Abstract
Delirium is a common, but an often underdiagnosed complication in the elderly following major surgery. Recognising delirium in early stages and diagnosing the condition based on established criteria can improve the outcome and management. Managing delirium with environmental, supportive and pharmacological interventions will possibly reduce the incidence and side-effects associated with post-operative delirium. The purpose of this article is to provide an over view of the current knowledge about the disease, diagnosis, pathogenesis, preventive strategies, and treatment of post-operative delirium.
Keywords: Delirium, elderly, geriatric, post-operative
INTRODUCTION
The longevity of the elderly has increased over recent years because of better medical care and living conditions.[1] However, anaesthetic complications in elderly still mount a considerable challenge to the attending anaesthesiologist. Delirium is one of the common post-operative complications in elderly patients associated with increased morbidity and mortality. It may result in prolonged hospital stay, additional investigations and increased cost of treatment.[2] The incidence ranges from 9%-87% depending on the patient population and the degree of operative stress.[3] The word “delirium” is derived from Latin word ‘delirare’, which means ‘to be out of one's furrow’. This syndrome was first reported during the period of Hippocrates. It is usually seen on the first or second post-operative day, and symptoms are often aggravated at night. Early investigation and treatment are important to reduce the associated complications.[3] An extensive literature search was performed through electronic databases such as PubMed, Google Scholar, and Medline, for articles published between 1918 and 2014 with keywords ‘post-operative’, ‘delirium’, ‘geriatric’, ‘elderly’. The articles were further searched from the cross references and full text articles relevant to modern practice were reviewed.
DEFINITION
Delirium can be defined as an ‘acute confusional state’, which can also be a part of fluctuating neuropsychiatric clinical syndrome and clinically manifesting as a disturbed state of consciousness, cognitive dysfunction or alteration in perception and behaviour. The onset is usually over 1–2 days and can deteriorate further with poor prognosis if appropriate intervention is delayed. However, it can be prevented and treated if dealt with urgently.[4] Delirium may be confused with dementia, which has an insidious onset and progressive course with clear consciousness until the end stages. Clinically speaking, delirium generally relates to awareness and dementia to memory.
Robinson et al.,[5] by using the validated tools of the confusion assessment method (CAM)-Intensive Care Unit (ICU) and Richmond Agitation - Sedation Scale (RASS) measured delirium and classified delirium into three different types as hyperactive, hypoactive, mixed type based on motor activity. The patients who show positive RASS scores are defined as hyperactive (pure agitation) and those with negative RASS scores are classified as hypoactive (pure lethargy) and patient with either positive or negative scores are classified as mixed type (fluctuation between lethargy and agitation). The more common hypoactive patients have higher mortality and poor outcome.
DIAGNOSIS
Delirium is diagnosed if the patient develops inattention, disorganised thinking or coma of abrupt onset and fluctuating course. They may have one or more combination of the following symptoms, which include alteration in consciousness, cognitive deficit, hallucinations, psychomotor disturbances, lethargy, agitation, alteration in the sleep-wake cycle and emotional disturbances.
Patient assessment for delirium can be performed with the help of tools such as mini mental scale examination (MMSE), CAM for the intensive care unit (CAM-ICU), delirium writing test and others [Table 1].
Table 1.
