Abstract
Background and Aims:
Mixing adjuvants with hyperbaric bupivacaine in a single syringe before injecting the drugs intrathecally is an age old practice. In doing so, the density of the hyperbaric solution and also of the adjuvant drugs may be altered, thus affecting the spread of drugs. Administering local anaesthetic and the adjuvants separately may minimise the effect of the changes in densities. We aimed to compare block characteristics, intraoperative haemodynamics and post-operative pain relief in parturients undergoing caesarean section (CS) after administering hyperbaric bupivacaine and clonidine intrathecally as a mixture and sequentially.
Methods:
In this single-blind prospective randomised controlled study at a tertiary care centre from 2010 to 12, 60 full-term parturients scheduled for elective CSs were divided into two groups on the basis of technique of intrathecal drug administration. Group M received mixture of clonidine (75 mcg) and hyperbaric bupivacaine 0.5% (10 mg) intrathecally, whereas Group B received clonidine (75 mcg) followed by hyperbaric bupivacaine 0.5% (10 mg) through separate syringes. Observational descriptive statistics, analysis of variance test, Wilcoxon test and Chi-square test were used as applicable.
Results:
Duration of analgesia was significantly longer in Group B (474.33 ± 20.79 min) in which the drug was given sequentially than in Group M (337 ± 18.22 min). Furthermore, the time to achieve highest sensory block and complete motor block was significantly less in Group B without any major haemodynamic instability and neonatal outcome.
Conclusions:
When clonidine and hyperbaric bupivacaine were administered in a sequential manner, block characteristics improved significantly compared to the administration of the mixture of the two drugs.
Keywords: Adjuvants, caesarean section, clonidine, hyperbaric bupivacaine, spinal anaesthesia
INTRODUCTION
Spinal anaesthesia has been widely used for caesarean section (CS) deliveries because of greater maternal safety, foetal benefits, higher parental satisfaction, and consumer demand.[1] However, to address the problem of limited duration of action and to improve the quality of analgesia, various adjuvants are added intrathecally with local anaesthetics (LA).[2,3] The adjuvants gained widespread popularity as they reduce the amount of LA and thus the incidence of side-effects.
Clonidine, a selective partial agonist for alpha-2 adrenoreceptors, is an attractive alternative to commonly used opioids, and is known to increase both sensory and motor block of LA.[4,5] Several studies have shown that clonidine also has antihyperalgesic effect and thus reduces the post-operative analgesic requirement.[6]
Commonly, adjuvants are mixed with LA in a single syringe before injecting the drugs intrathecally. Mixing of these drugs changes the density of both drugs, thus affecting their spread in the cerebrospinal fluid (CSF).[7] Density is known to influence the spread of LA, but the effect of adjuvant solution density on its movement in the CSF has not been studied extensively.[8,9] Therefore, we hypothesised that if we administer LA and the adjuvants separately, it may minimise the effect of the changes in densities and also hence their actions.
Thus, in this study we aimed to compare block characteristics, intra-operative haemodynamics and post-operative pain relief in parturients undergoing CS under subarachnoid block (SAB), after administering hyperbaric bupivacaine (HB) and clonidine as a mixture in single syringe and sequentially in two syringes.
METHODS
After approval by the institutional ethical committee and written informed consent, sixty parturients with singleton pregnancy, American Society of Anaesthesiologist (ASA) I and II physical status, scheduled for elective CS under SAB, were enrolled in this single-blind prospective randomised controlled trial. Patients having multiple pregnancy, intrauterine deaths or known foetal anomaly, severe pregnancy induced hypertension, contraindication to SAB, patients on cardiovascular medications and those having history of hypersensitivity to clonidine and LA were excluded from the study.
Using a sealed envelope technique, patients were randomly allocated to one of the two groups. Group M (n = 30) received hyperbaric bupivacaine (0.5%) 10 mg (2 mL) and clonidine 75 mcg (0.5 mL) as a mixture. Group B (n = 30) received clonidine 75 mcg (0.5 mL) followed by hyperbaric bupivacaine (0.5%) 10 mg (2 mL) in different syringes.
