Abstract
Background and Aims:
Dexmedetomidine (α2 adrenergic agonist) has been used for prevention of post anaesthesia shivering. Its use for the treatment of post-spinal anaesthesia shivering has not been evaluated. The aim of this study was to evaluate and compare the efficacy, haemodynamic and adverse effects of dexmedetomidine with those of tramadol, when used for control of post-spinal anaesthesia shivering.
Methods:
A prospective, randomised, and double-blind study was conducted in 50 American Society of Anaesthesiologists Grade I and II patients of either gender, aged between 18 and 65 years, scheduled for various surgical procedures under spinal anaesthesia. The patients were randomised in two groups of 25 patients each to receive either dexmedetomidine 0.5 μg/kg or tramadol 0.5 mg/kg as a slow intravenous bolus. Grade of shivering, onset of shivering, time for cessation of shivering, recurrence, response rate, and adverse effects were observed at scheduled intervals. Unpaired t-test was used for analysing the data.
Results:
Time taken for cessation of shivering was significantly less with dexmedetomidine when compared to tramadol. Nausea and vomiting was observed only in tramadol group (28% and; 20% respectively). There was not much difference in the sedation profile of both the drugs.
Conclusion:
We conclude that although both drugs are effective, the time taken for cessation of shivering is less with dexmedetomidine when compared to tramadol. Moreover, dexmedetomidine has negligible adverse effects, whereas tramadol is associated with significant nausea and vomiting.
Keywords: Dexmedetomidine, post-spinal anaesthesia shivering, tramadol
INTRODUCTION
Shivering, a common post-anaesthesia occurrence is defined as an involuntary, repetitive activity of skeletal muscles. The incidence of shivering has been found to be quite high, approximately 40-50% in different studies.[1] It can double or even treble oxygen consumption and carbon dioxide production.[2] Shivering also increases intraocular and intracranial pressure, and may contribute to increased wound pain, delayed wound healing, and delayed discharge from post-anaesthetic care.[3,4] Apart from being an uncomfortable experience, its deleterious effects deserve primary prevention and rapid control on occurrence.
Shivering is a physiological response to core hypothermia in an attempt to raise the metabolic heat production. The main causes of intra/post-operative shivering are temperature loss, increased sympathetic tone, pain, and systemic release of pyrogens.[5] Spinal anaesthesia significantly impairs the thermoregulation system by inhibiting tonic vasoconstriction, which plays a significant role in temperature regulation. It also causes a redistribution of core heat from the trunk (below the block level) to the peripheral tissues. These factors predispose patients to hypothermia and shivering.[5]
The treatment of shivering includes both pharmacological and non-pharmacological methods. The non-pharmacological management is by external heating like the use of forced air warming, warming blankets, warmed fluids etc., According to the results of a meta-analysis, the most frequently reported pharmacological interventions include clonidine, pethidine, tramadol, nefopam and ketamine.[6] Unfortunately, no gold standard treatment is known for shivering as the administration of all the available drugs is associated with various adverse effects.
During the last decade, Tramadol has become a favoured and commonly used drug for post-spinal anaesthesia shivering. However, it has many adverse effects like nausea, vomiting, dizziness etc., which cause further discomfort to the patient.[7,8] Clonidine is another agent which has gained popularity during the last few years. Various studies, which have been conducted to compare them have concluded that clonidine has better efficacy and less adverse effects as compared to tramadol.[7,8] But there was 5-10% incidence of hypotension and bradycardia with clonidine.[7] Dexmedetomidine, a congener of clonidine, is a highly selective α2-adrenoceptor agonist. It has been used as a sedative agent and is known to reduce the shivering threshold.[9] Few studies which have explored its anti-shivering potential have inferred that dexmedetomidine is an effective drug without any major adverse effect and provides good haemodynamic stability.[9,10,11] Hence, we planned to do a comparative study of the efficacy, haemodynamic, and adverse effects of tramadol and dexmedetomidine when used for the control of post-spinal anaesthesia shivering.
