Abstract
Re-expansion pulmonary oedema (REPE) is a rare complication following re-inflation of a chronically collapsed lung, which is often fatal. We present a case of a 22-year-old male who presented to the hospital with severe respiratory distress and a history of blunt abdominal trauma 3 months back. He was diagnosed to have left sided diaphragmatic hernia with a mediastinal shift to the right, and was posted for emergency repair of the same. After surgical decompression of the left hemi-thorax and reduction of the abdominal contents, re-expansion of the left lung was achieved, following which patient developed REPE. A left sided double lumen tube was then inserted to prevent flooding and cross contamination of the right lung and ventilation of both lungs was maintained intraoperatively. Post-operatively, REPE was successfully managed by differential lung ventilation with a lung salvage strategy to the left lung and a lung protective strategy to the right lung.
Keywords: Differential lung ventilation, double lumen tube, re-expansion pulmonary oedema
INTRODUCTION
Re-expansion pulmonary oedema (REPE) is a rare complication after re-inflation of a chronically collapsed lung, following pneumothorax or effusion. It has an incidence of about 1%, but is associated with high mortality (20%).[1,2,3]
Re-expansion pulmonary oedema is usually ipsilateral and the risk is highest after rapid re-expansion of the lung, which has been collapsed for 3 or more days.[3] Other contributory factors are large pneumothorax, young patients (between 20 and 39 years) and the method of re-expansion.[3] Although most patients recover within 7 days, severe REPE due to sequestration of large quantities of fluid in the lung may lead to hypoxaemia, hypotension, shock, and death.[4] Though conventional ventilatory therapy may be useful in mild cases of REPE, no definitive treatment is known for severe forms.[5] REPE has been described following re-expansion of lung after pneumothorax, pleural effusion, resection of mediastinal tumours, thorascopies and has also been reported after diaphragmatic hernia repair.[6,7,8] The aim of this case report is to emphasise the possibility of REPE arising after diaphragmatic hernia reduction, and the significance of differential lung ventilation (DLV) as a successful ventilatory management option in severe cases of REPE.
CASE REPORT
A 22-year-old male presented to the hospital with a history of blunt trauma to the abdomen 3 months ago, along with respiratory distress of 3 days duration. Radiological investigations revealed left sided diaphragmatic hernia with abdominal contents in the left hemi-thorax and mediastinal shift to the right.
Patient was posted for emergency laparotomy and repair of the diaphragmatic hernia. Pre-operatively, patient was anxious, unable to lie down supine with minimal chest expansion on the left side. He was having respiratory rate (RR) of 48/min, pulse rate 126/min, and blood pressure (BP) of 90/54 mmHg. Pulse oximetry revealed room air saturation of 89%. Auscultation revealed minimal breath sounds only in the left infraclavicular area. Modified rapid sequence induction was performed in a propped up position using 60 mg intravenous (IV) ketamine, 2 mg IV midazolam and 75 mg IV succinylcholine and the patient was intubated with 8.0 mm internal diameter cuffed endotracheal tube (ETT). Right radial artery was cannulated for the purpose of invasive arterial BP monitoring and left subclavian central venous catheter was secured and ionotropic support was initiated with IV dopamine 10 μg/kg/min. Surgery revealed a grossly distended stomach, transverse colon and spleen in the left hemi-thorax. Once the abdominal contents from the left hemi-thorax were reduced, re-expansion of the left lung was achieved. The saturation improved from 94% to 98% transiently, after which it suddenly decreased to 93% along with the drop in BP. The ionotropic support was increased and copious amount of pink frothy fluid in the ETT was noticed. There was incessant production of fluid, which was flooding the ETT and the anaesthesia circuit. Since it developed dramatically after left lung re-expansion, the possibility of REPE was considered. In order to avoid contamination of the right lung, we secured the airway with 39 French left sided double lumen tube (DLT), confirmed the correct position of the DLT with fibreoptic bronchoscope and ventilated both lungs intraoperatively. There was continuous drainage of the pink fluid from the bronchial lumen of the DLT, which was intermittently suctioned. The saturation gradually increased to 98%. The arterial blood gas (ABG) investigation revealed pH 7.18, partial pressure of carbon dioxide (pCO2) 59 mmHg, partial pressure of oxygen (pO2) 117 mmHg; bicarbonate 22 mEq/L on fractional of inspired oxygen concentration (FiO2) of 0.8. Rest of the intraoperative period was uneventful and the patient was shifted to intensive care unit (ICU) for further management, with DLT in situ. Patient was sedated with continuous infusion of IV morphine and midazolam.
In ICU, conventional ventilation with DLT was continued for first 6 h with FiO2 of 0.8 using synchronized intermittent mandatory ventilation (SIMV) with tidal volume (TV) of 500 mL, RR of 14/min, and positive end expiratory pressure (PEEP) of 8 mmHg. ABG revealed pH 7.34 with pCO2 35 mmHg, pO2 58 mmHg and bicarbonate 19.3 mEq/L. Pulse oximetry showed 89-90% saturation and chest radiograph revealed left REPE [Figure 1].
Figure 1.
