Abstract
Background and Aim:
Central venous catheter (CVC) insertion induces pain and discomfort to a conscious patient despite application of a local anaesthetic (LA) field block and this pain can be greatly lessened by using additional analgesics. The aim of this study was to evaluate the efficacy of dexmedetomidine along with LA field infiltration in controlling pain and discomfort associated with CVC insertion.
Methods:
A prospective, randomised, double-blind, placebo-controlled trial of 54 patients scheduled for planned CVC insertion was undertaken. Patients were randomly assigned into two groups of 27 each, to receive either dexmedetomidine (1 μg/kg) or 0.9% normal saline, along with LA field infiltration. Pain and discomfort score was measured at 5 time points.
Results:
The median pain score was worst for placebo group at local anaesthetic injection (6 [4-7]) and at the end of procedure (5 [4-5]), which was significantly attenuated in the dexmedetomidine group (4 [4-5] and 4 [3-5]; P = 0.007 and 0.040 respectively). The lower procedure related discomfort score in the immediate post-procedural period was statistically significant in dexmedetomidine group compared to placebo (4 [4-5] vs. 5 [4-6]; P = 0.008).
Conclusions:
Pre-procedural bolus dexmedetomidine infusion provides adequate analgesia and patient comfort for CVC insertion along LA field block. However, the tendency for excessive sedation and bradycardia associated with dexmedetomidine render it less desirable for this purpose.
Keywords: Analgesia, central venous catheter, dexmedetomidine, procedural pain
INTRODUCTION
Central venous catheter (CVC) insertion is a frequently performed clinical procedure associated with pain, anxiety and discomfort.[1] Clinical guidelines recommend infiltration of subcutaneous tissues at the insertion site with local anaesthetics (LA) such as 1% or 2% lignocaine. However, injection of LA itself induces considerable degree of pain on field infiltration.[2] Moreover, our experience suggests that in a conscious patient, even after the establishment of an effective field block, other subsequent procedural steps such as positioning for CVC insertion and anchoring the catheter to skin with suture may be associated with pain and discomfort. Ensuring adequate analgesia and sedation is therefore essential for patient comfort and satisfaction.
Many different types of analgesics can be added to prevent and/or control procedural pain. Yet, only very few investigators have examined the effect of additional analgesic usage, along with LA infiltration on pain associated with the procedure. Combination analgesic therapy for procedural pain has many potential advantages. Opioid antinociception can be initiated by activation of central opioid receptors; sedatives can augment opioid antinociception by providing anxiolysis, skeletal muscle relaxation, and amnesia,[3] while LA agents block pain transmission by interfering with nerve cell depolarization of peripheral pain fibres.
The use of short acting potent opioids such as fentanyl, along with a benzodiazepine sedative, administered intravenously as a bolus could alleviate such pain in a simple and effective manner, but their use would warrant additional nursing and medical care in the Intensive Care Units (ICU).
Dexmedetomidine, a highly selective α-2 adrenoreceptor agonist has sedative, sympatholytic, analgesic and anxiolytic properties without producing significant respiratory depression.[4,5,6,7] Intravenous (IV) dexmedetomidine is effective as a primary sedative in adult patients undergoing a variety of diagnostic or surgical procedures requiring monitored anaesthesia care.[8,9]
The purpose of this prospective randomised double-blind clinical trial was to evaluate objectively whether a single dose of dexmedetomidine before the procedure was efficient in prevention of pain and discomfort during CVC insertion.
METHODS
After obtaining approval from the Hospital Ethics Committee, 54 consecutive consenting adult patients requiring central venous access with planned placement in the internal jugular vein (IJV) as a part of normal care were enrolled in this study. Patients were included in the study if they were awake, alert, and oriented and their medical condition was stable enough to allow them to understand and use verbal numeric rating pain scale (VNRPS), systemic analgesics had not been administered for at least 4 h before the procedure, and were 18–65 years. Patients were excluded from the study if they had a known adverse reaction to the study drugs and not willing to participate in the study.
