Abstract
The concept of extended self refers to the idea that people incorporate self-relevant others or objects into one’s sense of self. Initial neural support for the notion of extended self was provided by fMRI evidence that medial prefrontal cortex (MPFC) showed greater activation while people imagined objects belonging to them compared with someone else (Kim & Johnson, 2012). This study investigated whether self-associated objects (i.e. ‘mine’) subsequently engage MPFC spontaneously when a task does not require explicit self-referential judgments. During fMRI scanning, participants detected ‘oddballs’ (objects with a specific frame color) intermixed with objects participants had previously imagined belonging to them or to someone else and previously unseen non-oddball objects. There was greater activity in MPFC and posterior cingulate cortex for those ‘self-owned’ objects that participants were more successful at imagining owning compared with ‘other-owned’ objects. In addition, change in object preference following the ownership manipulation (a mere ownership effect) was predicted by activity in MPFC. Overall, these results provide neural evidence for the idea that personally relevant external stimuli may be incorporated into one’s sense of self.
Keywords: extended self, ownership, spontaneous self-relevant processing, medial prefrontal cortex, fMRI
INTRODUCTION
A central feature of human experience is a sense of ‘self’ that provides stability and continuity to the flow of subjective experience across space and time (Neisser, 1988; Damasio, 1999). As noted by William James, each individual inevitably makes the ‘great splitting of the whole universe into two halves’ involving not only the distinction between parts unambiguously belonging to oneself (‘me’) from the immediate external environment (‘not me’) but also the distinction between other aspects of one’s experiences that bear relevance to oneself (‘mine’) from those with no or minimal self-relevance (‘not mine’) (James, 1890/1983, p. 289). That is, one’s sense of self can extend beyond the sense of body ownership and agency (minimal self: Gallagher, 2000), for example, when self-relevant people (Aron et al., 1991) or objects (Wicklund & Gollwitzer, 1982; Belk, 1988) are incorporated into one’s sense of self. In particular, Belk (1988) suggested that one’s possessions can be considered part of one’s extended self. The early development of an understanding of ownership and strong self-object associations provides support for the importance of ownership in human social-cognitive functioning (Ross, 1996; Fasig, 2000).
Acquiring ownership of an object triggers a range of cognitive and affective effects. Even transient, imagined ownership produces a memorial advantage (self-reference effect; Cunningham et al., 2008; Van den Bos et al., 2010) and higher value and desirability ratings for self-‘owned’ objects compared with similar objects not owned by the self (mere ownership effect, endowment effect; Kahneman et al., 1991; Beggan, 1992; Huang et al., 2009). Strikingly, the mere ownership effect extends beyond objects to non-material entities such as attitude positions (De Dreu & van Knippenberg, 2005), and even to artificial and inconsequential stimuli such as abstract symbols (Feys, 1991).
Neural substrates supporting the association between one’s self and objects have been explored recently using an imagined ownership paradigm (Turk et al., 2011; Kim & Johnson, 2012). When participants were assigned imaginary ownership of objects that could either belong to them or to a fictitious other person, medial prefrontal cortex (MPFC), the region most reliably recruited during explicit self-referential processing across various domains and stimuli (Lieberman, 2010), showed greater activity for self-owned objects compared with other-owned objects. In addition, increased preference for and superior subsequent source memory for self-owned objects were also associated with MPFC activity during imagined ownership (Kim & Johnson, 2012). Using a similar paradigm, Turk et al. (2011) found greater MPFC activity for self-owned vs other-owned objects and that superior recognition memory for self-owned objects was correlated with activity in MPFC. Taken together, these findings provided initial neural evidence for the incorporation of self-relevant objects into one’s sense of self.
Most previous studies examined neural underpinnings of self-relevant processing by requiring participants to explicitly process some, but not other, stimuli in reference to themselves. Two recent studies found that largely the same self-sensitive brain regions recruited during explicit self-referential processing, notably MPFC and other cortical midline structures [CMSs; e.g. posterior cingulate cortex (PCC), precuneus], are activated when the self-relevance of stimuli is presumably only implicitly processed, or at least not explicitly required by the task (Moran et al., 2009; Rameson et al., 2010). In Moran et al. (2009), MPFC selectively responded when individuals were presented with personal semantic facts (e.g. one’s initials) compared with non-self-related stimuli in a non-self-referential oddball detection task in which the self-related stimuli served as non-oddballs. In another study, MPFC was more active during non-self-referential judgments of pictures (i.e. ‘Is there a person in a scene?’) when pictures depicted a scene related to one’s self-schema (e.g. a picture of a gym for individuals with an athletic self-schema) compared with when they did not (Rameson et al., 2010). The recruitment of MPFC and other CMSs in the absence of explicit self-referential judgments suggest that these brain areas may signal the potential self-relevancy of incoming information. Such signals of self-relevance may reflect personal significance of incoming stimuli (D’Argembeau et al., 2012), or more general, spontaneous subjective valuation (Peters & Buckel, 2010; Rangel & Hare, 2010), both likely to involve MPFC (especially, ventral MPFC) as well as implicit and/or explicit activation of autobiographical/episodic memories, likely to involve PCC/precuneus (Svoboda et al., 2006).
The findings of spontaneous activity in self-sensitive brain regions during the presentation of information that is prototypically related to one’s sense/concept of self (e.g. one’s name, one’s self-schema) raise the question: are these regions similarly engaged spontaneously when people are presented with their possession, as would be predicted by the notion of extended self? Here, we set out to explore this question using an imagined ownership paradigm based on previous findings of (i) the involvement of MPFC during ownership imagination (Turk et al., 2011; Kim & Johnson, 2012) and (ii) spontaneous engagement of self-sensitive brain areas by stimuli that are pre-experimentally self-relevant and well-learned (Moran et al., 2009; Rameson et al., 2010). Specifically, this study investigated whether objects that are experimentally self-associated through imagined ownership later engender spontaneous activity in self-sensitive brain areas even when the task does not require explicit self-referential judgments. Similar to Moran et al. (2009), we used a color oddball detection task in which participants were required to respond only to object pictures with a specific frame color. We hypothesized that to the extent that MPFC activity during ownership imagination reflects acquiring associations between self and objects, self-associated objects should later spontaneously engage MPFC, and possibly other CMSs, compared with non-self-associated objects. We further sought to examine whether the strength of the association between the self and objects (self-reports of imagined ownership success and the mere ownership effect) is predicted by spontaneous activity in these brain regions.
