Abstract
Background and Aims:
The choice of anaesthetic agent for electroconvulsive therapy (ECT) depends on seizure duration, haemodynamic, and recovery parameters. The aim of the study was to assess the effects of ketamine-propofol induction with dexmedetomidine preadministration (ketofol-dex group) and without its preadministration (ketofol group) on haemodynamics, depression, seizure duration, recovery characteristics, and agitation following ECT in patients with depression.
Methods:
40 patients aged 18-60 years were scheduled for ECT for treatment of depression. Dexmedetomidine (0.5 μg/kg) diluted to a volume of 10 ml with 0.9% saline or 10 ml 0.9% saline were infused intravenously over 10 minutes before induction of anaesthesia with ketamine and propofol (ketofol). Statistical analysis was carried out using the Statistical Software for the Social Sciences (SPSS) package.
Results:
Motor seizure duration in ketofol group was significantly less compared to ketofol-dex group (35.8 ± 6.6s versus 38.9 ± 4.9s). Total ketofol used was significantly less in ketofol-dex group compared to ketofol group (78.5 ± 10.8mg versus 90 ± 13.2mg). The number of patients with agitation score >2 was significantly lower in ketofol-dex group (1.4%) compared to ketofol group (8.6%). There was significant decrease (P = 0.000) in mean arterial pressure (MAP) and heart rate (HR) in ketofol-dex group compared to ketofol group at 20, 30, and 40 minutes for MAP and at 10, 20, 30, and 40 minutes for HR.
Conclusions:
Ketofol-dex mixture in ECT is associated with longer mean seizure duration, effective anti-depression, less incidence of agitation, more patient satisfaction, and acceptable decreases in blood pressure and HR when compared to ketofol alone.
Keywords: Agitation, depression, dexmedetomidine, electroconvulsive therapy, ketofol
INTRODUCTION
ECT is very effective for many psychiatric disorders, such as severe depression, schizophrenia, and bipolar disorder. All ECTs are performed under general anaesthesia with neuromuscular blockade. The goals during general anaesthesia during ECT is to get an unconscious patient with muscle paralysis and amnesia.[1] ECT is associated with hyperdynamic response due to increased concentrations of catecholamines.[2] Anaesthetic agent for ECT includes ketamine, propofol, dexmedetomidine, thiopentone, methohexitone, etomidate, and sevoflurane.[3] The choice of anaesthetic agent may influence seizure quality and duration, and haemodynamic, and recovery parameters.[4]
Ketamine is a N-Methyl-D-aspartate (NMDA) receptor antagonist with seizure inducing properties and increases seizure duration.[5] Ketamine is also considered as neuroprotective during ECT preserving cognitive function by preventing excitotoxic neuronal damage (caused by glutamate action on NMDA receptors).[6] Ketamine has a rapid anti depressant effect represented by fewer sessions and better response.[4] Hallucinations and hyperdynamic response in the form of increased HR and blood pressure are common adverse effects of ketamine.[7]
Ketamine when used in combination with propofol decreases its consumption and preserves haemodynamic stability while propofol relieves hallucinations associated with ketamine. The mean recovery times from ketofol sedation is shorter than IV ketamine alone and longer than IV propofol alone. A better seizure quality was also reported with the ketamine-propofol combination compared with propofol alone.[8]
Dexmedetomidine is a potent α2-adrenergic agonist, used to attenuate the stress response, for haemodynamic stability and to reduce the dose of anaesthetic agent.[9] The hyperdynamic response to ECT reduced by intravenous administration of 1 μg/kg dexmedetomidine over 10 min before induction of anesthesia with preservation of seizure activity and recovery time.[2] Emergence agitation (excitement, restlessness, and panic) may occur in some patients after ECT. Dexmedetomidine is very effective in management of emergence agitation following ECT.[3,7] The aim of our work was to study the anaesthetic effect of combined ketofol-dex on haemodynamics, depression, seizure duration, recovery characteristics and agitation following ECT in patients with depression.