METHODS
This was a prospective, randomised, double-blind study, which was conducted at a tertiary care centre after taking approval from Institutional Ethics Committee. All subjects gave written informed consent to participate in the study. 50 American Society of Anaesthesiologists (ASA) Grade I and II consenting patients of either gender aged 18-65 years scheduled for elective as well as emergency lower abdominal, lower limb, orthopaedic and plastic surgeries under spinal anaesthesia were included in the study. Patients with known hypersensitivity to dexmedetomidine or tramadol, cardio-pulmonary, renal or hepatic disease, hyperthyroidism, psychiatric disorder, urinary tract infection, severe diabetes or autonomic neuropathies, known history of substance or alcohol abuse, patients receiving any pre-medication were not included in the study.
All patients who fulfilled the inclusion criteria and developed post-spinal anaesthesia shivering were enrolled and randomised using computer generated chart with allocation ratio of 1:1 into either of the two groups. Group D (n = 25) were administered dexmedetomidine 0.5 μg/kg intravenous (IV) and Group T (n = 25) received tramadol 0.5 mg/kg IV as per randomisation by index anaesthesiologist (not a part of the study) who prepared either of the drugs, at the onset of shivering (detailed below). The anaesthesiologist conducting the case as well as recording the data were unaware of the drug being administered.
Upon arrival in the operation theatre, an 18G venous cannula was inserted and preloading done with Ringer's Lactate solution 10 ml/kg before giving spinal anaesthesia and maintained at 6 ml/kg/h after spinal anaesthesia. Before starting the procedure, standard monitors were attached and all the baseline parameters such as heart rate (HR), non-invasive blood pressure (NIBP), oxygen saturation (SPO2), electrocardiography (ECG), and body temperature (axillary) were recorded. Subarachnoid anaesthesia was administered with 0.5% heavy bupivacaine (15 mg) at L3-4 or L4-5 interspace using 26G Quincke's spinal needle under aseptic conditions. All operation theatres were maintained at an ambient temperature of around 24°C-25°C. Supplemental oxygen was administered to all the patients at the rate of 5 l/min with face mask and patients were covered with drapes but not actively warmed. IV fluids and anaesthetics were administered at room temperature. Vital parameters such as HR, NIBP, and SPO2 were recorded at intervals of every 5 min for first 30 min and every 15 min for the rest of the observation period. Continuous ECG monitoring was done.
Shivering was graded using a four point scale as per Wrench.:[12] Grade 0: No shivering, Grade 1: One or more of the following: Piloerection, peripheral vasoconstriction, peripheral cyanosis, but without visible muscle activity, Grade 2: Visible muscle activity confined to one muscle group, Grade 3: Visible muscle activity in more than 1 muscle group and Grade 4: Gross muscle activity involving the whole body. Patients who developed either Grades 3 or 4 shivering were included in the study. Either of the two drugs was given as slow IV bolus injection. The drugs were diluted to a volume of 5 ml in a 5 ml syringe and presented as coded syringes as per randomisation list by an anaesthesiologist who was not aware of the group allocation. The attending anaesthesiologist recorded the time in minutes at which shivering started after spinal anaesthesia (onset of shivering), severity of the shivering, time to the disappearance of shivering and response rate (shivering ceasing within 15 min after treatment). Duration of surgery was recorded and duration of spinal anaesthesia was noted by assessing spontaneous recovery of sensory block using the pin-prick method and observing spontaneous movements of limbs in the post-operative period. Recurrence of shivering was also noted. In case there was recurrence of shivering, patients were treated with an additional dose of dexmedetomidine (0.5 μg/kg IV) or tramadol (0.5 mg/kg IV) in the respective groups. Adverse effects such as nausea, vomiting, bradycardia (<50/min), hypotension (>20% of baseline), dizziness; and sedation score were noted. The degree of sedation was graded on a four point scale as per Filos et al.:[13] Grade 1: Awake and alert, Grade 2: Drowsy, responsive to verbal stimuli, Grade 3: Drowsy, arousable to physical stimuli, Grade 4: Unarousable.
Nausea and vomiting were treated with injection metoclopramide 10 mg IV as and when required. The coding was opened after completion of the study to compile results.