METHODS
Participants and stimuli
Participants were 24 healthy right-handed young adults (14 females; mean age = 21 ± 3.01 years) who gave written informed consent in accordance with the Yale University School of Medicine Human Investigation Committee.
The stimuli were 200 pictures (250 × 250 pixels) of items available for purchase in a large offline/online market (e.g. clothing). The stimuli were divided into four sets of 50 objects that were matched for preference level, estimated cost and ease of identification based on data from a separate pilot study. Two sets served as critical items and were presented both during the object assignment task as ‘MINE’ or ‘OTHER’ items and during the oddball detection task as non-oddballs. The remaining two sets were only used during the oddball detection task and served as non-critical items that were neither self-relevant nor other-relevant (‘NEUTRAL’ or ‘ODDBALL’ items, see Procedure below). The assignment of critical sets to MINE and OTHER conditions and non-critical sets to NEUTRAL and ODDBALL conditions was counterbalanced across participants. Among the 50 objects assigned to ODDBALL condition, randomly chosen 25 items served as oddballs during the oddball detection task.
Experimental design and procedure
The study consisted of the following six phases:
Pre-ownership preference rating: participants were presented with 100 objects (critical MINE and OTHER items) one at a time for 5 s each and indicated how much they liked each object on a 1 (‘Lowest preference’) to 9 (‘Highest preference’) scale.
Object assignment task: on each 7 s trial, participants were presented with a picture of an object and two baskets labeled ‘Mine’ and ‘Alex’. Participants’ task was to move each item into one of the baskets according to the color of a dot appearing on the object by pressing one of the two buttons. The dot color matched the label color of one of the baskets. Importantly, participants were asked to imagine that they are going to own the items assigned to the ‘Mine’ basket but not those assigned to the ‘Alex’ basket. There were 50 MINE and 50 OTHER (‘Alex’) trials.
Oddball detection task: on each trial, an object picture was presented within a colored frame for 2 s, preceded by a 400 ms fixation dot. The frame was gray for the self-owned (MINE), other-owned (OTHER) and previously unseen non-oddball objects (NEUTRAL) and yellow for the previously unseen oddball objects (ODDBALL). Participants were asked to press a button whenever they saw an object with a yellow frame. The trials were separated by jittered intertrial intervals (ITIs; 8.6–10.6 s). The trials were randomly ordered and there were 35 trials (10 MINE, 10 OTHER, 10 NEUTRAL and 5 ODDBALL) in each of the five functional runs.
Source memory test: during scanning, each trial consisted of a 400 ms fixation dot, followed by a 2 s presentation of object picture. For each object, participants indicated to whom (i.e. self or Alex) it was assigned during the object assignment task. The trials were separated by 8.6–10.6 s ITIs. There were 10 MINE and 10 OTHER trials in each of the three functional runs. Right after the scanning, participants performed the same source memory test with a shorter ITI (1 s) on the remaining MINE and OTHER items. The items to be tested inside or outside the scanner were randomly assigned for both MINE and OTHER conditions.
Post-ownership preference rating: the procedure was the same as the pre-ownership preference rating. This phase was included to measure changes in preference ratings from before to after the ownership manipulation (mere ownership effect).
Imagined ownership rating: in this phase, only the 50 MINE items were presented one at a time. Participants rated how well (easily, vividly or successfully) they could imagine each object as belonging to themselves during the object assignment task on a 1 (‘not very well’) to 4 (‘very well’) scale. The trials were self-paced. This phase was included to measure relative strength of the association between the self and each of the to-be-owned objects.
Localizer ‘explicit self-referencing’ task
A trait adjective rating task was used to localize regions of interest (ROI) involved in explicit self-referencing. In a blocked design, participants rated how well trait adjectives describe themselves (self-referent) or former president G. W. Bush (other-referent) on a 4-point scale. Each block consisted of five sequential presentations of adjectives (2.7 s word presentation, 500 ms interstimulus interval). There were 10 blocks for each reference condition and an 8 s fixation period separated the blocks. A total of 100 trait adjectives were divided into two lists matched for number of syllables, word length and desirability (Anderson, 1968) and were assigned to the self- and other-referent conditions in a counterbalanced manner.
Image acquisition and preprocessing
Data were acquired using a 3 T Siemens TimTrio scanner with a 12-channel head coil. A total of 189 and 131 functional image volumes for each of five runs of the oddball detection task and for each of three runs of the source memory test, respectively, were acquired using a standard echo planar pulse sequence (TR = 2 s, TE = 25 ms, flip angle [α] = 90°, FOV = 240 mm, matrix = 642, slice thickness = 3.5 mm, 34 slices). For the localizer run, a total of 250 image volumes with the same imaging parameter as the main functional runs were acquired. Two sets of structural images were acquired for registration: coplanar images, using a T1 Flash sequence (TR = 300 ms, TE = 2.47 ms, α = 60°, FOV = 240 mm, matrix = 2562, slice thickness = 3.5 mm, 34 slices) and high-resolution images, using a 3D MP-RAGE sequence (TR = 2530 ms, TE = 3.34 ms, α = 7°, FOV = 256 mm, matrix = 2562, slice thickness = 1 mm, 160 slices).