10 min before the procedure, patients were allocated randomly to one of the two groups using a computer-generated random number table. An anaesthetist, who was not one of the observers, prepared syringes containing either dexmedetomidine 1 μg/kg, or 0.9% saline, all made to a total volume of 10 mL. All the solutions were labelled ‘study drug’ and coded to maintain the double-blind nature of the study. The study drugs were infused to the patients as per their group allocation over 10 min using a syringe infusion pump.
The procedure commenced at the end of infusion of the study drugs. Each patient was inserted a 7 Fr triple-lumen catheters via a nontunnelled approach in the right IJV using anterior approach.[10] All patients received subcutaneous infiltration of 5 mL of 2% lignocaine after confirming the anatomical landmark of the target jugular vein. The physician then injected 3 mL of the LA solution through a 25 gauge needle directly superficial to the IJV at the designated puncture site. This injection was deliberate and not rushed, lasting around 15 s. The needle was then repositioned to inject additional LA, 1 mL just to the left and 1 mL just to the right of the vein for anchoring stitches.
Scores for discomfort, pain, sedation, cardiovascular and respiratory events and peripheral oxygen saturation were recorded at rest by an anaesthesia resident at 5 time points: Time 1, at base line before infusion of study drugs (T1); Time 2, after initial local anaesthetic injection (LAI) (T2); Time 3, immediately after the procedure and the patient was asked to report the peak pain experienced during the procedure (T3); Time 4, 10 min after completion of the procedure (T10) and Time 5, 60 min after completion of the procedure (T60).
Discomfort was assessed using an 11-point verbal numeric rating discomfort scale (VNRDS) from 0 to 10 (0: None, 10: Extreme discomfort); pain was assessed by a VNRPS from 0 to 10 (0: No pain, 10: The worst pain imaginable.[11] Both the scales were explained to each patient by the investigator, while counselling the patient regarding the need for central venous access, before the start of infusion of study drugs. Sedation was assessed on a 6-point modified Observer's assessment of alertness/sedation (OAA/S) scale with 0: No response and 6: Agitated.[12] If OAA/S score was 0 or 1 (patient un-arousable), VNRPS and VNRDS was counted as 0 (no pain and no discomfort).
Respiratory events were defined as oxygen saturation by pulse oximetry (SpO2) <92% and/or respiratory rate (RR) <8 breaths/min. A decrease in SpO2 to <92% for >30 s was treated sequentially with verbal stimulation, head tilt -chin lift, Guedel airway, and bag-mask-assisted ventilation. A RR <8 breaths/min was treated sequentially with verbal stimulation, mild prodding, and nasopharyngeal stimulation. Cardiovascular events were defined as a single episode of variation in heart rate (HR) and systolic blood pressure (SBP) by >20% from patient baseline. Persistent (two reading 3 min apart) or recurrent SBP <90 mmHg, was treated with boluses of IV ephedrine 6 mg repeated and/or persistent (>30 s) or recurrent HR <50 bpm was treated with IV atropine 0.6 mg and repeated as necessary.
The primary outcomes of this study were assessment of pain and discomfort at five predefined time points. The secondary outcomes were sedation score and occurrence of predefined adverse cardiovascular and respiratory events.
Previous research suggests that a difference of 2.0 points on an 11-point VNRPS indicates a clinically important effect.[13] We calculated our sample size based on previously reported pain scores of 3.6 (standard deviation [SD] 2.6) on a 11-point VNRPS during CVC insertion using remifentanil infusion.[14] A reduction in pain scores by 2.1 on VNRPS was considered acceptable. A total of 25 patients were required in each group, for an alpha-error of 0.05 and beta-error of 0.20. We randomised and recruited 27 patients in each group to allow for withdrawals.