For our study, the two drugs used were sourced from same company to avoid manufacturer's difference. Hyperbaric bupivacaine used was HEAVY ANAWINTM® and clonidine used was CLONEONTM® manufactured by Neon Laboratories Limited, Mumbai. Patients were kept fasting overnight and antacid prophylaxis with oral ranitidine 150 mg at night and on the morning prior to surgery were given. The patients were familiarised with the concept of visual analogue scale (VAS) for pain assessment with 0 = no pain and 10 = worst possible pain.
In the operating room, monitor for heart rate (HR), non-invasive blood pressure, electrocardiography and oxygen saturation (SpO2) was connected and baseline parameters were recorded. After establishing 18 gauge venous cannula, patients were pre-loaded with 15 mL/kg of lactated Ringer's solution 15-20 min before spinal block. Under all aseptic precautions SAB was administered with 23 G Quincke spinal needle through mid-line approach in sitting position. Intrathecal (IT) drug was injected in L3-L4 interspace over 30 s (including the time for change of syringe in sequential administration). After the block was performed, the patients were made supine with 15°-20° left displacement of uterus until birth of baby by keeping a wedge under the right buttock. Fluid therapy was maintained with lactated Ringer's solution 10 mL/kg/h. An experienced anaesthesiologist who was unaware of the drug given evaluated the spinal block and other physiological parameters.
Haemodynamic parameters such as HR, systolic arterial pressure (SAP), diastolic arterial pressure (DAP) were monitored at every 2 min (min) for the first 20 min and then every 5 min subsequently until 75 min or until completion of surgery. Any episode of hypotension and bradycardia in 24 h was noted. Hypotension (decrease in SAP below 90 mmHg or a fall in blood pressure by >20% of baseline values) was treated with a rapid infusion of crystalloids (200 mL) and a bolus of ephedrine 5 mg intravenous (i.v) was administered if hypotension persisted. Bradycardia (HR <50 beats/min) was treated with injection atropine 10 mcg/kg i.v.
The onset of sensory block was assessed by loss of pin prick sensation along the mid clavicular line bilaterally. Dermatomal level was tested every 2 min after SAB until level was stabilised for four consecutive readings. The time from IT injection to highest sensory level (maximum block height) was noted. Furthermore, level was tested every 30 min until regression from highest level to T10 dermatome was noted. Degree of motor block was assessed by modified Bromage scale as follows; I – Free movement of legs and feet; II – Just able to flex knees with free movement of feet; III – Unable to flex knees but with free movement of feet; IV – Unable to move legs and feet. Motor block was assessed at the same interval as sensory block. Onset of motor block was assessed by time to reach modified Bromage II. Time to achieve complete motor block (modified Bromage IV) and its regression to modified Bromage I was noted.
Respiration was monitored and respiratory depression was defined as respiratory rate <10 breaths/min or SpO2 < 92%; oxygen was then supplemented through nasal prongs at 4 L/min.
Sedation score was assessed at the same interval as sensory block until 2 h post-operatively by Ramsay sedation score (RSS) as: Level 1 – awake, anxious, agitated, restlessness; Level 2 – awake, tranquil, co-operative; Level 3 – responds to commands; Level 4 – asleep, brisk response to stimuli; Level 5 n asleep, sluggish response to stimuli; Level 6 – asleep, no response to stimuli. Intra-operative pain was checked and expressed as VAS, whenever the parturients complained of any discomfort or pain. Duration of effective analgesia was defined as time from IT injection till VAS was ≥3, when rescue analgesia in the form of injection i.v diclofinac sodium 75 mg was administered.
Patients complaining of nausea or having any episode of vomiting were given injection ondansetron 0.15 mg/kg i.v. Any complaint of patient experiencing dry mouth was noted. At delivery, new-born's APGAR scores were determined by a paediatrician not otherwise involved in the study at 1, 5, and 10 min.
Post-operatively any incidence of bradycardia, hypotension, nausea/vomiting, prolonged sedation reported by the recruited post-operative care unit staff was taken into account and managed accordingly. The parturients were also interviewed for post-dural puncture headache (PDPH), backache, and examined for any neurological deficit.