Power analysis was based on the detectable difference between the means of time taken for cessation of post-spinal shivering after treatment with dexmedetomidine or tramadol = 180 s. With a standard deviation (SD) of 180 s considering α =0.05 and power of 90% (two-tailed test), sample size calculated to be 21.02 patients per group. To increase the power of study we enrolled 25 patients per group. Numerical data were presented as mean ± SD and categorical data as proportions (%). The comparison of the mean levels of all variables between two groups was made by the unpaired t-test. P value was calculated, and P < 0.05 was considered to be statistically significant.
RESULTS
In the present study, a total of 50 patients out of 69 consecutive patients met the inclusion criteria and consented for study. These 50 patients were randomized into two groups of 25 each. Out of the total patients, 25 were male, and 25 were female. As it was an intra-operative study, no patient was lost to follow-up [Figure 1].
Figure 1.
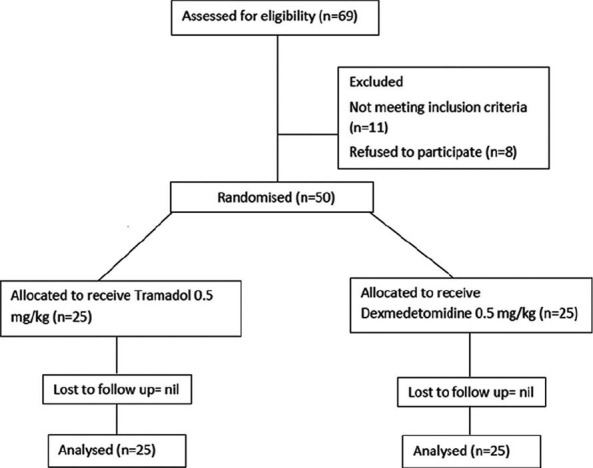
Patient flow (according to consort chart)
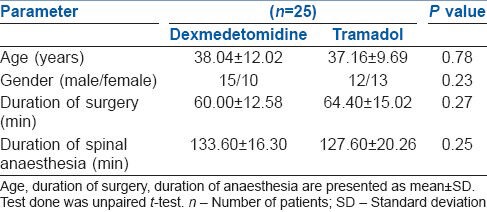
Both groups were comparable with respect to age, gender, ASA grade, duration of surgery and the duration of spinal anaesthesia. Duration of surgery varied from 30 min to 90 min. Duration of spinal anaesthesia ranged from 90 min to 150 min [Table 1].
Table 1.
Demographic profile of patients of both groups
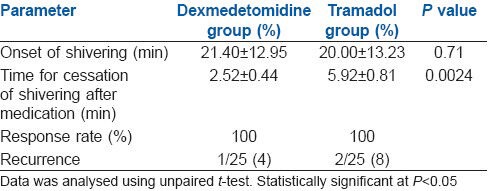
All the patients had Grade 3 shivering. There was no statistically significant difference in time for the onset of shivering between the two groups. However, the difference in the time interval between administration of drug after the onset of shivering and cessation of shivering was significantly shorter in the dexmedetomidine group when compared to tramadol group. There was recurrence of shivering in 1 patient in dexmedetomidine group and 2 patients in tramadol group. The patients were given rescue doses of dexmedetomidine or tramadol, respectively [Table 2].
Table 2.
Parameters for post-spinal anaesthesia shivering
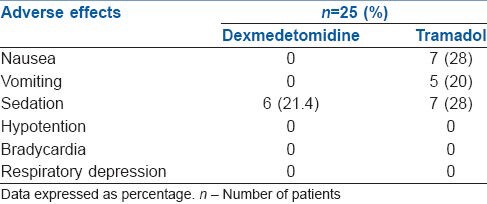
Nausea and vomiting was observed only in tramadol group, and there was no incidence in the dexmedetomidine group. Almost similar number of patients were sedated in both groups and the sedation score in all the patients was 2. There was no evidence of respiratory depression, hypotension or bradycardia in any of the patients. HR, mean blood pressure, body temperature, and SPO2 remained within normal limits throughout the procedure in both groups [Table 3].
Table 3.