Statistical analyses were performed using the statistical package for the social sciences (SPSS) version 10.0 (SPSS Inc. (1999). SPSS 10.0 for Windows. SPSS Inc., Chicago IL). Data are expressed as means (SD), or numbers (n), as appropriate. Approximate normality of the distribution was assessed using Shapiro–Wilk test before applying a particular statistical test. Unpaired Student's t-test (two-tailed) for equal variance was employed for comparison of demography, baseline hemodynamic and respiratory data; Chi-square and Fisher's exact tests were employed to compare gender distribution and differences between predefined adverse effects in the groups. Pain, discomfort and sedation score were analysed using the Mann–Whitney test. Statistical significance was accepted at P < 0.05.
RESULTS
Fifty-four patients were recruited, with all but two completing the study. 2 patients from placebo group were withdrawn due to failure in identifying a patent jugular vein and the physician opted to cannulate the subclavian vein. Finally, data from 27 patients from dexmedetomidine group and 25 patients from placebo group were tabulated for analysis.
There were no significant differences between the groups of patients in terms of patient demographics, baseline respiratory, cardiovascular parameters, level of consciousness and indications for CVC insertion [Table 1].
Table 1.
Demographic data, base line variables and indications for CVC in patients receiving dexmedetomidine or placebo
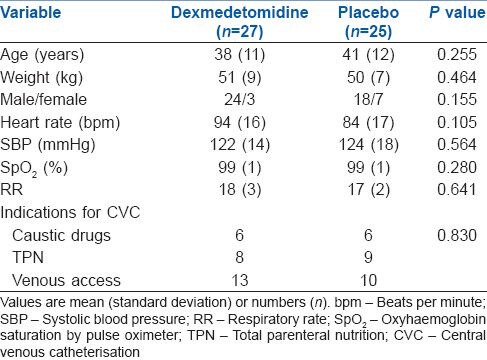
The median pain score, discomfort score and sedation score in dexmedetomidine and placebo group are shown in Table 2. Comparison between groups revealed that placebo group had worst pain scores at LAI, which was significantly attenuated by addition of study drugs in dexmedetomidine group (Dexmedetomidine, 4 [4-5] vs. Placebo, 6 [4-7]; P = 0.007). When compared with placebo group, dexmedetomidine appeared to be more analgesic-efficient in reducing the pain intensity to CVC insertion for all procedural steps including 60 min after the completion of the procedure. The procedure related discomfort was significantly lower in dexmedetomidine group compared with placebo group at each step of the procedure (T2 to T60) after LAI (T2, dexmedetomidine, 2 [1-2] vs. placebo, 2 [2-3]; P = 0.025, T3, dexmedetomidine, 4 [4-5] vs. placebo, 5 [4-6]; P = 0.008, T10, dexmedetomidine, 4 [3-5] vs. placebo, 5 [4.5-5]; P = 0.016 and T60 dexmedetomidine, 2 [1-2] vs. placebo, 3 [2–3]; P = 0.027).
Table 2.
Comparison of pain scores, discomfort score and sedation score in patients receiving dexmedetomidine or placebo
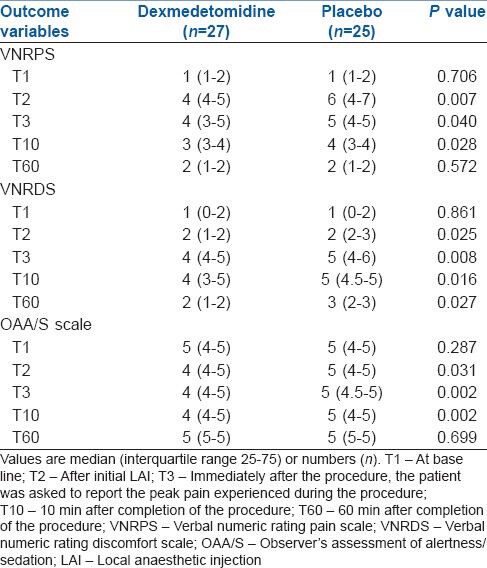
A significantly more number of patients from dexmedetomidine group had a fall in HR by >20% from base line and bradycardia during the study period. HR decreased by >20% from baseline in seven and 1 patient for dexmedetomidine and placebo group respectively (P = 0.029). SBP decreased by >20% of baseline in more number of patients in dexmedetomidine group (3/27) in contrast to placebo group (0/25) (P = 0.086). 2 patients from dexmedetomidine group (2/27) experienced episodes of SpO2 <92%, compared with placebo group (0/25) and required a sequential verbal stimulation to maintain oxygen saturation above 98%. However, the difference did not reach statistical significance (P = 0.165) [Table 3]. Of 7 patients from dexmedetomidine group who had HR below 20% of the base line, 4 patients experienced bradycardia requiring treatment with atropine (0.6 mg bolus IV). All 3 patients from dexmedetomidine group who had >20% fall in SBP below base line also required a single dose IV ephedrine (6 mg) because of persistent low SBP below 90 mm Hg.