Power analysis suggested that a sample size of 30 patients/group was required to achieve a power of 80% and a level significance of 0.05 to be able to detect a difference between the groups, based on the assumption that an increase in the mean duration of analgesia by 60 min in sequential group. Interpretation of the data was carried out and analysed using software Microsoft® Excel and SPSS® version 19. Data is represented as Mean ± standard deviation for continuous data and frequency (percentage %) or median (range) for non-parametric (categorical) data. The two groups were compared using analysis of variance. The proportion of adverse effects was compared using Chi-square test and level of sedation was compared with the help of Wilcoxon test. The P < 0.05 was considered significant and P < 0.01 was considered highly significant.
RESULTS
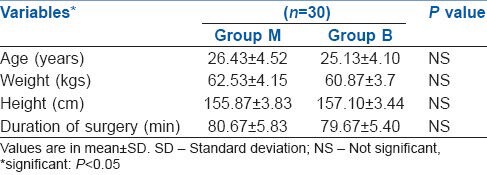
Demographic data in terms of age, height, weight, ASA physical status and duration of surgery were comparable in both groups [Table 1].
Table 1.
Demographic profile
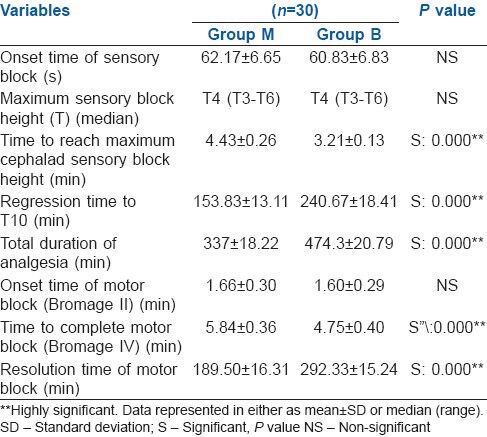
The onset time of sensory and motor block and also the highest level of block achieved (T4) were comparable in both groups [Table 2]. Mean time to reach maximal cephalad sensory block height was significantly less in Group B (3.21 ± 0.13 min) than in Group M (4.43 ± 0.26 min) and the total duration of analgesia lasted significantly longer in Group B (474.3 ± 20.79 min) as compared to Group M (337 ± 18.22 min) (P = 0.000). Complete motor blockade was achieved earlier in Group B (4.75 ± 0.40) than in Group M (5.84 ± 0.36) (P = 0.000). The resolution time of motor block back to modified Bromage I was significantly prolonged in Group B (292.23 ± 15.24 min) than in Group M (189.50 ± 16.31 min) [Table 2].
Table 2.
Characteristics of sensory and motor block
Haemodynamic parameters showed that the lowest values of the HR were after 45 min of the administration of SAB, but none of the patients had bradycardia [Figure 1]. There was a significant fall in SAP at 2 min and 4 min after administration of SAB in both groups [Figure 2]. A significant fall in DAP was seen at 2, 4, 6, and 8 min of administration of SAB. There was an overall trend of fall in SAP and DAP in both groups, except during the time intervals of 20 and 25 min (during delivery of baby) where there was rise in both SAP and DAP [Figures 2 and 3]. The falling trend of arterial blood pressures was more in the Group B than in Group M.
Figure 1.
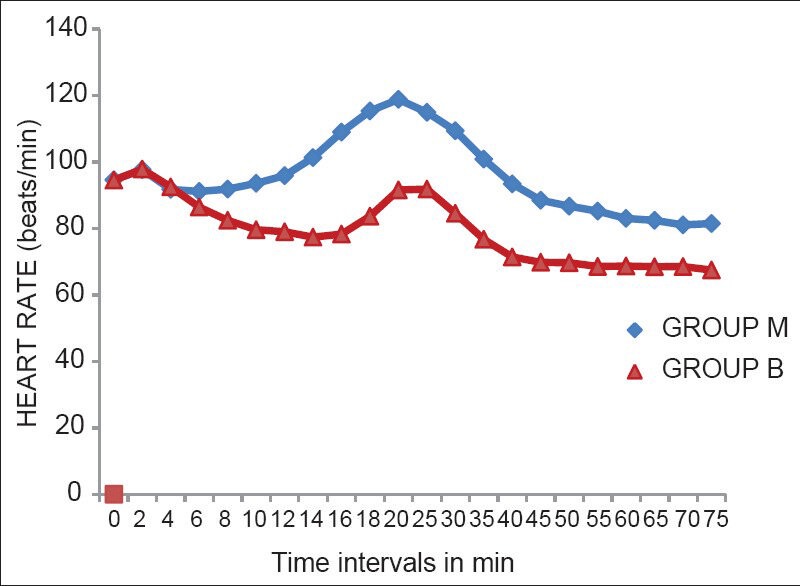
Heart rates at different time intervals
Figure 2.