Analyses were performed using the FMRIB software library (FSL, http://www.fmrib.ox.ac.uk/fsl). The first four volumes (8 s) of each functional dataset were discarded to allow for MR equilibration. Preprocessing included skull-stripping, slice-timing correction, motion correction using MCFLIRT, spatial smoothing (Gaussian, FWHM 5 mm) and high-pass temporal filtering (cut off = 50 s). Functional images were registered to coplanar images, which were then registered to high-resolution images, and normalized to the Montreal Neurological Institute’s MNI152 template.
fMRI data analysis
Whole-brain voxel-wise regression analyses were performed using FSL’s FEAT. First-level general linear model analysis was performed using a separate explanatory variable (EV) for each condition. For data from the oddball detection task, the initial model included EVs for each stimulus type (MINE, OTHER, NEUTRAL and ODDBALL). Based on the post-scan imagined ownership ratings, a separate model was constructed by including only the MINE items with ownership ratings of 3 and 4 (i.e. successfully ownership-imagined items) in the MINE EV. For data from the source memory test, the model included two EVs corresponding to trials with correct source memory for MINE and OTHER conditions. Each trial type was modeled with a boxcar function convolved with a single-gamma hemodynamic response function. The contrasts of particular interest in the oddball detection task analyses were the comparisons between MINE and OTHER conditions. For completeness, we also conducted contrasts with the NEUTRAL condition (MINE vs NEUTRAL, OTHER vs NEUTRAL, NEUTRAL vs MINE, and NEUTRAL vs OTHER trials).1 For the source memory test, the contrast involved a comparison between correctly source-attributed MINE items and correctly source-attributed OTHER items. Subject-level analyses combining multiple runs were conducted using a fixed effects model.
Group-level analyses were performed on the parameter estimates obtained from each of the contrasts calculated at the subject level using a mixed effects model, with a random effects component of variances estimated using FSL’s FLAME stage 1 only procedure (Beckmann et al., 2003). For significance testing, voxels were first thresholded at an entry level of Z > 2.3. Cluster correction (cluster probability P < 0.05) using a Gaussian Random Field theory was then applied to the thresholded voxels to correct for multiple comparisons (Worsley et al., 1996).
ROI definition and analysis
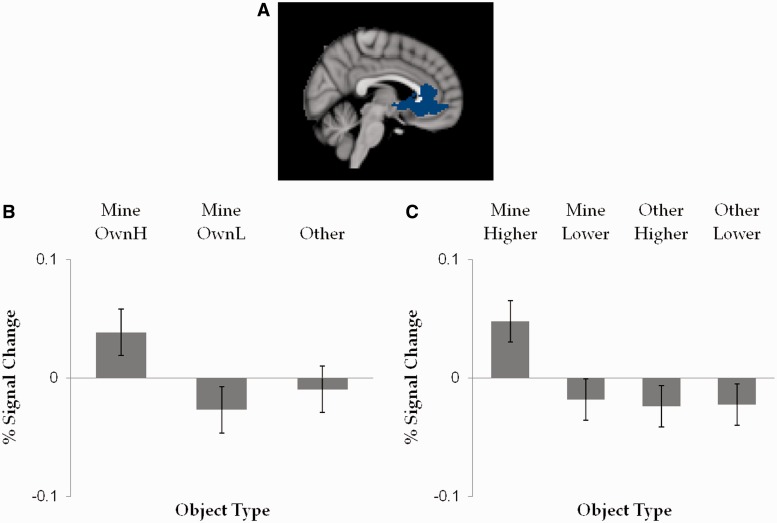
The data from the explicit self-referencing localizer run were analyzed using the same approach of preprocessing, subject- and group-level analyses as described for the main task analyses. The EVs consisted of Self-referent vs Other-referent task blocks. The group-level contrast map for Self-referent > Other-referent contrast (Z > 3.0, cluster probability P < 0.05) revealed four clusters in paracingulate gyrus/MPFC, posterior cingulate gyrus, occipital pole/superior lateral occipital cortex and intracalcarine cortex/lingual gyrus. For the purpose of this study, we created an ROI brain mask containing only the cluster containing MPFC [peak voxel: 4 26 −6, Z-score = 5.35 (center of gravity: −6.49 19.5 0.456), see Figure 3A].
Fig. 3.
Results from the ROI analyses: (A) MPFC ROI cluster derived from the Self-referent > Other-referent contrast in an independent trait-descriptiveness rating task, (B) percent signal change in the ROI as a function of high vs low ownership ratings for MINE (MineOwnH or MineOwnL) and all OTHER items, (C) percent signal change in the ROI as a function of owner type (MINE or OTHER) and post- vs pre-ownership preference change (higher or lower). Error bars represent SEM.
The fMRI signal from each voxel in each participant’s functional data was calculated across peri-events created separately for MINE and OTHER object types in each contrast of interest. The fMRI signals were then converted to percent signal change relative to an intertrial baseline and averaged over the voxels contained in our ROI for three time points (epochs) of interest expected to show the maximal BOLD effect (4–8 s post stimulus onset).
RESULTS
Behavioral results
Oddball detection
The average oddball detection accuracy was 99.44% (s.d. = 1.52) with a mean response time of 636 ms (s.d. = 96), suggesting that participants were fully attentive throughout the task.
Source memory
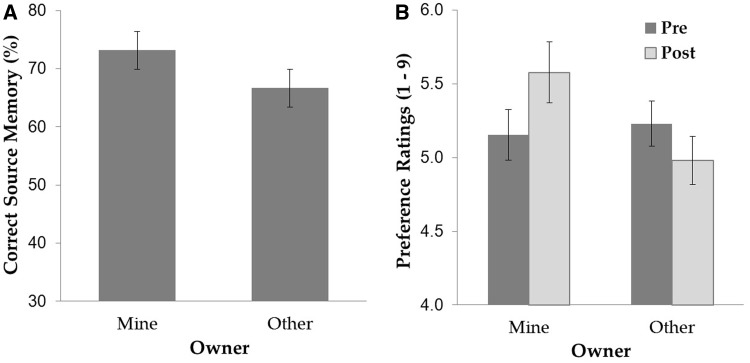
Source memory was calculated by dividing the number of correct source assignments to each owner condition (MINE or OTHER) by the total number of items of that owner type. As shown in Figure 1A, participants demonstrated a typical self-reference effect exhibiting better memory of an object’s source for MINE (73.17%) compared with OTHER items (66.67%), t(23) = 2.28, P = 0.032.