Table 3.
Comparison of adverse effects in patients receiving dexmedetomidine or placebo
The median sedation score for dexmedetomidine group was significantly less compared to placebo group at the insertion of LAI (T2) and immediate post procedure period (T3 and T10) (dexmedetomidine: T2, 4 [4-5]; T3, 4 [4-5]; T10, 4 [4-5] vs. placebo: T2, 5 [4-5]; T3, 5 [4.5-5]; T10, 5 [4-5]; P = 0.031, 0.002 and 0.002 respectively) but for the rest of the procedural steps we did not find any significant difference between the groups. At the end of study period (T 60), all the patients were responding to verbally spoken words (OAA/S score 4/5). No patient from any group manifested uncontrolled movement strong enough to hinder performance of the procedure.
DISCUSSION
Pain during CVC insertion is of moderate intensity and short duration but is a definite source also of discomfort and anxiety to the conscious patient and the pain component is often reduced with the use of LA.
For our study, we have defined pain as an unpleasant sensory and emotional experience that arises from actual or potential tissue damage associated with CVC insertion. The sensory and emotional components of pain were assessed with VNRPS and VNRDS, respectively.
The primary findings of the study are field infiltration with LA for CVC insertion induces considerable degree of pain in conscious patients and dexmedetomidine can attenuate this pain response significantly if given before such invasive procedure. In addition to this, dexmedetomidine resulted in a better discomfort score compared with the control for patients in the post-procedural period.
Literature search revealed two studies,[1,15] describing pain and discomfort as two separate perceptions experienced by patients during CVC insertion. Morrison et al.[1] in their 5-point numeric rating scale described CVC insertion as a moderately painful and severely uncomfortable procedure.
The reason for choosing the study drug is the additional hypnotic, sedative, and anxiolytic properties of dexmedetomidine with minimal respiratory depression.[4,16]
Although generally effective for sedation during noninvasive procedures, dexmedetomidine as the sole agent has not been uniformly successful for invasive procedures. Jalowiecki et al.,[17] showed that, sole use of dexmedetomidine infusion (1 μg/kg followed by 0.2 μg/kg/h) did not provide satisfactory analgesia for outpatient colonoscopy. Supplemental fentanyl was required in 47% of patients receiving dexmedetomidine for procedural analgesia. Puntillo et al.,[15,17] demonstrated a big positive surge in mean pain intensity score at the time of CVC insertion over the base line pre-procedural pain. In their analysis, they found that, only 20% of patients received opioids either alone or in combination with sedation before or during a CVC insertion depending on the pain intensity at the beginning of the procedure. However, they suggested use of preemptive analgesia to avoid central sensitisation, which can lead to persistent pain that continues for some time after a noxious event. In our study, we provided base line analgesia with pre-procedural infusion of dexmedetomidine and achieved the targeted decrease in pain score that was prospectively agreed on at the time of study power calculation.