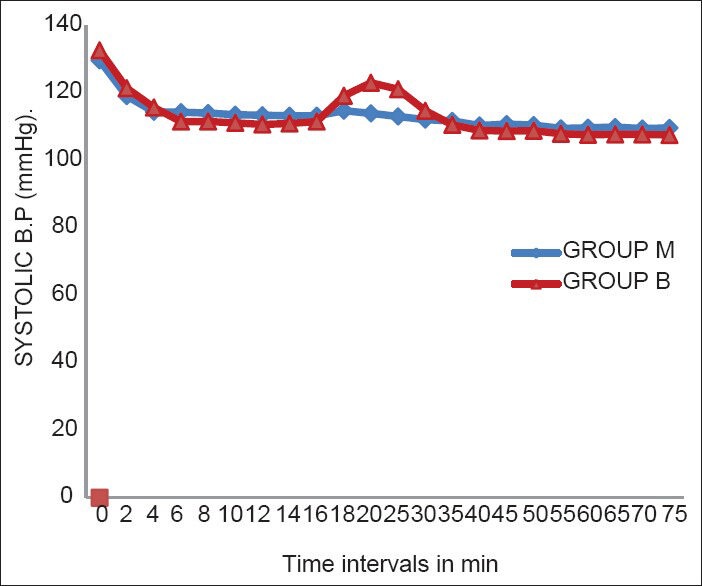
Systolic blood pressure at different time intervals
Figure 3.
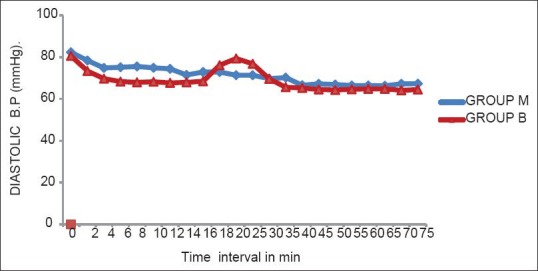
Diastolic blood pressure at different time intervals
There was no noticeable fall in SpO2 in both groups. It was observed that only one patient in Group M and 3 in Group B had sedation score of 4 according to RSS. None of the patients had score of 5 or 6.
Intra-operative incidence of hypotension, bradycardia, respiratory depression, nausea/vomiting, dry mouth and additional analgesic requirement are comparable in both groups [Figure 4]. Newborn's well-being, assessed by the APGAR scoring system was observed in both groups. The median value was 8 at 1 min, 9 at 5 min and 10 at 10 min in both groups.
Figure 4.
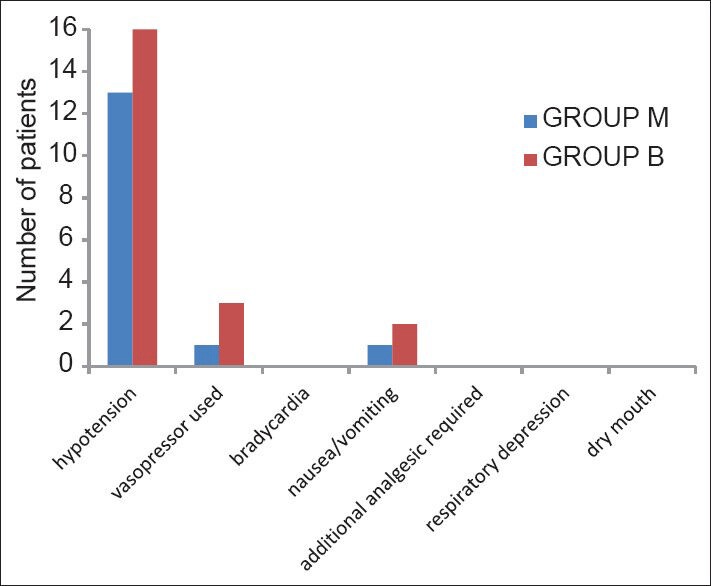
Incidence of intra-operative adverse effects
DISCUSSION
Activation of post-synaptic alpha-2 receptors in the substantia gelatinosa of the spinal cord is the presumed mechanism by which clonidine produces analgesia. These receptors are located on primary afferent terminals (both at peripheral and spinal endings), on neurons in the superficial lamina of the spinal cord, and within several brainstem nuclei implicated in analgesia, supporting the possibility of analgesic action at peripheral, spinal, and brainstem sites.[10,11]
Various authors have used different doses of IT clonidine ranging from 15 mcg to 300 mcg along with local anaesthetics. Kaabachi et al., in their study used 2 mcg/kg of IT clonidine and reported extended duration of post-operative analgesia, but with moderate side-effects.[12] Sethi et al., used 70 mcg of clonidine and found a significant decrease in mean arterial pressure and HR in clonidine group, but no therapeutic intervention was required for either.[13] Since marked decrease in blood pressure is observed only with intermediate doses of spinal clonidine (150 mcg) and relative haemodynamic stability is maintained after larger doses (300-400 mcg), we were interested to test a dose of lower range of efficacy. Hence, we selected a 75 mcg of preservative free clonidine as an adjuvant for spinal anaesthesia in CS. Patients scheduled for CS were chosen for the study because it is a well-known fact that visceral discomfort and pain is common occurence in CS under SAB.[14]
The observations and results obtained in the study are based on the assumption that the original densities of hyperbaric bupivacaine and clonidine are lost when they are premixed in a syringe thus, they exert suboptimal actions when compared to their IT administration in a sequential manner. The above assumption is supported by the work of Desai et al. who studied the same effect by adding opioids to LA solution intrathecally.[7]