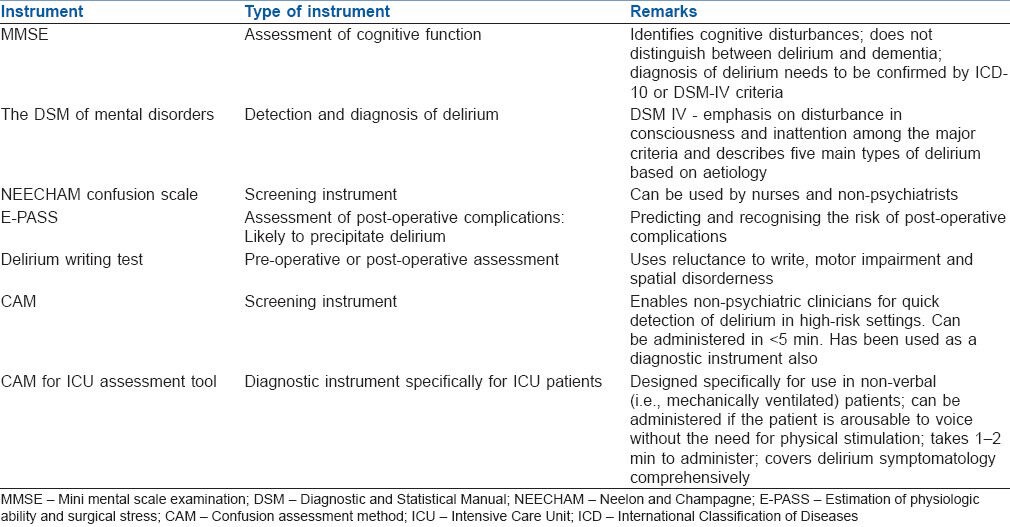
Mini mental scale examination[6]
Among the earliest assessments, the MMSE is an 11 question bedside screening tool to measure only the cognitive function. MMSE assesses attention, calculation, recall, orientation and language. A score of 0 represents profound cognitive impairment, and a score of 30 suggest intact cognition. However, it does not differentiate between delirium and dementia.
Confusion assessment method-intensive care unit[2]
This modified tool for ICU patients, which can be used in mechanically ventilated and nonverbal patients, consists of four diagnostic criteria: Acute onset, fluctuating course (primary symptoms), disorganised thinking and altered level of consciousness (secondary symptoms). Patients with two primary symptoms and one secondary symptom are considered to be suffering from delirium.
The Neelon and Champagne (NEECHAM) Confusion scale[7]
It is commonly used as a screening tool to assess the psychological state on daily basis, while providing routine nursing care to the patients by non-specialist doctors, nurses and paramedical staff. It mainly comprises of 3 subscales to evaluate the mental state. Cognitive functions such as attention, orientation and ability to follow the command are measured by subscale-1 and relates to scores between 0 and 14. Behavioural assessment is measured by subscale-2, which comprises of three items namely appearance, motor and verbal behaviour corresponding to scores between 0 and 10. Temperature, blood pressure heart rate and respiration stability, oxygen saturation and urinary control are measured by subscale-3 as a component of physiological functioning. Rating is done on the basis of scores between 0 (minimal responsiveness) and 30 (normal function). A score below 20 points indicate moderate to severe delirium, a score between 20 and 24 suggests mild or early development of delirium. A score of 25 and 26 suggests that the patient is ‘not delirious’ but the patients are at high-risk for development of delirium and a score of 27–30 indicates normal function.[7]
The Diagnostic and Statistical Manual (DSM) of mental disorders[8]
The American Psychiatric Association developed a tool for detection and diagnosis of delirium. Its updated version retains the emphasis on disturbance in consciousness and inattention among the major criteria and describes five main types of delirium based on aetiology.
Estimation of physiologic ability and surgical stress[9]
Basically, these scores emphasise the importance of surgical stress in eliciting various immune response from the host body so as to maintain physiological milieu. The measurement is based on the combination of pre-operative risk score, a surgical stress score, and a comprehensive risk score. This can be extremely useful in predicting post-operative complications; thus, enabling a proactive approach to recognise and treat the post-operative complications with a suitable therapeutic intervention thereby reducing morbidity and mortality. This scoring system is brief, simple, and reproducible and can be useful in all types of hospitals.
Delirium writing test[10]
This test may be used on the pre-operative day (for baseline) or whenever the patient seems mentally disturbed in the post-operative period and the criteria assessed are reluctance to write, motor impairment and spatial disorderliness.
Differential diagnosis and risk factors
Sub-syndromal delirium
This condition was first defined by Ouimet et al. Though it resembles delirium, the features do not meet the criteria for full-blown clinical delirium[11] and also they do not have the same risk factors. Sub-syndromal delirium constitutes a graded step in the spectrum of brain dysfunction severity.
The differential diagnosis of delirium includes alcohol withdrawal syndrome, delirium tremens and fat embolism syndrome. The cause of delirium is multifactorial and the risk factors for developing delirium are additive and it is important to identify the various risk factors at the earliest to prevent the onset and shorten the duration of delirium.
When people first present to the hospital for long-term care, the following risk factors are assessed namely age 65 years or older, cognitive impairment (past or present) and/or dementia, type of surgery, severe illness, metabolic derangements, alcohol abuse and sleep deprivation.