In our study, dexmedetomidine was able to reduce procedure specific pain and resulted in better comfort score for most of the time points during insertion of CVC. This action of dexmedetomidine can be partly explained by multidimensional model of procedural pain.[15] Dexmedetomidine, not only acts as an analgesic by modulating the sensory-discriminative component of the pain, but also has a greater magnitude of effect in attenuating the motivational-affective and cognitive component of pain.
Although effective for analgesia and reduced discomfort, our study showed that dexmedetomidine was associated with increased sedation scores. 2 patients from dexmedetomidine group needed to be called repeatedly with mild prodding (OAA/S score-2) to ascertain level of sedation after initial LA injection (T2), which some time may hinder the patient cooperation needed by the physician, while inserting CVC.
The most significant adverse reactions associated with dexmedetomidine are hypotension and bradycardia, resulting from its sympatholytic activity. The incidence of postoperative bradycardia has been reported to be as high as 40% in healthy surgical patients who received dexmedetomidine, especially high doses.[18] Venn and Grounds,[19] compared dexmedetomidine with propofol in 20 adults expected to require artificial ventilation. Patients sedated with dexmedetomidine required 3 times less analgesia than did those receiving propofol. They also found no differences in arterial pressures between groups, but HR was significantly lower in the dexmedetomidine group. We had similar finding in our study.
Two patients from dexmedetomidine group had SpO2 <92% during procedure and required a sequential verbal stimulation to maintain oxygen saturation above 98%. This finding confers the advantage of dexmedetomidine in providing adequate sedation with clinically insignificant respiratory effects, including apnoea, hypoxemia, or airway obstruction during ICU procedures.
The major limitation of the study is the non-uniformity in the indication for CVC insertion in the study sample selected. For example, patients who required a chemotherapeutic agent (caustic drugs) or parenteral nutrition were frequent visitors to the hospital for their illness and well acquainted with different painful hospital procedures in the past. These patients might have had an entirely different perception of pain and discomfort during their procedures than the eligible patients who came to the hospital for the 1st time. Nevertheless, the numbers of patients requiring CVC insertion for different indications were equally distributed among groups.
CONCLUSIONS
Dexmedetomidine provides adequate analgesia and reduces the level of discomfort for the insertion of CVC along with field infiltration with LA. However the tendency for excessive sedation, unwanted cardiovascular events associated with dexmedetomidine render it less desirable for this purpose.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Morrison RS, Ahronheim JC, Morrison GR, Darling E, Baskin SA, Morris J, et al. Pain and discomfort associated with common hospital procedures and experiences. J Pain Symptom Manage. 1998;15:91–101. [PubMed] [Google Scholar]
- 2.Morris RW, Whish DK. A controlled trial of pain on skin infiltration with local anaesthetics. Anaesth Intensive Care. 1984;12:113–4. doi: 10.1177/0310057X8401200204. [DOI] [PubMed] [Google Scholar]
- 3.Puntillo K, Casella V, Reid M. Opioid and benzodiazepine tolerance and dependence: Application of theory to critical care practice. Heart Lung. 1997;26:317–24. doi: 10.1016/s0147-9563(97)90089-3. [DOI] [PubMed] [Google Scholar]
- 4.Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705. doi: 10.1097/00000539-200003000-00035. [DOI] [PubMed] [Google Scholar]