The densities of the drugs that we used (HB and clonidine) were 1.0260 and 0.9930, respectively. The density of the mixture of 2 mL (10 mg) of hyperbaric bupivacaine and 0.5 mL (75 mcg) clonidine was also estimated and it was found to be 1.0189.
In our study, we observed that the mean onset time of sensory and motor block was similar in both groups. However, onset of sensory block does not get any better after a particular dose as supported by a study done by Heo et al. who did not report any difference in onset time even after using 150 mcg clonidine.[15]
The time to reach maximum sensory block height and maximum motor block was significantly less in Group B (sequential drugs) than in Group M (mixed drugs) in this study. This difference might have existed because of the preferential cephalad spread of clonidine when we administered it through a separate syringe, owing to its hypobaric nature which is lost when the drugs are premixed. Desai et al. also observed that the time to reach highest level of block was less when morphine and fentanyl were administered sequentially with HB than when given as a mixture.[7]
In our study, we found that the mean time taken for sensory block to regress to T10 level was significantly longer in Group B (240.67 ± 18.47 min) than in Group M (153.83 ± 13.11 min). Similarly, the mean duration of analgesia lasted significantly longer in Group B (474.33 ± 20.79 min) than in Group M (337 ± 18.22 min), depicting significant prolongation of analgesic effect in the group receiving drugs in a sequential fashion. This difference might be due to the fact that injecting clonidine and bupivacaine as a mixture dilutes clonidine and receptor occupancy might decrease leading to less pronounced effect. However, if clonidine is administered separately, we expect a greater spread and therefore formation of stronger bonds with the receptor leading to a denser and prolonged block.
According to Desai et al., dextrose in a HB solution slow the movement of morphine molecules in the CSF, reducing the exposure of supraspinal centres to morphine.[7] Clonidine also being hypobaric drug, acting on both spinal and supraspinal receptors, might exhibit similar properties. Gray et al. observed that duration of analgesia is increased when IT morphine is administered with normal saline (hypobaric) than with dextrose saline (hyperbaric).[16]
Clonidine decreases HR by a presynaptic mediated inhibition of nor epinephrine release and by a direct depression of atrioventricular nodal conduction after systemic absorption.[17] The maximum fall in the HR when compared to the baseline was 28% in Group B, whereas it was only 13% in Group M which was statistically significant (P < 0.001). This fall in HR was more pronounced after about 40-60 min of administration of SAB and toward end of the surgery. However, in our study none of the patients had bradycardia.