METHODS
Forty patients aged 18-60 years scheduled for ECT for treatment of depression between January and June 2013 in our hospital were enrolled for the study. The sample size was calculated using Epi info program version 6.02. Out of totally available 210 patients that formed our sample frame for randomization, only 53 were eligible for allocation as shown in the CONSORT flow diagram [Figure 1].[10] With 50% reported increase in duration of relief from depression in ketofol[11] and an expected 60% with ketofol-dex group, the sample size needed was estimated to be at least 35 for each group at 0.05 margin of (5% ∝significance level) at 80% power. The sample size was increased to 53 in both groups to overcome non-compliances and dropouts. Of the 53 patients who fulfilled the inclusion criteria, only 40 underwent analysis. The study population was randomly assigned to either ketofol-dex group (20 patients, each one received 7 sessions) or ketofol group (20 patients, each one received 7 sessions).
Figure 1.
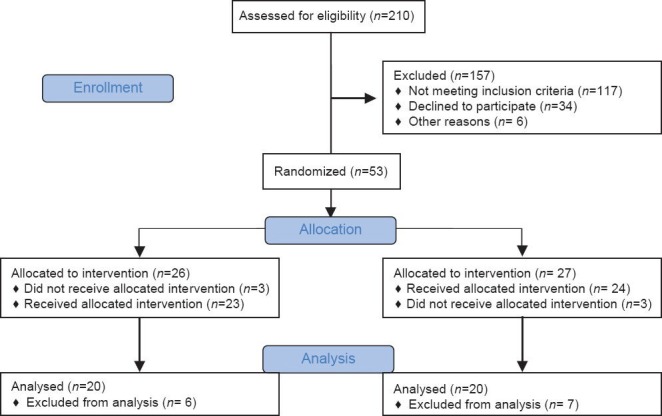
CONSORT 2010 flow diagram
Ethical committee approval and written informed consent were obtained in this prospective randomized double-blind study. The exclusion criteria included serious physical disease, such as cardiovascular disease, cerebrovascular disorder, intracranial hypertension, respiratory tract disease, or a previous fracture. Glaucoma, arterial aneurysm, or cerebrovascular malformation, presence of a pacemaker, history of seizures, ASA III –V physical status, history allergy to the study drugs, and pregnancy were also excluded. Chronic antidepressant medications were continued, fasting guidelines were followed, and patients were encouraged to empty their bladder before ECT.
The objective of anaesthesia was to provide rapid onset and offset of both unconsciousness and muscle relaxation for the duration of electrical stimulation and subsequent seizure. After premedication with 0.5 mg intravenous atropine sulfate, 0.5 μg/kg dexmedetomidine (diluted to 10 ml with 0.9% saline) for ketofol-dex group or 10 ml 0.9% saline for ketofol group was infused intravenously over 10 minutes before induction of anaesthesia by an anaesthesiologist not involved in the recording of data. The patient was pre-oxygenated with 100% oxygen and ketofol was prepared as a 1:1 mixture of 10 mg/ml ketamine and 10 mg/ml propofol mixed in a 20 ml syringe and was given slowly (20 mg/10 s) until the patient no longer responded to his/her name being called loudly and there was loss of the eyelash reflex. Additional ketofol was given in 10 mg increments if the responsiveness to verbal command had not been lost within 60 s after drug administration. The required total dose of ketofol was recorded.
Succinylcholine in a dose of 0.5 mg/kg was administered after induction of anesthesia with ketofol and manual ventilation was performed with face mask using 100% oxygen at flow rate of 8L/min. A bite block was used to protect the patient's teeth, lips, and tongue. A suprathreshold electrical stimulus was given via bifrontotemporal electrodes and ventilation was assisted with oxygen during the procedure. Mean arterial pressure (MAP), heart rate (HR), and oxygen saturation were recorded at baseline, 5, 10, 20, 30, 40, and 50 minutes after the end of the seizure. The duration of the motor seizure was defined as the time from the beginning of ECT to cessation of tonic–clonic motor activity in the ‘isolated’ arm. The time from the end of succinylcholine administration until spontaneous breathing, eye opening, and obeying commands were recorded.
Probable side effects including nausea, vomiting, bradycardia, tachycardia, hypotension/hypertension, respiratory depression, and hypoxemia were recorded after the electrical stimulus until the patient was discharged from the post anesthetic care unit (PACU) to the psychiatry department. In the PACU, standard monitoring was applied during recovery and oxygen supplementation continued until oxygen saturation was adequate on room air. Respiratory depression was defined as respiratory rate less than 10 breaths/min, hypoxaemia was defined as oxygen saturation (SpO2) of 90% or less, bradycardia was defined as HR less than 50 beats/min, tachycardia was defined as HR more than 100 beats/min, hypotension was defined as MAP less than 60 mmHg, and hypertension was defined as MAP more than 120 mmHg.
