] [Google Scholar]
- Foo H, Helmstetter FJ. Hypoalgesia elicited by a conditioned stimulus is blocked by a mu, but not a delta or a kappa, opioid antagonist injected into the rostral ventromedial medulla. Pain. 1999;83(3):427–31. doi: 10.1016/S0304-3959(99)00125-6. [DOI] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP. A neural representation of categorization uncertainty in the human brain. Neuron. 2006;49(5):757–63. doi: 10.1016/j.neuron.2006.01.032. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. Journal of Chemical Neuroanatomy. 2003;26(4):317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Hayden BY, Heilbronner SR, Pearson JM, Platt ML. Surprise signals in anterior cingulate cortex: neuronal encoding of unsigned reward prediction errors driving adjustment in behavior. Journal of Neuroscience. 2011;31(11):4178–87. doi: 10.1523/JNEUROSCI.4652-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innis RB, Cunningham VJ, Delforge J, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. Journal of Cerebral Blood Flow & Metabolism. 2007;27(9):1533–9. doi: 10.1038/sj.jcbfm.9600493. [DOI] [PubMed] [Google Scholar]
- Ito S, Stuphorn V, Brown JW, Schall JD. Performance monitoring by the anterior cingulate cortex during saccade countermanding. Science. 2003;302(5642):120–2. doi: 10.1126/science.1087847. [DOI] [PubMed] [Google Scholar]
- Jewett DM. A simple synthesis of [11C]carfentanil using an extraction disk instead of HPLC. Nuclear Medicine and Biology. 2001;28(6):733–4. doi: 10.1016/s0969-8051(01)00226-8. [DOI] [PubMed] [Google Scholar]
- Keltner JR, Furst A, Fan C, Redfern R, Inglis B, Fields HL. Isolating the modulatory effect of expectation on pain transmission: a functional magnetic resonance imaging study. Journal of Neuroscience. 2006;26(16):4437–43. doi: 10.1523/JNEUROSCI.4463-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist. 1985 1985. [Google Scholar]
- Klinger R, Soost S, Flor H, Worm M. Classical conditioning and expectancy in placebo hypoalgesia: a randomized controlled study in patients with atopic dermatitis and persons with healthy skin. Pain. 2007;128(1–2):31–9. doi: 10.1016/j.pain.2006.08.025. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage. 2003;18(2):263–72. doi: 10.1016/s1053-8119(02)00057-5. [DOI] [PubMed] [Google Scholar]
- Kong J, Gollub RL, Rosman IS, et al. Brain activity associated with expectancy-enhanced placebo analgesia as measured by functional magnetic resonance imaging. Journal of Neuroscience. 2006;26(2):381–8. doi: 10.1523/JNEUROSCI.3556-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel TR, Reiss E, Cherniak R. Concomitant but not causal association between surface charge and inhibition of phagocytosis by cryptococcal polysaccharide. Infection and Immunity. 1980;29(2):295–300. doi: 10.1128/iai.29.2.295-300.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauwereyns J, Takikawa Y, Kawagoe R, Kobayashi S, Koizumi M, Coe B, Sakagami M, Hikosaka O. Feature-based anticipation of cues that predict reward in monkey caudate nucleus. Neuron. 2002;33(3):463–73. doi: 10.1016/s0896-6273(02)00571-8. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. Journal of Cerebral Blood Flow & Metabolism. 1996;16(5):834–40. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447(7148):1111–5. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- Matsumoto M, Matsumoto K, Abe H, Tanaka K. Medial prefrontal cell activity signaling prediction errors of action values. Nature Neuroscience. 2007;10(5):647–56. doi: 10.1038/nn1890. [DOI] [PubMed] [Google Scholar]
- Melzack R, Torgerson WS. On the language of pain. Anesthesiology. 1971;34(1):50–9. doi: 10.1097/00000542-197101000-00017. [DOI] [PubMed] [Google Scholar]
- Meyer CR, Boes JL, Kim B, et al. Demonstration of accuracy and clinical versatility of mutual information for automatic multimodality image fusion using affine and thin-plate spline warped geometric deformations. Medical Image Analysis. 1997;1(3):195–206. doi: 10.1016/s1361-8415(97)85010-4. [DOI] [PubMed] [Google Scholar]
- Minoshima S, Koeppe RA, Mintun MA, et al. Automated detection of the intercommissural line for stereotactic localization of functional brain images. Journal of Nuclear Medicine. 1993;34(2):322–9. [PubMed] [Google Scholar]
- Narendran R, Martinez D. Cocaine abuse and sensitization of striatal dopamine transmission: a critical review of the preclinical and clinical imaging literature. Synapse. 2008;62(11):851–69. doi: 10.1002/syn.20566. [DOI] [PubMed] [Google Scholar]
- Pecina M, Stohler CS, Zubieta JK. Role of mu-opioid system in the formation of memory of placebo responses. Molecular Psychiatry. 2013;18(2):135–7. doi: 10.1038/mp.2012.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price DD, Barrell JJ. Mechanisms of analgesia produced by hypnosis and placebo suggestions. Progress in Brain Research. 2000;122:255–71. doi: 10.1016/s0079-6123(08)62144-5. [DOI] [PubMed] [Google Scholar]
- Reiss S. Transfer effects of success and failure training from one reinforcement agent to another. Journal of Abnormal Psychology. 1973;82(3):435–45. doi: 10.1037/h0035393. [DOI] [PubMed] [Google Scholar]
- Reiss S. Pavlovian conditioning and human fear: an expectancy model. Behavior Therapy. 1980;11:380–96. [Google Scholar]
- Reiss S, Levitan GW, Szyszko J. Emotional disturbance and mental retardation: diagnostic overshadowing. American Journal of Mental Deficiency. 1982;86(6):567–74. [PubMed] [Google Scholar]
- Reiss S, Szyszko J. Diagnostic overshadowing and professional experience with mentally retarded persons. American Journal of Mental Deficiency. 1983;87(4):396–02. [PubMed] [Google Scholar]