A significant fall in arterial blood pressure after SAB was observed in our study. The fall from baseline SAP and DAP in Group M was 15% and 18% and in Group B was 19% and 20%, respectively. Haemodynamic effects of clonidine after neuraxial or systemic administration begin within 30 min, reach maximum within 1-2 h, and last approximately 6-8 h after a single injection.[18] We observed hypotension in 13% patients in Group M and 16% patient in Group B. Hypotention was managed by i.v fluids and vasopressors were needed for only 1% and 3% parturients in Groups M and B, respectively which was comparable in both groups, suggesting that the clonidine groups did not have a higher predisposition for the development of hypotension if administered sequentially. In our study, the level of sedation provided by IT clonidine (RSS 2 and 3) was not only acceptable, but also beneficial owing to its anxiolytic role.
None of the patients needed any additional analgesics during the intra-operative period. In line with our observations, Benhamou et al. found that when IT clonidine was administered with HB, none of patients required additional analgesics to obtain an adequate sensory block.[2] None of the patients complained of dry mouth.
In the post-operative period one patient each in Group M and B developed PDPH, which was managed conservatively. There was no incidence of hypotension, bradycardia and nausea/vomiting, neurological deficit, prolonged sedation in the post-operative period.
The APGAR scores in our study were statistically comparable in both groups. Benhamou et al. and Neves et al. also concluded that addition of IT clonidine did not adversely affect the neonatal outcome in terms of APGAR scores.[2,19,20]
The limitation of our study was that we measured the densities of solutions in vitro; but, we could not measure the densities when injected into the CSF. Hence, we could not assess what actually happens to the drug densities intrathecally. Similarly, effects of temperature of drugs when injected were not considered.
CONCLUSION
Sequential administration of clonidine reduces the time to achieve complete sensory and motor block and significantly prolongs the total duration of analgesia. Addition of clonidine to hyperbaric bupivacaine provided a dense surgical anaesthesia irrespective of the technique of administration. However, we noticed that sequential technique did not increase the level of sedation and incidence of hypotension or bradycardia as compared to the administration of drugs as mixture. New-born outcome also remained unaffected.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hawkins JL, Koonin LM, Palmer SK, Gibbs CP. Anesthesia-related deaths during obstetric delivery in the United States, 1979-1990. Anesthesiology. 1997;86:277–84. doi: 10.1097/00000542-199702000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Benhamou D, Thorin D, Brichant JF, Dailland P, Milon D, Schneider M. Intrathecal clonidine and fentanyl with hyperbaric bupivacaine improves analgesia during cesarean section. Anesth Analg. 1998;87:609–13. doi: 10.1097/00000539-199809000-00022. [DOI] [PubMed] [Google Scholar]
- 3.Roelants F. The use of neuraxial adjuvant drugs (neostigmine, clonidine) in obstetrics. Curr Opin Anaesthesiol. 2006;19:233–7. doi: 10.1097/01.aco.0000192812.56161.f8. [DOI] [PubMed] [Google Scholar]
- 4.Gecaj-Gashi A, Terziqi H, Pervorfi T, Kryeziu A. Intrathecal clonidine added to small-dose bupivacaine prolongs postoperative analgesia in patients undergoing transurethral surgery. Can Urol Assoc J. 2012;6:25–9. doi: 10.5489/cuaj.11078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thakur A, Bhardwaj M, Kaur K, Dureja J, Hooda S, Taxak S. Intrathecal clonidine as an adjuvant to hyperbaric bupivacaine in patients undergoing inguinal herniorrhaphy: A randomized double-blinded study. J Anaesthesiol Clin Pharmacol. 2013;29:66–70. doi: 10.4103/0970-9185.105804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavand‘homme PM, Roelants F, Waterloos H, Collet V, De Kock MF. An evaluation of the postoperative antihyperalgesic and analgesic effects of intrathecal clonidine administered during elective cesarean delivery. Anesth Analg. 2008;107:948–55. doi: 10.1213/ane.0b013e31817f1595. [DOI] [PubMed] [Google Scholar]