Indicators of delirium at presentation
A recent change or alteration in behaviour in people at risk (within hours or days) can be an indicator of delirium during initial clinical presentation. The symptomatology may be reported by the person at risk, or by the carer or relative. Vigilance for behaviour indicating hypoactive delirium is important. These behaviour changes may affect:
Cognitive functions: Presenting as worsened concentration, slow responses, and confusion.
Perception: Visual or auditory hallucinations may get affected
Physical function: Deterioration may present as reduced mobility, reduced movement, restlessness, agitation, changes in appetite, and sleep disturbance.
Social behaviour: May manifest as lack of cooperation with reasonable requests, withdrawal, or alterations in communication, mood and/or attitude.
Peri-operative triggers
Uncontrolled pain and non-judicious use of opioids are independent risk factors for possible development of delirium post-operatively. This clinical entity is further augmented by presence of hypoxemia, hypercarbia, metabolic disorders and/or sepsis. Other possible triggering mechanism may include but are not limited to use of physical restraints, malnutrition, urinary catheterisation, electrolyte and fluid abnormalities, anaemia, increased surgical blood loss and greater intraoperative transfusion.[12]
AETIOLOGY AND PATHOPHYSIOLOGY
Delirium most probably results from an imbalance in the synthesis, release and inactivation of neuro-transmitters, which are normally responsible for coordinating cognition, mood and behaviour. It is augmented by an acute imbalance of the neurotransmitter pool in vivo, alteration in oxidative metabolism and inflammatory process in the body. Three specific neurotransmitter systems involved in the development of delirium include glutaminergic, dopaminergic and cholinergic pathways.[13] Among various hypotheses proposed for development of delirium, increased level of dopamine and reduced level of acetylcholine[14] (causing increased neural excitability) are commonly implicated. Tryptophan which is a precursor for serotonin and melatonin production plays a role in the development of delirium.[15] Phenylalanine, is also associated in the development of delirium by competing with tryptophan and also reduces levels of serotonin and melatonin. Phenylalanine is actively transported across the blood-brain barrier and is converted into DOPA thereby producing delirium.[16]
The inflammatory response causes release of cytokines interleukin-1 (IL-1), IL-2, IL-6, tumour necrosis factor-α, and interferon-α which leads to a prothrombotic state, that can result in reduced cerebral blood flow possibly triggering delirium. It also increases the dopamine and decreases the acetylcholine levels.[17] In addition, it also affects the permeability of the blood-brain barrier. In normal individual, thalamus act as a filter for the information flowing to the cerebral cortex.[14] Neurotransmitter imbalance caused by the disease or medication results in thalamic dysfunction and leads on to delirium.
DRUGS IMPLICATED IN CAUSATION OF DELIRIUM
Opioid and anticholinergic drugs are most commonly associated with development of delirium. The opioids, particularly meperidine alter the neurotransmitter level (acetylcholine and serotonin) and by its direct neurotoxic and anticholinergic activity may produce delirium (indirect effects can be by its metabolite, normeperidine). The post-operative sedation induces delirium by interfering with normal sleep, which results in acetylcholine depletion[18] and diminished melatonin formation.[19]
The role of anaesthesia in development of delirium remains unclear. A recent meta-analysis concluded that general anaesthesia has an increased risk of developing post-operative cognitive dysfunction as compared to regional anaesthesia.[20] The plausible reason for the increased incidence for delirium following general anaesthesia may be attributed to hypoxemia resulting from residual effects of non-depolarising muscle relaxants. The drugs such as central nervous system depressant drugs, H2-antagonists, anti-cholinergics, digitalis, phenytoin, lignocaine, first-generation antihistamines (hydroxyzine), anti-hypertensives (b-blockers, methyldopa) and aminophylline are some of the drugs implicated in causing delirium and should be used with caution.[21]
PREVENTIVE STRATEGIES AND MANAGEMENT
Delirium is preventable or its severity can be lessened. Morandi et al. introduced ‘ABCDE bundle’ in the prevention of delirium, that includes: Awake and breathing, choice of sedation, daily delirium monitoring, early mobility and exercise.[22] The Yale Delirium Prevention Trial[23] targeted six risk factors, which include cognitive impairment, hearing impairment, visual impairment, sleep deprivation, immobility and dehydration.