Fig. 1.
(A) Source memory performance and (B) pre-and post-ownership preference ratings as a function of owner type. Error bars indicate standard error of the mean (SEM).
Preference rating
A 2 (owner; mine or other) × 2 (time of rating; pre- or post-ownership) repeated-measures analysis of variance (ANOVA) revealed a significant main effect of owner, F(1, 23) = 6.83, P = 0.016, 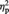 = 0.23, which was qualified by a significant two-way interaction, F(1, 23) = 7.45, P = 0.012,
= 0.23, which was qualified by a significant two-way interaction, F(1, 23) = 7.45, P = 0.012, 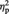 = 0.25. Simple effects analyses revealed that MINE items were given significantly higher preference in the post-ownership rating (5.58) than in the pre-ownership rating (5.15), F(1, 23) = 5.48, P = 0.028,
= 0.25. Simple effects analyses revealed that MINE items were given significantly higher preference in the post-ownership rating (5.58) than in the pre-ownership rating (5.15), F(1, 23) = 5.48, P = 0.028, 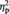 = 0.19 (Figure 1B). In contrast, the opposite pattern was revealed for OTHER items (5.23 and 4.98 for pre- and post-ownership ratings, respectively), F(1, 23) = 6.26, P = 0.020,
= 0.19 (Figure 1B). In contrast, the opposite pattern was revealed for OTHER items (5.23 and 4.98 for pre- and post-ownership ratings, respectively), F(1, 23) = 6.26, P = 0.020, 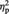 = 0.21. Pre-ownership ratings for the objects assigned to MINE and OTHER did not differ, P > 0.4. Interestingly, the amount of post-ownership preference increase for MINE items was positively correlated with the amount of post-ownership preference decrease for OTHER items, Pearson r = 0.50, P = 0.012. These findings are in line with a proposal that the self-enhancement motive is an underlying mechanism for the mere ownership effect (Beggan, 1992): the desire to see oneself in a positive light extends to overvaluing objects associated with self, which may be accompanied by relative devaluation of objects associated with others, especially in situations where an explicit comparison is present between the self and other as in our object assignment task.
= 0.21. Pre-ownership ratings for the objects assigned to MINE and OTHER did not differ, P > 0.4. Interestingly, the amount of post-ownership preference increase for MINE items was positively correlated with the amount of post-ownership preference decrease for OTHER items, Pearson r = 0.50, P = 0.012. These findings are in line with a proposal that the self-enhancement motive is an underlying mechanism for the mere ownership effect (Beggan, 1992): the desire to see oneself in a positive light extends to overvaluing objects associated with self, which may be accompanied by relative devaluation of objects associated with others, especially in situations where an explicit comparison is present between the self and other as in our object assignment task.
Imagined ownership rating
Imagined ownership ratings from one participant failed to be properly collected, leaving a final sample of 23. The average rating score was 2.81, significantly higher than the midpoint ‘2.5’ on a 4-point Likert scale, t(22) = 4.30, P < 0.001, suggesting that, in general, participants were successful at imagining owning the MINE items. In addition, when the MINE items were divided into two groups based on low (1–2) and high (3–4) ratings, there was a trend for a greater post-ownership preference increase for the high items (0.60) compared with the low items (0.29), t(22) = 2.06, P = 0.052, suggesting that participants tended to show a greater mere ownership effect for objects that were more successfully imagined as belonging to them. The mean number of items (26.34 and 23.66 for the high and low items, respectively) and source memory accuracy (73.40% and 68.87% for the high and low items, respectively) did not differ between high and low items, Ps > 0.5.
Imaging results
Oddball detection task
We first report the results from the initial whole-brain analysis in which all the trials of each stimulus type were modeled for each of the contrasts among MINE, OTHER and NEUTRAL. We then report the results from a separate whole-brain analysis involving trial sorting based on subsequent imagined ownership ratings, followed by the results from the ROI analyses, focusing on the contrasts between MINE and OTHER conditions.
Stimulus type contrasts without trial sorting based on behavioral performance
No cluster survived the cluster-corrected significance level for MINE > OTHER or OTHER > MINE contrasts. The MINE > NEUTRAL contrast revealed greater activity in MPFC and PCC along with frontal pole, middle frontal gyrus, angular gyrus and superior lateral occipital cortex. The OTHER > NEUTRAL contrast revealed activity in precuneus, middle frontal gyrus, angular gyrus and superior lateral occipital cortex. The NEUTRAL > MINE contrast revealed greater activity in inferior lateral occipital cortex, inferior temporal gyrus and temporal occipital fusiform cortex. The NEUTRAL > OTHER contrast revealed activity in inferior lateral occipital cortex and occipital pole (Table 1).
Table 1.