Agitation score[12] and patient satisfaction[13] were evaluated when the patients were completely awake after ECT. The agitation was evaluated using an emergence agitation score in which 1 = sleeping, 2 = awake and calm, 3 = irritable and crying, 4 = inconsolable crying, 5 = severe restlessness and disorientation. Patient satisfaction was assessed using a satisfaction scale, 1 for pleased and calm patient, 2 for patient without any complaint (satisfaction is not bad), 3 the patient has some complaints (middling quality of satisfaction), and 4 patient complained that the treatment was unpleasant (he/she does not want to undergo the same technique any more). The patients were assessed for depression before and after every ECT session by a psychiatrist unaware of the anesthetic study groups using the Hamilton Depression Rating Scale (HDRS) scores (1 day before ECT as a baseline and days 1, 2, 3, 4 and 5 after ECT treatment).[5]
Data analysis was carried out using the SPSS package (Version 13, Chicago, Illinois, USA). Quantitative variables were tested for normality distribution by the Kolmogorov–Smirnov test. Quantitative variables were presented as mean ± SD and the differences were assessed using an independent sample t-test. Qualitative variables were presented as numbers and percentages and Chi-square test or Fisher's Exact Test was used for comparison. The alternative hypothesis was assumed and value of P ≤ 0.05 was considered to be statistically significant.
RESULTS
The mean age, weight and gender were comparable between the two groups. Spontaneous breathing time (261.6 ± 42.5 s versus 255.7 ± 39.9 s), eye-opening time (459.4 ± 67.7 s versus 447.9 ± 73.5 s), and time to respond to oral command (543.9 ± 39.6 s versus 536.9 ± 61.8 s) were not significantly higher in ketofol group compared to ketofol-dex group respectively. Motor seizure duration in ketofol group was significantly lower compared to ketofol-dex group (35.8 ± 6.6 s versus 38.9 ± 4.9 s). Total (mean) ketofol used was significantly less in ketofol-dex group compared to ketofol group (78.5 ± 10.8mg versus 90 ± 13.2mg). Patients’ satisfaction score was significantly higher in ketofol-dex group (1.75 ± 0.44) compared to ketofol group (1.53 ± 0.61). The number of patients with agitation score > 2 was significantly lower in ketofol-dex group (1.4%) compared to ketofol group (8.6%) [Table 1].
Table 1.
Demographic data, seizure duration, recovery characteristics, patient's satisfaction and agitation score (mean±SD, number (%))
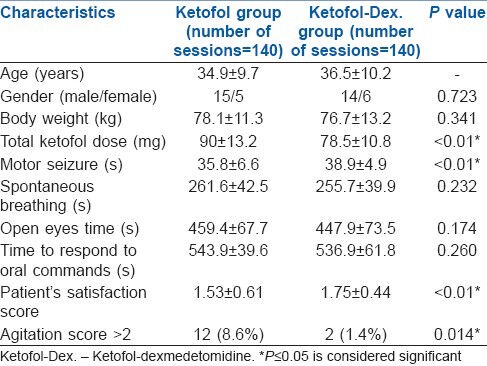
There was a significant decrease (P < 0.01) in MAP (mmHg) in ketofol-dex group compared to ketofol group at (71.9 ± 5.1 versus 75.8 ± 6.3), 20 min (72.0 ± 5.3 versus 75.9 ± 4.4), 30 min (70.2 ± 5.5 versus 74.6 ± 6.9), and 40 min (71.8 ± 6.0 versus 76.3 ± 4.6) respectively [Figure 2].
Figure 2.