Management of delirium
Prevention and management of delirium may include non-pharmacological and pharmacological methods depending upon the stage or severity of the clinical symptomatology
Non-pharmacological management
Formulation of pre-operative strategies should be the first-line of therapeutic intervention in the management of delirious patients. To ensure a calm and quiet environment the patient is usually provided with clocks, calendars and frequent re-orientation material. The presence of familiar family members, limiting room and staff changes, and minimising the night time disruptions help in uninterrupted sleep and maintain normal sleep-wake cycles. Providing access to glasses and hearing aids can largely help in communication and maintenance of nurse to patient or doctor to patient rapport besides a smooth communication with relatives and friends. Cognitive impairment can be treated with orientation protocol and therapeutic activities protocol.
Pharmacological management
Main aims of the pharmacological intervention in delirium are directed at prophylaxis (to prevent the development of delirium) or therapeutic management (after delirium has developed). Before starting the drugs one should rule out the reversible causes of delirium such as hypoxia, hypoglycaemia, infection and sepsis. Pharmacological management should be reserved for those who are potentially dangerous to themselves or to others.
Haloperidol is a convenient drug, which is most frequently used in the treatment of delirium.[24] Because of increased sensitivity of elderly patients, the drugs should be administered in small doses and titrated to the effective dose.[25] Haloperidol antagonises the D2 receptor in numerous higher pathways, leading to restoration of hippocampal function and reversing hallucination.[26] However, in hypoactive delirium it can worsen the symptoms due to dopamine deficiency. The optimal dosing schedule of haloperidol has not yet been established. However, a commonly used schedule is 2.5–5 mg intravenously (IV) every 6 h. It has been used as a continuous infusion in severe cases; however, this does not represent routine practice.[27] IV administration of haloperidol is preferable to intramuscular administration since drug absorption after intramuscular administration may be inconsistent, particularly in critically ill patients with hemodynamic instability and variable muscle perfusion characteristics. In addition, painful intramuscular injections can exacerbate paranoia, agitation, and treatment noncompliance. Moreover, use of IV haloperidol is less likely to produce extrapyramidal symptoms than intramuscular or even oral haloperidol.[28] If agitation is mild, it is reasonable to start with 0.5–2.5 mg. of IV haloperidol, if it is moderate, 5–10 mg IV is given, and if severe, 10 mg IV is given at the outset. The side-effects of haloperidol includes torsades de-pointes, malignant-hyperthermia and extra pyramidal movement disorders, which should be watched for during the treatment of delirium. Electrocardiogram monitoring is essential for patients receiving IV injection of haloperidol. Haloperidol and heparin should not be given in the same IV line because of the possibility of forming a precipitate.
Atypical anti-psychotic drugs such as risperidone, olanzapine, ziprasidone are considered as possible alternatives to haloperidol. The molecular action is exerted not only on dopamine receptors but also influences serotonin, acetylcholine and norepinephrine neurotransmission. Atypical anti-psychotics also have severe adverse effects like that of haloperidol; as such titrated minimal effective doses should be used. Enteral administration is required as there are no IV preparations available. They are metabolised in the liver and have active metabolites. Patients with hypo active delirium may benefit by the use of atypical anti-psychotics (such as olanzapine, quetiapine)[29] because of global effect on neurotransmitter equilibrium.