Peak coordinates from the contrasts among the stimulus types in the oddball detection task
| Brain region | MNI coordinates (mm) |
Z-score | ||
|---|---|---|---|---|
| x | y | z | ||
| MINE > NEUTRAL | ||||
| MPFC | 6 | 58 | 2 | 3.72 |
| Posterior cingulate gyrus | 4 | −44 | 20 | 4.87 |
| Right frontal pole | 44 | 54 | −12 | 4.01 |
| Right middle frontal gyrus | 36 | 22 | 46 | 4.07 |
| Left middle frontal gyrus | −28 | 20 | 44 | 4.03 |
| Right angular gyrus | 44 | −56 | 40 | 4.37 |
| Left superior lateral occipital cortex | −50 | −64 | 34 | 4.95 |
| OTHER > NEUTRAL | ||||
| Right precuneus cortex | 4 | −64 | 36 | 4.36 |
| Right middle frontal gyrus | 44 | 26 | 36 | 3.72 |
| Right angular gyrus | 52 | −54 | 22 | 4.02 |
| Left superior lateral occipital cortex | −44 | −60 | 40 | 4.15 |
| NEUTRAL > MINE | ||||
| Right inferior lateral occipital cortex | 40 | −82 | −4 | 3.62 |
| Right inferior temporal gyrus | 52 | −52 | −20 | 4.04 |
| Left temporal occipital fusiform cortex | −40 | −54 | −12 | 3.41 |
| NEUTRAL > OTHER | ||||
| Right inferior lateral occipital cortex | 34 | −88 | 4 | 4.53 |
| Left occipital pole | −22 | −92 | −8 | 4.16 |
MINE and OTHER contrasts based on imagined ownership rating
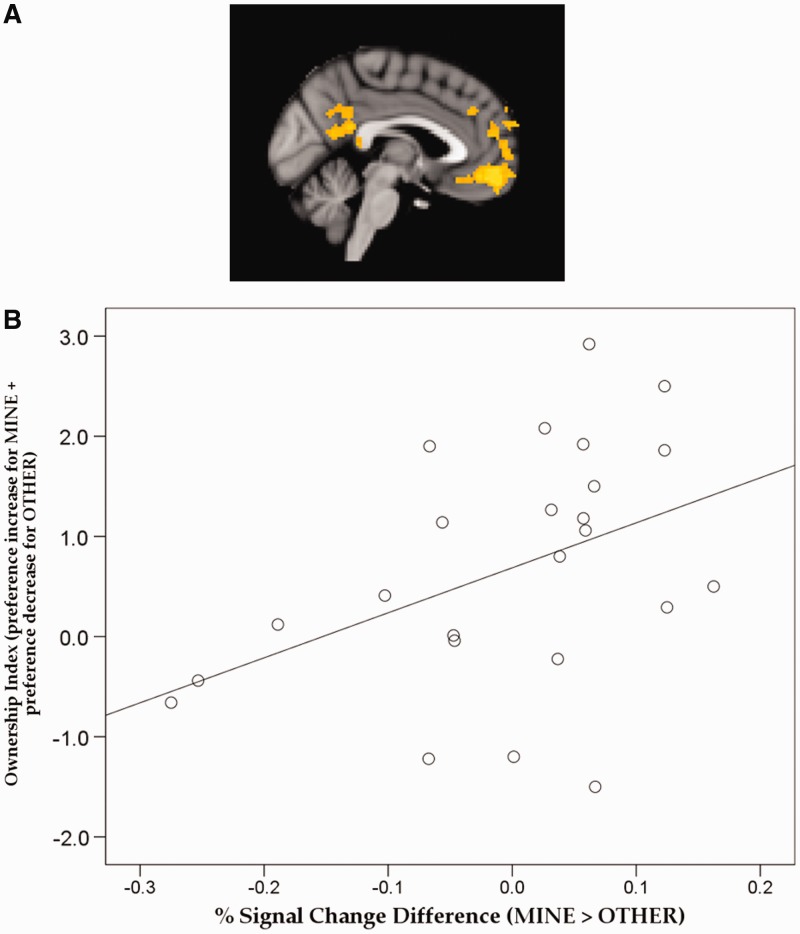
To explore the possibility that spontaneous activation of self-sensitive areas would more likely to be prompted by those objects that were strongly associated with the self, we contrasted only the MINE objects that were reported to have been successfully imagined to belong to the participants (i.e. high imagined ownership ratings; MineOwnH) to the OTHER items in the whole-brain regression analysis. As shown in Figure 2A, the MineOwnH > OTHER contrast revealed greater activity in ventral MPFC (−4 54 −16, Z-score = 3.87) extending to a more dorsal portion (local maxima: −12 58 18, Z-score = 3.67) and in PCC (4 −44 14, Z-score = 3.37). No cluster was found for the reverse contrast.
Fig. 2.
Results from the oddball detection task based on the subsequent imagined ownership ratings: (A) Activation map from whole-brain regression analysis for MINE items with high ownership ratings (MineOwnH) > OTHER contrast and (B) percent signal change difference between MINE and OTHER in MPFC cluster in relation to participants’ ownership index (i.e. sum of the amount of preference increase for MINE items and the amount of preference decrease for OTHER items). Error bars represent SEM.
We further explored whether the behaviorally observed post-ownership preference change would be predicted by activity in these MPFC and PCC clusters. Given the positive correlation between the amount of preference increase for MINE objects and the corresponding preference decrease for OTHER objects, we created an ownership index for each participant by summing the post-ownership preference increase for all the MINE items and post-ownership preference decrease for all the OTHER items. Then, we correlated this ownership index with the percent signal change difference between the MINE and OTHER items within the MPFC and PCC clusters, separately. In the MPFC cluster, the ownership index was positively correlated with the percent signal change difference between MINE and OTHER items, Pearson r = 0.43, P = 0.038 (Figure 2B). The MINE > OTHER percent signal change difference in PCC exhibited a trend for a positive correlation with the ownership index, Pearson r = 0.36, P = 0.088.
These findings indicate that when presented with self-associated objects (even transiently ‘owned’), self-sensitive areas activate, as they do when people are presented with typically self-related information such as semantic autobiographical facts (Moran et al., 2009). In addition, the mere ownership effect, a behavioral manifestation of self-object association, was predicted by the difference in spontaneous MPFC activity during the presentation of self-associated objects vs other-associated objects. This finding further suggests that being associated with self, the ‘self-owned’ objects were conferred greater subjective value or personal significance (e.g. D’Argembeau et al., 2012).
MINE and OTHER contrasts based on imagined ownership rating and pre- vs post-ownership preference change in the MPFC ROI independently identified by a localizer task
The percent signal change for MINE items with high imagined ownership ratings (MineOwnH) was significantly greater compared with OTHER items, F(1, 22) = 10.09, P = 0.004, 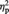 = 0.31 and compared with the MINE items with low imagined ownership ratings (MineOwnL), F(1, 22) = 23.81, P < 0.001,
= 0.31 and compared with the MINE items with low imagined ownership ratings (MineOwnL), F(1, 22) = 23.81, P < 0.001, 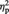 = 0.52 (Figure 3B). The percent signal change for the MineOwnL and OTHER items did not significantly differ from each other, P > 0.1.