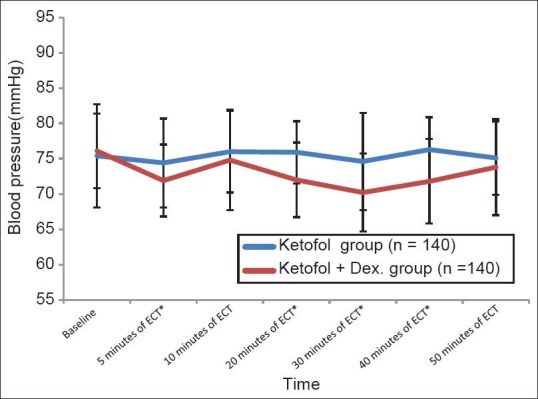
Blood pressure during the study period (Mean±SD). *significant : P≤0.01
There was a significant decrease in heart rate (beats per min) (P < 0.01) in ketofol-dex group compared to ketofol group at 5 (72.8 ± 6.4 versus 78.3 ± 6.8), 10 (71.9 ± 7.1 versus 77.1 ± 5.0), 20 (72.8 ± 4.7 versus 76.7 ± 6.5), 30 (72.5 ± 5.1versus 75.8 ± 5.8), and 40 (70.9 ± 5.7 versus 76.5 ± 6.6) minutes respectively [Figure 3].
Figure 3.
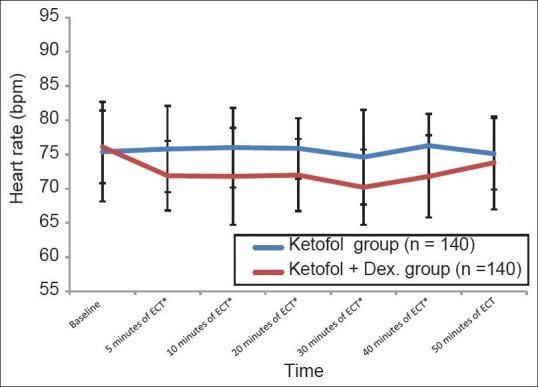
Heart rate during the study period (Mean±SD) *significant P≤0.01
There was no difference in oxygen saturation among the groups and none of the patients complained of awareness during anaesthesia. Two patients in ketofol group and one patient in ketofol-dex group developed coughing. Headache occurred in one patient in ketofol group. No patient experienced respiratory depression, hypoxaemia, bradycardia, hypotension, or hypertension.
Regarding Hamilton Depression Rating Scale (HDRS) scores, there was a significant decrease (P ≤ 0.01) in the two groups from 1 to 5 days after ECT treatment when compared to pre-ECT. However there was a significant difference between the two groups only after the first day of the first ECT [Figure 4].
Figure 4.
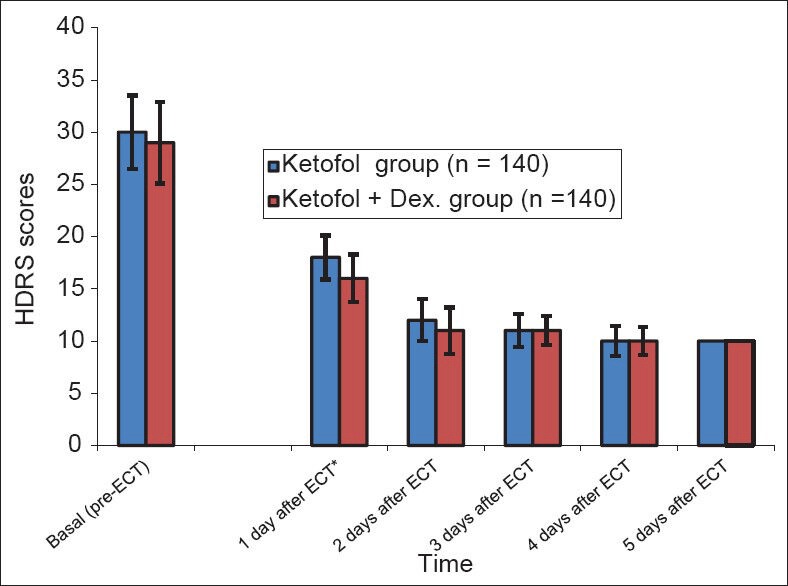
Hamilton depression rating scale scores during the study period (Mean±SD). *significant: P≤0.01
DISCUSSION
Ketamine seems to be an appropriate anaesthetic for ECT due to preservation of cognitive function, antidepressant effect,[14] seizure induction and improvements of response in resistant depression to ECT clinical non-response.[15] Okamato et al., showed that ketamine is useful when the early antidepressant effect is needed in severe cases. Possible mechanism of the antidepressant effect is suppression of excitotoxicity and neuroprotective action due to NMDA antagonism. Cardiotoxicity, transient psychotic episodes, and delayed recovery, are the main drawbacks of ketamine.[5] Ketamine causes systemic release of catecholamines and inhibition of norepinephrine re-uptake at peripheral nerves and myocardium.[15]
Propofol is considered as one of the most popular anaesthetic agents in ECT due to rapid recovery, minor haemodynamic response and antiemetic effect.[2]
Ketofol might be advantageous for haemodynamic stability and analgesia. The total amount of ketamine required for ECT is reduced when ketofol is used compared to ketamine alone, and also reduces the recovery time without any significant side effects. Additionally, it is assumed that the sedative and antiemetic effects of propofol may counterbalance the nausea and psychomimetic effects related to ketamine.[16]
Dexmedetomidine has been reported to be effective in ECT without serious side effects.[17] Dexmedetomidine is α2-adrenergic agonist and has anaesthetic, sedative, analgesic effects, and can reduce shivering and emergence delirium.[18] Rapid administration of dexmedetomidine can lead to tachycardia, bradycardia, and hypertension because of the sudden exogenous release of catecholamine. The minimum time needed to administer dexmedetomidine is 10 minutes.[17]