Other pharmacological agents
Lorazepam 1–2 mg IV every 2–4 h is useful in alcohol withdrawal syndrome to control agitation. The use of benzodiazepines in other patients’ sub-groups has been identified as independent risk factors for delirium development. Physostigmine is effective in delirium caused by anti-cholinergic syndrome. Vitamin B12 is useful as a replacement in alcoholic patients. Newer alpha 2 agonist dexmedetomidine appears to be a potentially better agent in providing sedation and anxiolysis without respiratory depression.[30] The mechanism of action is focussed on the receptors of the locus coeruleus for the anxiolytic effects, whereas pain control is through spinal cord receptors.[31]
SUMMARY
Delirium is a common feature in the post-operative period in the elderly patients associated with acute alteration in attention and cognitive impairment, which is responsible for the significant increase in both morbidity and mortality. Multiple factors predispose a patient to different forms of delirium. The reversible causes must be identified and treated promptly. Anti-psychotic drugs are helpful in the treatment of delirium and haloperidol given IV remains the treatment of choice. No non-pharmacological approach or drugs has been shown to be beneficial once delirium is established. A wider knowledge of the various drug actions and interactions, pathophysiological knowledge and clinical awareness can help an anaesthesiologist to counter the possible deleterious effects of delirium during the peri-operative period.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Vaupel JW. Biodemography of human ageing. Nature. 2010;25(464):536–42. doi: 10.1038/nature08984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: Validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–10. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 3.Ansaloni L, Catena F, Chattat R, Fortuna D, Franceschi C, Mascitti P, et al. Risk factors and incidence of postoperative delirium in elderly patients after elective and emergency surgery. Br J Surg. 2010;97:273–80. doi: 10.1002/bjs.6843. [DOI] [PubMed] [Google Scholar]
- 4.National Institute for Health Care and Excellence; 2010. NICE Clinical Guideline 103. Delirium: Diagnosis, Prevention and Management; p. 34. [PubMed] [Google Scholar]
- 5.Robinson TN, Raeburn CD, Tran ZV, Brenner LA, Moss M. Motor subtypes of postoperative delirium in older adults. Arch Surg. 2011;146:295–300. doi: 10.1001/archsurg.2011.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer A. New York: Bloomingdale Hospital Press; 1918. Outlines of Examinations. [Google Scholar]
- 7.Grover S, Kate N. Assessment scales for delirium: A review. World J Psychiatry. 2012;2:58–70. doi: 10.5498/wjp.v2.i4.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Text Revision (DSM-IV) 4th ed. Washington DC: American Psychiatric Association; 1994. American Psychiatric Association. Covering both the cognitive and noncognitive aspects of delirium. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 9.Oka Y, Nishijima J, Oku K, Azuma T, Inada K, Miyazaki S, et al. Usefulness of an estimation of physiologic ability and surgical stress (E-PASS) scoring system to predict the incidence of postoperative complications in gastrointestinal surgery. World J Surg. 2005;29:1029–33. doi: 10.1007/s00268-005-7719-y. [DOI] [PubMed] [Google Scholar]
- 10.Aakerlund LP, Rosenberg J. Writing disturbances: An indicator for postoperative delirium. Int J Psychiatry Med. 1994;24:245–57. doi: 10.2190/PTP2-GPB2-GA6Q-NM87. [DOI] [PubMed] [Google Scholar]
- 11.Ouimet S, Riker R, Bergeron N, Cossette M, Kavanagh B, Skrobik Y. Subsyndromal delirium in the ICU: Evidence for a disease spectrum. Intensive Care Med. 2007;33:1007–13. doi: 10.1007/s00134-007-0618-y. [DOI] [PubMed] [Google Scholar]
- 12.Marcantonio ER, Goldman L, Orav EJ, Cook EF, Lee TH. The association of intraoperative factors with the development of postoperative delirium. Am J Med. 1998;105:380–4. doi: 10.1016/s0002-9343(98)00292-7. [DOI] [PubMed] [Google Scholar]