Pattern of adverse reactions in both groups
DISCUSSION
Intra and post-operative shivering is a distressing experience for the patient. The exact mechanism of shivering during spinal anaesthesia has not been fully recognized. The possible mechanisms include impairment of central thermoregulation, internal redistribution of body heat, and heat loss to the environment. Potential risk factors for hypothermia in spinal anaesthesia include ageing, level of sensory block, temperature of the operation theatre and IV solutions. In this study, all operation theatres (OTs) maintained an ambient temperature of 23-25°C, and all fluids and drugs were at room temperature during the surgery. Demographic factors such as age, gender, duration of anaesthesia and surgery have also been matched to reduce any confounding bias.
The neurotransmitter pathways involved in shivering are multiple and involve opioids, α2 adrenergic, serotenergic, and anticholinergic receptors. Hence, drugs acting on these systems which include opioids (pethidine, nalbuphine, or tramadol), ketanserin, propofol, doxapram, clonidine, ketamine and nefopam are utilized in the treatment of shivering. However, adverse effects such as hypotension, hypertension, sedation, respiratory depression, nausea and vomiting limit their use. Hence, the hunt for an ideal anti-shivering agent is continuing.
Tramadol is an opioid analgesic with opioid effect mainly mediated via mu receptor with minimal effect on kappa and delta receptors. It also activates the monoaminergic receptors of the descending spinal inhibitory pain pathway. The anti-shivering action of tramadol is probably mediated via its opioid or serotonergic and noradrenergic activity or both.[14,15,16] It is a well-established agent in the treatment of post-anaesthetic shivering.
Alpha-2 adrenergic agonists are widely used nowadays in anaesthesia and intensive care settings. Dexmedetomidine is an α2 adrenoceptor agonist, with antihypertensive, sedative, analgesic, and anti-shivering properties.[17] The anti-shivering effects of alpha adrenoceptor agonists are mediated by binding to α2 receptors that mediate vasoconstriction and the anti-shivering effect. In addition, it has hypothalamic thermoregulatory effects.[18] Dexmedetomidine comparably reduces the vasoconstriction and shivering thresholds, thus suggesting that it acts on the central thermoregulatory system rather than preventing shivering peripherally.[19] It has been successfully used as an adjunct to local anaesthetics in spinal anaesthesia and peripheral nerve blockade, for the sedation of mechanically ventilated patients in the Intensive Care Unit, as well as supplementation of post-operative analgesia.[17,20] The role of dexmedetomidine in the treatment of shivering has been evaluated in a few studies.[9,10,11] It may be a good choice because of its dual effects related ‘anti-shivering’ and sedation.
With same dose, that is, 0.5 mg/kg of tramadol, the response rate reported by Shukla et al. was 92.5%, by Reddy and Chiruvella as 95.56% and by Tsai and Chu, 87%.[7,8,16] We found 100% response rate in our study. Maheshwari et al. reported similar recurrence rate with tramadol as in our study (8%) but the dose used in their study was 1 mg/kg.[21] The recurrence rate in the study by Shukla et al. was 5%, which is similar to our results. The incidence of nausea and vomiting with tramadol in our study was 28% and 20%, respectively. The results correspond with that of other studies by Reddy and Chiruvella, Tsai and Chu; Bansal and Jain[8,16,22] However, in the study by Shukla et al.,[7] the incidence of nausea was quite high (77.5%), whereas Wason et al. have reported the incidence of nausea as only 4%.[23] These variations could be explained by the peculiar patient characteristics in different studies. Maheshwari et al. have reported a very high incidence of sedation to the extent of 84%, which as mentioned earlier could be due to the higher dose as opposed to 28% sedation in our study.[21]
In a study by Easley, all children who had post-anaesthesia shivering were treated with a single IV bolus dose of dexmedetomidine 0.5 μg/kg over 3-5 min.[24] All children had cessation of shivering behaviour within 5 min following the completion of dexmedetomidine administration. There was no recurrence of shivering and no adverse effects occurred. We found a response rate of 100% and apart from sedation, there was no other adverse effect observed. But there was recurrence in 1 patient who had to be given a rescue dose. All other studies have used higher dose of dexmedetomidine (1 μg/kg), so comparison of results would not be appropriate. In our study, the incidence of sedation was 21.4%, which is similar to other studies. One contradictory report was by Karaman et al. according to whom intra-operative dexmedetomidine infusion caused negligible sedation inspite of using a loading dose of 1 μg/kg followed by a maintenance infusion of 0.5 μg/kg/h.[11]
The otherα agonist clonidine is associated with a high incidence of hypotension and bradycardia. In this respect, dexmedetomidine has an edge as it causes less variations in haemodynamics.