- Reiss SK, Ross-Degnan D, Zhang F, et al. Effect of switching to a high-deductible health plan on use of chronic medications. Health Services Research. 46(5):1382–401. doi: 10.1111/j.1475-6773.2011.01252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. New York: Appleton Century Crofts; 1972. pp. 64–99. [Google Scholar]
- Rushworth MF, Behrens TE, Rudebeck PH, Walton ME. Contrasting roles for cingulate and orbitofrontal cortex in decisions and social behaviour. Trends in Cognitive Sciences. 2007;11(4):168–76. doi: 10.1016/j.tics.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Sallet J, Quilodran R, Rothe M, Vezoli J, Joseph JP, Procyk E. Expectations, gains, and losses in the anterior cingulate cortex. Cognitive Affective & Behavioral Neuroscience. 2007;7(4):327–36. doi: 10.3758/cabn.7.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samejima K, Ueda Y, Doya K, Kimura M. Representation of action-specific reward values in the striatum. Science. 2005;310(5752):1337–40. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Setlow B, Saddoris MP, Gallagher M. Encoding predicted outcome and acquired value in orbitofrontal cortex during cue sampling depends upon input from basolateral amygdala. Neuron. 2003;39(5):855–67. doi: 10.1016/s0896-6273(03)00474-4. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80(1):1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual differences in reward responding explain placebo-induced expectations and effects. Neuron. 2007;55(2):325–36. doi: 10.1016/j.neuron.2007.06.028. [DOI] [PubMed] [Google Scholar]
- Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Placebo and nocebo effects are defined by opposite opioid and dopaminergic responses. Archives of General Psychiatry. 2008;65(2):220–31. doi: 10.1001/archgenpsychiatry.2007.34. [DOI] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, et al. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–21. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychology Bulletin. 2004;130(2):324–40. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- Stohler CS, Kowalski CJ. Spatial and temporal summation of sensory and affective dimensions of deep somatic pain. Pain. 1999;79(2–3):165–73. doi: 10.1016/s0304-3959(98)00171-7. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cortical afferents. Journal of Comparative Neurology. 1994;350(4):497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Takahashi YK, Roesch MR, Wilson RC, et al. Expectancy-related changes in firing of dopamine neurons depend on orbitofrontal cortex. Nature Neuroscience. 2011;14(12):1590–7. doi: 10.1038/nn.2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashkin DP, Reiss S, Shapiro BJ, Calvarese B, Olsen JL, Lodge JW. Bronchial effects of aerosolized delta 9-tetrahydrocannabinol in healthy and asthmatic subjects. American Review of Respiratory Disease. 1977;115(1):57–65. doi: 10.1164/arrd.1977.115.1.57. [DOI] [PubMed] [Google Scholar]
- Thielscher A, Pessoa L. Neural correlates of perceptual choice and decision making during fear-disgust discrimination. Journal of Neuroscience. 2007;27(11):2908–17. doi: 10.1523/JNEUROSCI.3024-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler PN, Fiorillo CD, Schultz W. Adaptive coding of reward value by dopamine neurons. Science. 2005;307(5715):1642–5. doi: 10.1126/science.1105370. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Mauguiere F, Isnard J, Dreher JC. The hippocampus codes the uncertainty of cue-outcome associations: an intracranial electrophysiological study in humans. Journal of Neuroscience. 2009;29(16):5287–94. doi: 10.1523/JNEUROSCI.5298-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. Conditioned placebo responses. Journal of Personality and Social Psychology. 1985;48(1):47–53. doi: 10.1037//0022-3514.48.1.47. [DOI] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. Conditioned response models of placebo phenomena: further support. Pain. 1989;38(1):109–16. doi: 10.1016/0304-3959(89)90080-8. [DOI] [PubMed] [Google Scholar]
- Wager TD, Rilling JK, Smith EE, et al. Placebo-induced changes in FMRI in the anticipation and experience of pain. Science. 2004;303(5661):1162–7. doi: 10.1126/science.1093065. [DOI] [PubMed] [Google Scholar]
- Wager TD, Scott DJ, Zubieta JK. Placebo effects on human mu-opioid activity during pain. Proceedings of the National Academy of Sciences U S A. 2007;104(26):11056–61. doi: 10.1073/pnas.0702413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickramasekera I. A conditioned response model of the placebo effect predictions from the model. Biofeedback Self-Regulation. 1980;5(1):5–18. doi: 10.1007/BF00999060. [DOI] [PubMed] [Google Scholar]
- Wiech K, Ploner M, Tracey I. Neurocognitive aspects of pain perception. Trends in Cognitive Sciences. 2008;12(8):306–13. doi: 10.1016/j.tics.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Williams ZM, Bush G, Rauch SL, Cosgrove GR, Eskandar EN. Human anterior cingulate neurons and the integration of monetary reward with motor responses. Nature Neuroscience. 2004;7(12):1370–5. doi: 10.1038/nn1354. [DOI] [PubMed] [Google Scholar]
- Zhang X, Ashton-Miller JA, Stohler CS. A closed-loop system for maintaining constant experimental muscle pain in man. IEEE Transactions on Biomedical Engineering. 1993;40(4):344–52. doi: 10.1109/10.222327. [DOI] [PubMed] [Google Scholar]
- Zubieta JK, Bueller JA, Jackson LR, et al. Placebo effects mediated by endogenous opioid activity on mu-opioid receptors. Journal of Neuroscience. 2005;25(34):7754–62. doi: 10.1523/JNEUROSCI.0439-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293(5528):311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]