Premedication with low-dose dexmedetomidine reduces the total propofol requirement which in turn increases seizure duration, ensures rapid recovery without altering patient satisfaction and causes attenuation of agitation.[13,18] In our study, number of patients with agitation score more than 2 was significantly less in ketofol-dex group (1.4%) compared to ketofol group (8.6%). The combination of dexmedetomidine and ketamine is associated with stable cardiovascular and respiratory functions, good sedation, analgesia, decreased salivation and reduced incidence of emergence agitation.[19] Dexmedetomidine use was associated with decreases in the total dose of ketofol (78.5 ± 10.8 mg) in ketofol-dex group when compared to ketofol group (90 ± 13.2 mg). Also there was significant decrease of MAP (at 20, 30, and 40 minutes) and HR (at 10, 20, 30, and 40 minutes) in ketofol-dex group when compared to ketofol group, in a clinically acceptable range.
This is the first time ketofol-dex combination has been used during ECT for anesthesia. Decreased requirement of propofol was reflected by increased seizure duration. The major disadvantages of propofol in ECT is increased seizure threshold and decreased duration of seizure.[19,20] Ketofol-dex combination has advantages in the form of increased seizure duration, cardiovascular and respiratory stability, antiemetic effect, prevention of excessive salivation, rapid recovery, control of agitation, effective antidepressant effect, good analgesia, sedation and patient satisfaction. In this current study, patient satisfaction score was significantly higher in ketofol-dex group compared to ketofol group (1.75 ± 0.44 versus1.53 ± 0.61).
CONCLUSION
Ketofol-dexmedetomidine combination for ECT is associated with a longer mean seizure time, effective antidepressive effect following 1st session, lower incidence of agitation, more patient satisfaction, and acceptable decrease in heart rate and blood pressure when compared to ketofol and without any significant side effects.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Ding Z, White PF. Anesthesia for electroconvulsive therapy. Anesth Analg. 2002;94:1351–64. doi: 10.1097/00000539-200205000-00057. [DOI] [PubMed] [Google Scholar]
- 2.Mizrak A, Koruk S, Ganidagli S, Bulut M, Oner U. Premedication with dexmedetomidine and midazolam attenuates agitation after electroconvulsive therapy. J Anesth. 2009;23:6–10. doi: 10.1007/s00540-008-0695-2. [DOI] [PubMed] [Google Scholar]
- 3.Uppal V, Dourish J, Macfarlane A. Anaesthesia for electroconvulsive therapy. CEACCP. 2010;10:192–196. [Google Scholar]
- 4.Fink M. Post-ECT delirium. ConvulsTher. 1993;9:326–330. [PubMed] [Google Scholar]
- 5.Okamoto N, Nakai T, Sakamoto K, Nagafusa Y, Higuchi T, Nishikawa T. Rapid antidepressant effect of ketamine anesthesia during electroconvulsive therapy of treatment-resistant depression: Comparing ketamine and propofol anesthesia. J ECT. 2010;26:223–7. doi: 10.1097/YCT.0b013e3181c3b0aa. [DOI] [PubMed] [Google Scholar]
- 6.Waxman EA, Lynch DR. N-methyl-D-aspartate receptor subtypes: Multiple roles in excitotoxicity and neurological disease. Neuroscientist. 2005;11:37–49. doi: 10.1177/1073858404269012. [DOI] [PubMed] [Google Scholar]