= 0.52 (Figure 3B). The percent signal change for the MineOwnL and OTHER items did not significantly differ from each other, P > 0.1.
When the percent signal changes for items showing a post-ownership preference increase/decrease for each of the MINE and OTHER conditions were entered into a 2 (owner; mine or other) × 2 (preference change; increase or decrease) repeated-measures ANOVA, significant main effects of owner, F(1, 23) = 5.31, P = 0.031, 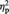 = 0.19, and of preference change, F(1, 23) = 7.48, P = 0.012,
= 0.19, and of preference change, F(1, 23) = 7.48, P = 0.012, 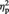 = 0.25, were obtained. Importantly, there was a significant two-way interaction, F(1, 23) = 6.12, P = 0.021,
= 0.25, were obtained. Importantly, there was a significant two-way interaction, F(1, 23) = 6.12, P = 0.021, 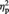 = 0.21. Simple effects analyses revealed that the percent signal change for MINE items with a post-ownership preference increase (MineHigher) was significantly greater compared with MINE items with a post-ownership preference decrease (MineLower), F(1, 23) = 16.25, P = 0.001,
= 0.21. Simple effects analyses revealed that the percent signal change for MINE items with a post-ownership preference increase (MineHigher) was significantly greater compared with MINE items with a post-ownership preference decrease (MineLower), F(1, 23) = 16.25, P = 0.001, 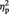 = 0.41 (Figure 3C). In contrast, for OTHER items, the percent signal change for items with a post-ownership increase and for those with a post-ownership decrease did not differ, P > 0.9.
= 0.41 (Figure 3C). In contrast, for OTHER items, the percent signal change for items with a post-ownership increase and for those with a post-ownership decrease did not differ, P > 0.9.
Source memory test
Mirroring previous findings of greater MPFC activity for subsequently remembered self-referenced information than other-referenced information during encoding (Macrae et al., 2004) and during retrieval (Zysset et al., 2002; Lou et al., 2004), the correctly source-attributed MINE > correctly source-attributed OTHER contrast revealed greater activity in MPFC (4 62 2, Z-score = 3.32). No cluster was found for the reverse contrast.
DISCUSSION
This study investigated whether objects made self-relevant by an imagined ownership procedure spontaneously engage MPFC in a non-self-referential oddball detection task. As would be predicted if the MPFC activity during the imagined ownership of objects reflects associating external objects with oneself, we found greater activity in MPFC (and PCC) subsequent to the imagined ownership for to-be-owned objects that the participants were successful at imagining owning compared with objects assigned to another person. In addition, the amount of preference increase for the objects assigned to self and corresponding preference decrease for objects assigned to another person was predicted by greater activity in MPFC. Finally, self-reports of imagined ownership success and the mere ownership effect were positively related to activity in a MPFC cluster independently drawn from an explicit self-referencing task.
Our results extend previous findings of spontaneous activation of self-sensitive brain regions by well-established self-related stimuli such as one’s initials (Moran et al., 2009; Rameson et al., 2010). The present findings demonstrate that even transiently self-associated objects can spontaneously trigger MPFC and PCC activity in a non-self-referential task context. Furthermore, our results argue against one potential interpretation of such effects in terms of relative familiarity of stimuli to the participants rather than self-relevancy. For instance, previous studies found a regional overlap between self-relevance and familiarity in the MPFC and PCC/precuneus, despite some differences in the neural processing of self-relevant and familiar stimuli (Seger et al., 2004; Qin et al., 2012). In this study, we found greater MPFC and PCC activity for self-associated than other-associated objects even when relative stimulus familiarity was controlled by presenting objects in each condition an equal number of times prior to the main oddball detection task.
Our finding of greater activity in precuneus but not in MPFC for previously seen other-associated objects (OTHER) than for previously unseen novel objects (NEUTRAL) suggests that precuneus activity reflected relative stimulus familiarity. Recently, by directly contrasting self-referential processing with episodic memory retrieval, Sajonz et al. (2010) found that whereas self-referential processing was more associated with PCC, as in our finding of greater PCC activity for self-owned than other-owned objects, episodic memory retrieval was more associated with precuneus, as in our finding of greater precuneus activity for other-owned than novel objects. Assuming familiar stimuli generate reactivation (Johnson, 1992) of prior occurrences of those stimuli (i.e. reminding, Hintzman, 2004; Kim et al., 2012), our finding of greater activity in precuneus for other-owned than novel objects would be consistent with the findings of Sajonz et al. (2010).
The current finding of spontaneous activity in self-sensitive brain areas induced by self-associated objects is in line with behavioral and neural findings suggesting incorporation of close others in one’s self-concept (Aron et al., 1991; Mashek et al., 2003; Krienen et al., 2010). The self-reference effect in memory is reduced or eliminated when memory for self-referenced information is compared with memory for information referenced to a close other (Bower & Gilligan, 1979; Kuiper & Rogers, 1979). Similarly, when remembering about whom the information was initially processed, more source confusions occur between self and an intimate other than between self and a familiar, yet less well known, other (Mashek et al., 2003). Furthermore, regardless of perceived similarity with the self, processing information in relation to close others results in greater activity in MPFC (Krienen et al., 2010). Based on our findings, an interesting possibility is that when presented with information associated with a close other, a similar ‘extended self’ effect occurs. From the present findings of positive relations between MPFC activity and the self-reported strength of self-object associations and between MPFC activity and the mere ownership effect, one would expect MPFC activity to be predicted by one’s perceived interpersonal closeness with the target person (e.g. ratings on the Inclusion of Other in the Self Scale; Aron et al., 1992).