The results of this study indicate that dexmedetomidine takes lesser time to control shivering. The incidence of adverse effects like nausea and vomiting was found to be higher in case of tramadol compared to dexmedetomidine. More studies need to be undertaken with varying dose ranges to extrapolate the results of this study.
There was not much difference in the number of patients who were sedated in either group. The sedation seen with dexmedetomidine, in the absence of nausea and vomiting, is beneficial for the surgeon, anaesthetist as well as the patient. It provided more comfort to the patient, maintained more cardio-respiratory stability, improved surgical conditions and also provided amnesia during surgery.
The major limitation of our study is the small sample size. A bigger sample size would have increased the robustness of our results. Another limitation was that the present study included short duration surgeries as the mean duration of surgical period was calculated to be approximately 1 h in both groups. The anti-shivering effect of dexmedetomidine needs to be seen in surgeries of longer duration where chances of developing hypothermia are more. Also, we did not assess different doses of dexmedetomidine; further studies are needed to evaluate the anti-shivering and haemodynamic effects of dexmedetomidine with various doses.
The search continues for drugs that sufficiently improve the tolerance of thermoregulation without simultaneously producing respiratory depression, or haemodynamic instability. Dexmedetomidine might prove to be a valuable addition in the current armamentarium of the available drugs.
CONCLUSION
Both dexmedetomidine (0.5 μg/kg) and tramadol (0.5 mg/kg) are effective in treating patients with post-spinal anaesthesia shivering, but time taken for complete cessation of shivering was shorter with dexmedetomidine as compared to tramadol, the difference being statistically significant. Furthermore, dexmedetomidine causes fewer adverse effects like nausea and vomiting. Sedation caused by dexmedetomidine provides additional comfort to the patient.
More studies of different dose ranges of dexmedetomidine need to be conducted in order to cement its position as an efficient anti-shivering agent.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.De Witte J, Sessler DI. Perioperative shivering: Physiology and pharmacology. Anesthesiology. 2002;96:467–84. doi: 10.1097/00000542-200202000-00036. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharya P, Bhattacharya L. Postanaesthetic shivering (PAS): A review. Indian J Anaesth. 2003;47:88–93. [Google Scholar]
- 3.Katyal S, Tewari A. Shivering: Anaesthetic considerations. J Anaesth Clin Pharmacol. 2002;18:363–76. [Google Scholar]
- 4.Kranke P, Eberhart LH, Roewer N, Tramèr MR. Pharmacological treatment of postoperative shivering: A quantitative systematic review of randomized controlled trials. Anesth Analg. 2002;94:453–60. doi: 10.1097/00000539-200202000-00043. [DOI] [PubMed] [Google Scholar]
- 5.Sessler DI. Temperature regulation and monitoring. In: Millar RD, editor. Textbook of Anaesthesia. 7th ed. New York: Churchill Livingstone Inc; 2010. pp. 1533–56. [Google Scholar]
- 6.Park SM, Mangat HS, Berger K, Rosengart AJ. Efficacy spectrum of antishivering medications: Meta-analysis of randomized controlled trials. Crit Care Med. 2012;40:3070–82. doi: 10.1097/CCM.0b013e31825b931e. [DOI] [PubMed] [Google Scholar]
- 7.Shukla U, Malhotra K, Prabhakar T. A comparative study of the effect of clonidine and tramadol on post-spinal anaesthesia shivering. Indian J Anaesth. 2011;55:242–6. doi: 10.4103/0019-5049.82666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reddy VS, Chiruvella S. Clonidine versus tramadol for post spinal shivering during caesarean section: A randomized double blind clinical study. J Obstet Anaesth Crit Care. 2011;1:26–9. [Google Scholar]