- 5.Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8. doi: 10.1213/01.ANE.0000093225.39866.75. [DOI] [PubMed] [Google Scholar]
- 6.Unlugenc H, Gunduz M, Guler T, Yagmur O, Isik G. The effect of pre-anaesthetic administration of intravenous dexmedetomidine on postoperative pain in patients receiving patient-controlled morphine. Eur J Anaesthesiol. 2005;22:386–91. doi: 10.1017/s0265021505000669. [DOI] [PubMed] [Google Scholar]
- 7.Talke P, Richardson CA, Scheinin M, Fisher DM. Postoperative pharmacokinetics and sympatholytic effects of dexmedetomidine. Anesth Analg. 1997;85:1136–42. doi: 10.1097/00000539-199711000-00033. [DOI] [PubMed] [Google Scholar]
- 8.Bergese SD, Candiotti KA, Bokesch PM, Zura A, Wisemandle W, Bekker AY, et al. A Phase IIIb, randomized, double-blind, placebo-controlled, multicenter study evaluating the safety and efficacy of dexmedetomidine for sedation during awake fiberoptic intubation. Am J Ther. 2010;17:586–95. doi: 10.1097/MJT.0b013e3181d69072. [DOI] [PubMed] [Google Scholar]
- 9.Candiotti KA, Bergese SD, Bokesch PM, Feldman MA, Wisemandle W, Bekker AY, et al. Monitored anesthesia care with dexmedetomidine: A prospective, randomized, double-blind, multicenter trial. Anesth Analg. 2010;110:47–56. doi: 10.1213/ane.0b013e3181ae0856. [DOI] [PubMed] [Google Scholar]
- 10.Chudhari LS, Karmarkar US, Dixit RT, Sonia K. Comparison of two different approaches for internal jugular vein cannulation in surgical patients. J Postgrad Med. 1998;44:57–62. [PubMed] [Google Scholar]
- 11.Katz J, Melzack R. Measurement of pain. Surg Clin North Am. 1999;79:231–52. doi: 10.1016/s0039-6109(05)70381-9. [DOI] [PubMed] [Google Scholar]
- 12.Chernik DA, Gillings D, Laine H, Hendler J, Silver JM, Davidson AB, et al. Validity and reliability of the Observer's Assessment of Alertness/Sedation Scale: Study with intravenous midazolam. J Clin Psychopharmacol. 1990;10:244–51. [PubMed] [Google Scholar]
- 13.Todd KH, Funk JP. The minimum clinically important difference in physician-assigned visual analog pain scores. Acad Emerg Med. 1996;3:142–6. doi: 10.1111/j.1553-2712.1996.tb03402.x. [DOI] [PubMed] [Google Scholar]
- 14.Burlacu CL, McKeating K, McShane AJ. Remifentanil for the insertion and removal of long-term central venous access during monitored anesthesia care. J Clin Anesth. 2011;23:286–91. doi: 10.1016/j.jclinane.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 15.Puntillo KA, White C, Morris AB, Perdue ST, Stanik-Hutt J, Thompson CL, et al. Patients’ perceptions and responses to procedural pain: Results from Thunder Project II. Am J Crit Care. 2001;10:238–51. [PubMed] [Google Scholar]
- 16.Techanivate A, Dusitkasem S, Anuwattanavit C. Dexmedetomidine compare with fentanyl for postoperative analgesia in outpatient gynecologic laparoscopy: A randomized controlled trial. J Med Assoc Thai. 2012;95:383–90. [PubMed] [Google Scholar]
- 17.Jalowiecki P, Rudner R, Gonciarz M, Kawecki P, Petelenz M, Dziurdzik P. Sole use of dexmedetomidine has limited utility for conscious sedation during outpatient colonoscopy. Anesthesiology. 2005;103:269–73. doi: 10.1097/00000542-200508000-00009. [DOI] [PubMed] [Google Scholar]
- 18.Aho M, Erkola O, Kallio A, Scheinin H, Korttila K. Comparison of dexmedetomidine and midazolam sedation and antagonism of dexmedetomidine with atipamezole. J Clin Anesth. 1993;5:194–203. doi: 10.1016/0952-8180(93)90014-6. [DOI] [PubMed] [Google Scholar]
- 19.Venn RM, Grounds RM. Comparison between dexmedetomidine and propofol for sedation in the intensive care unit: Patient and clinician perceptions. Br J Anaesth. 2001;87:684–90. doi: 10.1093/bja/87.5.684. [DOI] [PubMed] [Google Scholar]