Although our findings suggest that in becoming associated with self, objects can be imbued with positivity and activate brain areas that are active when one explicitly thinks about oneself, the exact mechanisms underlying this ‘incorporation’ of objects into one’s self remain to be investigated. The fact that the participants in our study were more successful at imagining owning some of the to-be-owned objects than others suggests that various person- and object-related factors may interact, influencing the degree to which external objects become part of one’s extended self. For example, it has been suggested that one’s possession can be used to maintain important self-definitions (i.e. symbolic self-completion; Wicklund & Gollwitzer, 1982). In this case, objects possessing attributes that correspond to already existing self-views (‘me’ aspects) that are important to oneself will be more successfully incorporated into one’s sense of self. In contrast, when there is a discrepancy between one’s present self and what one would ‘ideally’ like to be (e.g. Higgins, 1987), objects that symbolize the attributes that a person lacks at present but pursues (‘not me’ aspects) may be more readily incorporated into one’s sense of self than those possessing the present ‘me’ aspects. Another possibility arises when an individual does not have a clearly defined, internally consistent and stable self-concept (i.e. low self-concept clarity; Campbell et al., 1996). For individuals with low self-concept clarity, the match between object attributes and one’s self-view may not be a strong determinant of the degree to which an object becomes incorporated into one’s sense of self (cf. failure to use the self-prototype to guide choice behavior, Setterlund & Niedenthal, 1993).
How might these different mechanisms be orchestrated neurally? Insights come from a recent study showing differential engagement of subregions within MPFC according to the type of investment individuals have in a particular self-view (D’Argembeau et al., 2012). Whereas dorsal MPFC was related to the degree of certainty people have that they possess given personality traits (i.e. one’s epistemic investment), ventral MPFC was related to the degree of importance people place on possessing relevant personality traits (i.e. one’s emotive investment). These findings suggest the interesting possibility that among individuals with high self-concept clarity, the strength of self-object associations will be predicted by activity in both the dorsal and ventral MPFC, reflecting the perceived match/mismatch between object attributes and the currently held self-view (‘surely me’ as well as ‘surely not me’) and the importance people place on the current or ideal self-view. In comparison, only activity in ventral MPFC would be likely to predict the strength of self-object associations among individuals with low self-concept clarity.
By demonstrating (i) spontaneous engagement of MPFC and PCC in response to the sight of objects that were successfully imagined as belonging to oneself and (ii) a positive relation between spontaneous MPFC activity and the strength of the mere ownership effect, the current findings provide strong neural evidence for the incorporation of personally relevant external stimuli into one’s sense of self. However, there remain many yet to be answered questions as to the extent/types of self-relevant external entities that are incorporated into one’s sense of self and the underlying mechanisms supporting this incorporation. People function in a world where they distinguish not only between ‘me vs not me’ but also between ‘mine vs not mine.’ Our understanding of human social-cognitive functions should be advanced by exploring how viewing through the ‘eyes’ of the self colors objects, ideas and other individuals with value-laden qualities meaningful to oneself.
FUNDING
This work was supported by the National Institutes of Health [grant number R37AG009253] and the Yale University FAS Imaging Fund.
ACKNOWLEDGEMENTS
We thank Elizabeth Ankudowich for help in data collection. We also wish to thank the members of the Memory and Cognition and Human Neuroscience Labs at Yale for helpful discussions of the study reported in this article.
Footnotes
1 Contrasts involving comparisons between the ODDBALL and the other three conditions were also carried out. Given the reliably reported task-negative activity pattern in cortical midline structures (lower activation during a cognitive task than during rest), we reasoned that the MINE vs ODDBALL contrast might reflect both the non-targetnesss and self-associatedness of MINE items. Not surprisingly, the MINE > ODDBALL whole-brain contrast revealed two clusters centered in MPFC (−2 48 −14) and in PCC/precuneus (0 −62 26), respectively. For brevity the results from contrasts involving the ODDBALL condition are not further reported.
REFERENCES
- Anderson NH. Likableness ratings of 555 personality trait words. Journal of Personality and Social Psychology. 1968;9:272–9. doi: 10.1037/h0025907. [DOI] [PubMed] [Google Scholar]
- Aron A, Aron EN, Smollan D. Inclusion of other in the self scale and the structure of interpersonal closeness. Journal of Personality and Social Psychology. 1992;63:596–612. [Google Scholar]
- Aron A, Aron EN, Tudor M, Nelson G. Close relationships as including other in the self. Journal of Personality and Social Psychology. 1991;60:241–53. [Google Scholar]
- Beckmann C, Jenkinson M, Smith SM. General multilevel linear modeling for group analysis in FMRI. Neuroimage. 2003;20:1052–63. doi: 10.1016/S1053-8119(03)00435-X. [DOI] [PubMed] [Google Scholar]
- Beggan JK. On the social nature of nonsocial perception: the mere ownership effect. Journal of Personality and Social Psychology. 1992;62:229–37. [Google Scholar]
- Belk RW. Possessions and the extended self. Journal of Consumer Research. 1988;15:139–68. [Google Scholar]
- Bower GH, Gilligan SG. Remembering information related to one’s self. Journal of Research in Personality. 1979;13:420–32. [Google Scholar]
- Campbell JD, Trapnell PD, Heine SJ, Katz IM, Lavallee LF, Lehman DR. Self-concept clarity: measurement, personality correlates, and cultural boundaries. Journal of Personality and Social Psychology. 1996;70:141–56. [Google Scholar]
- Cunningham SJ, Turk DJ, Macdonald LM, Macrae CN. Yours or mine? Ownership and memory. Consciousness and Cognition. 2008;17:312–18. doi: 10.1016/j.concog.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Damasio AR. The Feeling of What Happens: Body and Emotion in the Making of Consciousness. New York, NY: Harcourt Brace; 1999. [Google Scholar]