- 9.Bajwa SJ, Gupta S, Kaur J, Singh A, Parmar S. Reduction in the incidence of shivering with perioperative dexmedetomidine: A randomized prospective study. J Anaesthesiol Clin Pharmacol. 2012;28:86–91. doi: 10.4103/0970-9185.92452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Usta B, Gozdemir M, Demircioglu RI, Muslu B, Sert H, Yaldiz A. Dexmedetomidine for the prevention of shivering during spinal anesthesia. Clinics (Sao Paulo) 2011;66:1187–91. doi: 10.1590/S1807-59322011000700011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karaman S, Gunusen I, Ceylan MA, Karaman Y, Cetin EN, Derbent A, et al. Dexmedetomidine infusion prevents postoperative shivering in patients undergoing gynaecologic laparoscopic surgery. Turk J Med Sci. 2013;43:232–7. [Google Scholar]
- 12.Wrench IJ, Singh P, Dennis AR, Mahajan RP, Crossley AW. The minimum effective doses of pethidine and doxapram in the treatment of post-anaesthetic shivering. Anaesthesia. 1997;52:32–6. doi: 10.1111/j.1365-2044.1997.006-az006.x. [DOI] [PubMed] [Google Scholar]
- 13.Filos KS, Goudas LC, Patroni O, Polyzou V. Hemodynamic and analgesic profile after intrathecal clonidine in humans. A dose-response study. Anesthesiology. 1994;81:591–601. doi: 10.1097/00000542-199409000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Lee CR, McTavish D, Sorkin EM. Tramadol. A preliminary review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in acute and chronic pain states. Drugs. 1993;46:313–40. doi: 10.2165/00003495-199346020-00008. [DOI] [PubMed] [Google Scholar]
- 15.Mathews S, Al Mulla A, Varghese PK, Radim K, Mumtaz S. Postanaesthetic shivering – A new look at tramadol. Anaesthesia. 2002;57:394–8. doi: 10.1046/j.1365-2044.2002.2457_3.x. [DOI] [PubMed] [Google Scholar]
- 16.Tsai YC, Chu KS. A comparison of tramadol, amitriptyline, and meperidine for postepidural anesthetic shivering in parturients. Anesth Analg. 2001;93:1288–92. doi: 10.1097/00000539-200111000-00052. [DOI] [PubMed] [Google Scholar]
- 17.Grewal A. Dexmedetomidine: New avenues. J Anaesthesiol Clin Pharmacol. 2011;27:297–302. doi: 10.4103/0970-9185.83670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bajwa SJ, Bajwa SK, Kaur J, Singh G, Arora V, Gupta S, et al. Dexmedetomidine and clonidine in epidural anaesthesia: A comparative evaluation. Indian J Anaesth. 2011;55:116–21. doi: 10.4103/0019-5049.79883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Talke P, Tayefeh F, Sessler DI, Jeffrey R, Noursalehi M, Richardson C. Dexmedetomidine does not alter the sweating threshold, but comparably and linearly decreases the vasoconstriction and shivering thresholds. Anesthesiology. 1997;87:835–41. doi: 10.1097/00000542-199710000-00017. [DOI] [PubMed] [Google Scholar]
- 20.Kamibayashi T, Maze M. Clinical uses of alpha2 -adrenergic agonists. Anesthesiology. 2000;93:1345–9. doi: 10.1097/00000542-200011000-00030. [DOI] [PubMed] [Google Scholar]
- 21.Maheshwari BS, Shah SK, Chadha IA. Tramadol and butrophanol for control of shivering: Randomised double blind comparative study. J Anaesth Clin Pharmacol. 2008;24:343–6. [Google Scholar]
- 22.Bansal P, Jain G. Control of shivering with clonidine, butorphanol, and tramadol under spinal anesthesia: A comparative study. Local Reg Anesth. 2011;4:29–34. doi: 10.2147/LRA.S15366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wason R, Jain N, Gupta P, Gogia AR. Randomized double-blind comparison of prophylactic ketamine, clonidine and tramadol for the control of shivering under neuraxial anaesthesia. Indian J Anaesth. 2012;56:370–5. doi: 10.4103/0019-5049.100821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blaine Easley R, Brady KM, Tobias JD. Dexmedetomidine for the treatment of postanesthesia shivering in children. Paediatr Anaesth. 2007;17:341–6. doi: 10.1111/j.1460-9592.2006.02100.x. [DOI] [PubMed] [Google Scholar]