- 7.Desai S, Lim Y, Tan CH, Sia AT. A randomised controlled trial of hyperbaric bupivacaine with opioids, injected as either a mixture or sequentially, for spinal anaesthesia for caesarean section. Anaesth Intensive Care. 2010;38:280–4. doi: 10.1177/0310057X1003800209. [DOI] [PubMed] [Google Scholar]
- 8.Hocking G, Wildsmith JA. Intrathecal drug spread. Br J Anaesth. 2004;93:568–78. doi: 10.1093/bja/aeh204. [DOI] [PubMed] [Google Scholar]
- 9.Imbelloni LE, Moreira AD, Gaspar FC, Gouveia MA, Cordeiro JA. Assessment of the densities of local anesthetics and their combination with adjuvants: An experimental study. Rev Bras Anestesiol. 2009;59:154–65. doi: 10.1590/s0034-70942009000200003. [DOI] [PubMed] [Google Scholar]
- 10.van Tuijl I, van Klei WA, van der Werff DB, Kalkman CJ. The effect of addition of intrathecal clonidine to hyperbaric bupivacaine on postoperative pain and morphine requirements after Caesarean section: A randomized controlled trial. Br J Anaesth. 2006;97:365–70. doi: 10.1093/bja/ael182. [DOI] [PubMed] [Google Scholar]
- 11.Roh DH, Kim HW, Yoon SY, Seo HS, Kwon YB, Han HJ, et al. Intrathecal clonidine suppresses phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit in spinal dorsal horn neurons of rats with neuropathic pain. Anesth Analg. 2008;107:693–700. doi: 10.1213/ane.0b013e31817e7319. [DOI] [PubMed] [Google Scholar]
- 12.Kaabachi O, Ben Rajeb A, Mebazaa M, Safi H, Jelel C, Ben Ghachem M, et al. Spinal anesthesia in children: Comparative study of hyperbaric bupivacaine with or without clonidine. Ann Fr Anesth Reanim. 2002;21:617–21. doi: 10.1016/s0750-7658(02)00704-9. [DOI] [PubMed] [Google Scholar]
- 13.Sethi BS, Samuel M, Sreevastava D. Efficacy of analgesic effects of low dose intrathecal clonidine as adjuvant to bupivacaine. Indian J Anaesth. 2007;51:415. [Google Scholar]
- 14.Zahir J, Syed S, Jabeen N, Anjum Q, Rehman SU. Maternal and neonatal outcome after spinal versus general anaesthesia for caesarean delivery. Ann Pak Inst Med Sci. 2011;7:115–8. [Google Scholar]
- 15.Heo GJ, Kim YH, Oh JH, Joo JC. Effect of intrathecal clonidine in hyperbaric bupivacaine spinal anesthesia. Korean J Anesthesiol. 1997;33:304–8. [Google Scholar]
- 16.Gray JR, Fromme GA, Nauss LA, Wang JK, Ilstrup DM. Intrathecal morphine for post-thoracotomy pain. Anesth Analg. 1986;65:873–6. [PubMed] [Google Scholar]
- 17.Baker A, Klimscha W, Eisenach JC, Li XH, Wildling E, Menth-Chiari WA, et al. Intrathecal clonidine for postoperative analgesia in elderly patients: The influence of baricity on hemodynamic and analgesic effects. Anesth Analg. 2004;99:128–34. doi: 10.1213/01.ANE.0000114549.17864.36. [DOI] [PubMed] [Google Scholar]
- 18.Eisenach JC, De Kock M, Klimscha W. alpha (2)-adrenergic agonists for regional anesthesia. A clinical review of clonidine (1984-1995) Anesthesiology. 1996;85:655–74. doi: 10.1097/00000542-199609000-00026. [DOI] [PubMed] [Google Scholar]
- 19.Neves JF, Monteiro GA, Almeida JR, Sant‘anna RS, Saldanha RM, Moraes JM, et al. Postoperative analgesia for cesarean section: Does the addiction of clonidine to subarachnoid morphine improve the quality of the analgesia? Rev Bras Anestesiol. 2006;56:370–6. doi: 10.1590/s0034-70942006000400005. [DOI] [PubMed] [Google Scholar]
- 20.Singh R, Gupta D, Jain A. The effect of addition of intrathecal clonidine to hyperbaric bupivacaine on postoperative pain after lower segment caesarean section: A randomized control trial. Saudi J Anaesth. 2013;7:283–90. doi: 10.4103/1658-354X.115360. [DOI] [PMC free article] [PubMed] [Google Scholar]