- D’Argembeau A, Jedidi H, Balteau E, Bahri M, Phillips C, Salmon E. Valuing one’s self: Medial prefrontal involvement in epistemic and emotive investments in self-views. Cerebral Cortex. 2012;22:659–67. doi: 10.1093/cercor/bhr144. [DOI] [PubMed] [Google Scholar]
- De Dreu CKW, van Knippenberg D. The possessive self as a barrier to conflict resolution: effects of mere ownership, process accountability, and self-concept clarity on competitive cognitions and behavior. Journal of Personality and Social Psychology. 2005;89:345–57. doi: 10.1037/0022-3514.89.3.345. [DOI] [PubMed] [Google Scholar]
- Fasig LG. Toddler’s understanding of ownership: implications for self-concept development. Social Development. 2000;9:370–82. [Google Scholar]
- Feys J. Briefly induced belongingness to self and preference. European Journal of Social Psychology. 1991;21:547–52. [Google Scholar]
- Gallagher II. Philosophical conceptions of the self: implications for cognitive science. Trends in Cognitive Sciences. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Higgins ET. Self-discrepancy: a theory relating self and affect. Psychological Review. 1987;94:319–40. [PubMed] [Google Scholar]
- Hintzman DL. Judgment of frequency versus recognition confidence: repetition and recursive reminding. Memory & Cognition. 2004;32:336–50. doi: 10.3758/bf03196863. [DOI] [PubMed] [Google Scholar]
- Huang Y, Wang L, Shi J. When do objects become more attractive? The individual and interactive effects of choice and ownership on object evaluation. Personality and Social Psychology Bulletin. 2009;35:713–22. doi: 10.1177/0146167209333046. [DOI] [PubMed] [Google Scholar]
- James W. The Principles of Psychology. Cambridge, MA: Harvard University Press; 1890/1983. [Google Scholar]
- Johnson MK. MEM: mechanisms of recollection. Journal of Cognitive Neuroscience. 1992;4:268–80. doi: 10.1162/jocn.1992.4.3.268. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Knetsch JL, Thaler RH. The endowment effect, loss aversion, and status quo bias. The Journal of Economic Perspectives. 1991;5:193–206. [Google Scholar]
- Kim K, Johnson MK. Extended self: medial prefrontal activity during transient association of self and objects. Social Cognitive and Affective Neuroscience. 2012;7:199–207. doi: 10.1093/scan/nsq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K, Yi D-J, Raye CL, Johnson MK. Negative effects of item repetition on source memory. Memory & Cognition. 2012;40:889–901. doi: 10.3758/s13421-012-0196-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Tu PC, Buckner RL. Clan mentality: evidence that the medial prefrontal cortex responds to close others. Journal of Neuroscience. 2010;30:13906–15. doi: 10.1523/JNEUROSCI.2180-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper NA, Rogers TB. Encoding of personal information: self-other differences. Journal of Personality and Social Psychology. 1979;37:499–514. doi: 10.1037//0022-3514.35.9.677. [DOI] [PubMed] [Google Scholar]
- Lieberman MD. Social cognitive neuroscience. In: Fiske ST, Gilbert DT, Lindzey G, editors. Handbook of Social Psychology. 3rd edn. New York, NY: McGraw-Hill; 2010. pp. 143–93. [Google Scholar]
- Lou HC, Luber B, Crupain M, et al. Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:6827–32. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cerebral Cortex. 2004;14:647–54. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Mashek DJ, Aron A, Boncimino M. Confusions of self with close others. Personality and Social Psychology Bulletin. 2003;29:382–92. doi: 10.1177/0146167202250220. [DOI] [PubMed] [Google Scholar]
- Moran JM, Heatherton TF, Kelly WM. Modulation of cortical midline structures by implicit and explicit self-relevance evaluation. Social Neuroscience. 2009;4:197–211. doi: 10.1080/17470910802250519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neisser U. Five kinds of self-knowledge. Philosophical Psychology. 1988;1:35–59. [Google Scholar]
- Peters J, Buchel C. Neural representations of subjective reward value. Behavioral Brain Research. 2010;213:135–41. doi: 10.1016/j.bbr.2010.04.031. [DOI] [PubMed] [Google Scholar]
- Qin P, Liu Y, Shi J, et al. Dissociation between anterior and posterior cortical regions during self-specificity and familiarity: a combined fMRI-meta-analytic study. Human Brain Mapping. 2012;33:154–64. doi: 10.1002/hbm.21201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rameson LT, Satpute AB, Lieberman MD. The neural correlates of implicit and explicit self-relevant processing. Neuroimage. 2010;50:701–8. doi: 10.1016/j.neuroimage.2009.12.098. [DOI] [PubMed] [Google Scholar]
- Rangel A, Hare T. Neural computations associated with goal-directed choice. Current Opinion in Neurobiology. 2010;20:262–70. doi: 10.1016/j.conb.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Ross HS. Negotiating principles of entitlement in sibling property disputes. Developmental Psychology. 1996;32:90–101. [Google Scholar]
- Sajonz B, Kahnt T, Margulies DS, et al. Delineating self-referential processing from episodic memory retrieval: common and dissociable networks. Neuroimage. 2010;50:1606–17. doi: 10.1016/j.neuroimage.2010.01.087. [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical activations during judgments about the self and an other person. Neuropsychologia. 2004;42:1168–77. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Setterlund MB, Niedenthal PM. “Who am I? Why am I here?”: self-esteem, self-clarity, and prototype matching. Journal of Personality and Social Psychology. 1993;65:769–80. doi: 10.1037//0022-3514.65.4.769. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia. 2006;44:2189–208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DJ, van Bussel K, Waiter GD, Macrae CN. Mine and me: exploring the neural basis of object ownership. Journal of Cognitive Neuroscience. 2011;23:3657–68. doi: 10.1162/jocn_a_00042. [DOI] [PubMed] [Google Scholar]
- Van den Bos M, Cunningham SJ, Conway MA, Turk DJ. Mine to remember: the impact of ownership on recollective experience. Quarterly Journal of Experimental Psychology. 2010;63:1065–71. doi: 10.1080/17470211003770938. [DOI] [PubMed] [Google Scholar]
- Wicklund RA, Gollwitzer PM. Symbolic Self-completion. Hillsdale, NJ: Lawrence Erlbaum; 1982. [Google Scholar]
- Worsley KJ, Marrett S, Neelin P, Vandal AC, Friston KJ, Evans AC. A unified statistical approach for determining significant signals in images of cerebral activation. Human Brain Mapping. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The anterior frontomedian cortex and evaluative judgment: An fMRI study. Neuroimage. 2002;15:983–91. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]