- 7.Strayer RJ, Nelson LS. Adverse events associated with Ketamine for procedural sedation in adults. Am J Emerg Med. 2008;26:985–1028. doi: 10.1016/j.ajem.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Chen Y, Zhou X, Liu F, Zhang T, Zhang C. Effects of propofol and ketamine as combined anesthesia for electroconvulsive therapy in patients with depressive disorder. J ECT. 2012;28:128–132. doi: 10.1097/YCT.0b013e31824d1d02. [DOI] [PubMed] [Google Scholar]
- 9.Begec Z, Toprak HI, Demirbilek S, Erdil F, Onal D, Ersoy MO. Dexmedetomidine blunts acute hyperdynamic responses to electroconvulsive therapy without altering seizure duration. Acta Anaesthesiol Scand. 2008;52:302–306. doi: 10.1111/j.1399-6576.2007.01462.x. [DOI] [PubMed] [Google Scholar]
- 10.Moher D, Hopewell S, Schulz KF, Montori V, Gøtzsche PC, Devereaux PJ, et al. CONSORT 2010 Explanation and Elaboration: Updated guidelines for reporting parallel group randomised trials. J ClinEpidemiol. 2010;63:1–37. [Google Scholar]
- 11.Suresh KP, Chandrashekara S. Sample size estimation and power analysis for clinical research studies. J Hum Reprod Sci. 2012;5:7–13. doi: 10.4103/0974-1208.97779. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Mizrak A, Koruk S, Ganidagli S, Bulut M, Oner U. Premedication with dexmedetomidine and midazolam attenuates agitation after electroconvulsive therapy. J Anesth. 2009;23:6–10. doi: 10.1007/s00540-008-0695-2. [DOI] [PubMed] [Google Scholar]
- 13.Tobias JD. Dexmedetomidine: Applications in pediatric critical care and pediatric anesthesiology. Pediatr Crit Care Med. 2007;8:115–31. doi: 10.1097/01.PCC.0000257100.31779.41. [DOI] [PubMed] [Google Scholar]
- 14.Kranaster L, Kammerer-Ciernioch J, Hoyer C, Sartorius A. Clinically favourable effects of ketamine as an anaesthetic for electroconvulsive therapy: A retrospective study. Eur Arch Psychiatry Clin Neurosci. 2011;261:575–82. doi: 10.1007/s00406-011-0205-7. [DOI] [PubMed] [Google Scholar]
- 15.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 16.Levänen J, Mäkelä ML, Scheinin H. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology. 1995;82:1117–25. doi: 10.1097/00000542-199505000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Timm C, Linstedt U, Weiss T, Zenz M, Maier C. Sympathomimetic effects of low-dose S(?)-ketamine. effect of propofol dosage. Anaesthesist. 2008;57:338–346. doi: 10.1007/s00101-008-1331-0. [DOI] [PubMed] [Google Scholar]
- 18.Grant SA, Breslin DS, MacLeod DB, Gleason D, Martin G. Dexmedetomidine infusion for sedation during fiberoptic intubation: A report of three cases. J Clin Anesth. 2004;16:124–6. doi: 10.1016/j.jclinane.2003.05.010. [DOI] [PubMed] [Google Scholar]
- 19.Geretsegger C, Rochowanski E, Kartnig C, Unterrainer AF. Propofol and methohexital as anesthetic agents for electroconvulsive therapy (ECT): A comparison of seizure-quality measures and vital signs. J ECT. 1998;14:28–35. [PubMed] [Google Scholar]
- 20.Fu W, White PF. Dexmedetomidine failed to block the acute hyperdynamic response to electroconvulsive therapy. Anesthesiology. 1999;90:422–4. doi: 10.1097/00000542-199902000-00015. [DOI] [PubMed] [Google Scholar]