- 13.Gaudreau JD, Gagnon P, Roy MA, Harel F, Tremblay A. Association between psychoactive medications and delirium in hospitalized patients: A critical review. Psychosomatics. 2005;46:302–16. doi: 10.1176/appi.psy.46.4.302. [DOI] [PubMed] [Google Scholar]
- 14.Pavlov VA, Wang H, Czura CJ, Friedman SG, Tracey KJ. The cholinergic anti-inflammatory pathway: A missing link in neuroimmunomodulation. Mol Med. 2003;9:125–34. [PMC free article] [PubMed] [Google Scholar]
- 15.Pandharipande PP, Morandi A, Adams JR, Girard TD, Thompson JL, Shintani AK, et al. Plasma tryptophan and tyrosine levels are independent risk factors for delirium in critically ill patients. Intensive Care Med. 2009;35:1886–92. doi: 10.1007/s00134-009-1573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van der Mast RC, van den Broek WW, Fekkes D, Pepplinkhuizen L, Habbema JD. Is delirium after cardiac surgery related to plasma amino acids and physical condition? J Neuropsychiatry Clin Neurosci. 2000;12:57–63. doi: 10.1176/jnp.12.1.57. [DOI] [PubMed] [Google Scholar]
- 17.van der Mast RC. Pathophysiology of delirium. J Geriatr Psychiatry Neurol. 1998;11:138–45. doi: 10.1177/089198879801100304. [DOI] [PubMed] [Google Scholar]
- 18.Maldonado JR. Delirium in the acute care setting: Characteristics, diagnosis and treatment. Crit Care Clin. 2008;24:657–722. doi: 10.1016/j.ccc.2008.05.008. vii. [DOI] [PubMed] [Google Scholar]
- 19.Olofsson K, Alling C, Lundberg D, Malmros C. Abolished circadian rhythm of melatonin secretion in sedated and artificially ventilated intensive care patients. Acta Anaesthesiol Scand. 2004;48:679–84. doi: 10.1111/j.0001-5172.2004.00401.x. [DOI] [PubMed] [Google Scholar]
- 20.Edelstein DM, Aharonoff GB, Karp A, Capla EL, Zuckerman JD, Koval KJ. Effect of postoperative delirium on outcome after hip fracture. Clin Orthop Relat Res. 2004;422:195–200. doi: 10.1097/01.blo.0000128649.59959.0c. [DOI] [PubMed] [Google Scholar]
- 21.Rudra A, Chatterjee S, Kirtania J, Sengupta S, Moitra G, Sirohia S, et al. Postoperative delirium. Indian J Crit Care Med. 2006;10:235–40. [Google Scholar]
- 22.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: The ‘ABCDE‘ approach. Curr Opin Crit Care. 2011;17:43–9. doi: 10.1097/MCC.0b013e3283427243. [DOI] [PubMed] [Google Scholar]
- 23.Inouye SK, Bogardus ST, Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med. 1999;340:669–76. doi: 10.1056/NEJM199903043400901. [DOI] [PubMed] [Google Scholar]
- 24.Ely EW, Stephens RK, Jackson JC, Thomason JW, Truman B, Gordon S, et al. Current opinions regarding the importance, diagnosis, and management of delirium in the intensive care unit: A survey of 912 healthcare professionals. Crit Care Med. 2004;32:106–12. doi: 10.1097/01.CCM.0000098033.94737.84. [DOI] [PubMed] [Google Scholar]
- 25.Pandharipande P, Jackson J, Ely EW. Delirium: Acute cognitive dysfunction in the critically ill. Curr Opin Crit Care. 2005;11:360–8. doi: 10.1097/01.ccx.0000170503.76528.4b. [DOI] [PubMed] [Google Scholar]
- 26.Rivera R, Antognini JF. Perioperative drug therapy in elderly patients. Anesthesiology. 2009;110:1176–81. doi: 10.1097/ALN.0b013e3181a10207. [DOI] [PubMed] [Google Scholar]
- 27.Riker RR, Fraser GL, Cox PM. Continuous infusion of haloperidol controls agitation in critically ill patients. Crit Care Med. 1994;22:433–40. doi: 10.1097/00003246-199403000-00013. [DOI] [PubMed] [Google Scholar]
- 28.Cassem NH, Murray GB, Lafayette JM. Delirious patients. In: Stern TA, Fricchione GL, Cassem NH, editors. Massachusets General Hospital Handbook of General Hospital Psychiatry. 5th ed. Philadelphia, PA: Mosby/Elsevier; 2004. pp. 119–34. [Google Scholar]
- 29.Skrobik YK, Bergeron N, Dumont M, Gottfried SB. Olanzapine vs haloperidol: Treating delirium in a critical care setting. Intensive Care Med. 2004;30:444–9. doi: 10.1007/s00134-003-2117-0. [DOI] [PubMed] [Google Scholar]
- 30.Sudheesh K, Harsoor S. Dexmedetomidine in anaesthesia practice: A wonder drug? Indian J Anaesth. 2011;55:323–4. doi: 10.4103/0019-5049.84824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maldonado JR, van der Starre PJ, Wysong A. Post-operative sedation and the incidence of ICU delirium in cardiac surgery patients. Anesthesiology. 2003;99:A465. [Google Scholar]