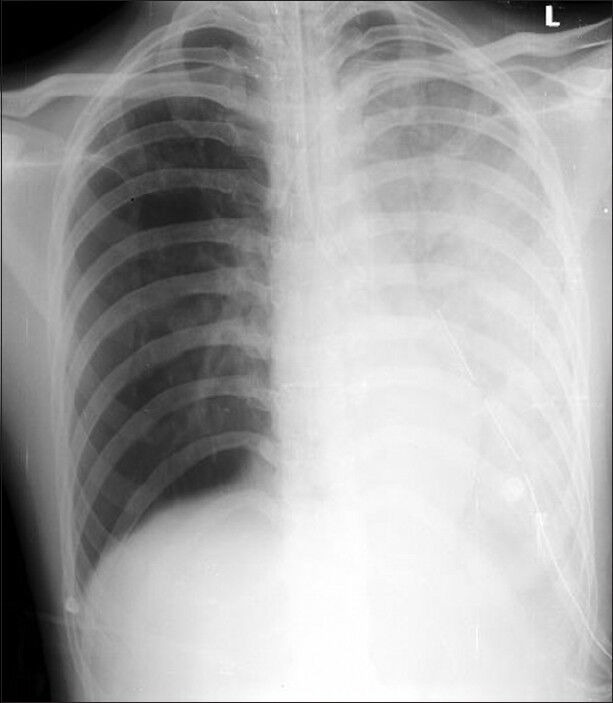
Chest radiograph showing left sided re-expansion pulmonary oedema
As there was deterioration in oxygenation on conventional ventilation, DLV was initiated using two ventilators of the same company (GE Datex Ohmeda Engstrom Carestation®) [Figure 2].
Figure 2.
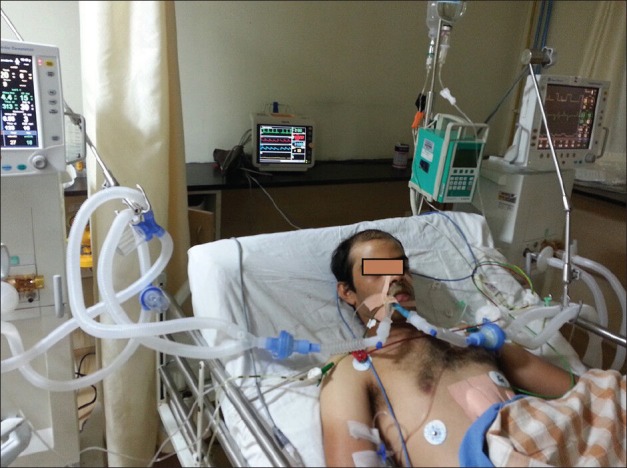
Differential lung ventilation being administered using two ventilators
Lung salvage ventilatory strategy was adopted for ventilating the left affected lung, with FiO2 of 0.8 using SIMV with pressure control (PC) 17 cm H2O, RR 14/min, PEEP of 14 mmHg. The ventilatory setting for the right lung was FiO2 of 0.4 using SIMV, PS of 14 cmH2O, RR 14/min, and PEEP of 5 mmHg. Patient tolerated the DLV with morphine and midazolam infusion, without the requirement for paralysis. The saturation and haemodynamics gradually improved, and ABG after 24 h on same ventilatory settings revealed pH 7.42 with pCO2 36 mmHg, pO2 127 mmHg and bicarbonate 24.2 mEq/L. Chest radiograph showed significant improvement of REPE [Figure 3].
Figure 3.
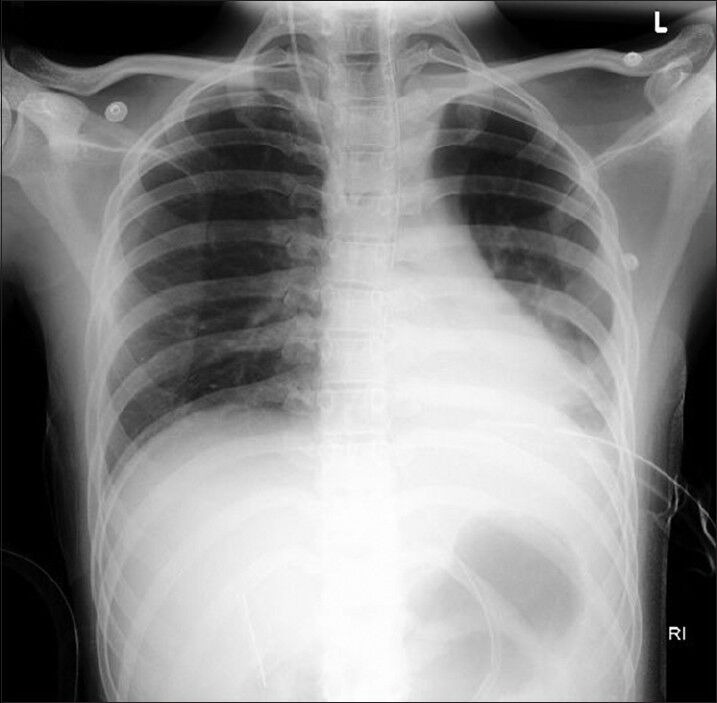
Chest radiograph showing the resolution of left sided re-expansion pulmonary oedema after differential lung ventilation
Compliance of the left lung gradually improved, and DLV was continued for 72 h until both lungs were ventilated with identical ventilatory settings of FiO2 0.4 using SIMV, PS of 14 cmH2O, RR 14/min, and PEEP of 5 mmHg. There was further improvement in the clinical condition of the patient, and trachea was extubated. After 2 days of intermittent non-invasive ventilation, patient was put on 40% Venturi and was shifted to step down ICU.
DISCUSSION
Re-expansion pulmonary oedema is a rare but fatal complication, which occurs after rapid re-expansion of a chronically collapsed lung.[1,5] Multiple possible aetiologies play a role in development of REPE, but the exact cause is not yet understood.[1] Contributory factors include increased pulmonary vasculature permeability due to anoxic damage to capillary endothelium and neovascularisation, reperfusion related free radical injury and mechanical damage to blood vessels due to overstretching during re-expansion.[1,9]
The patient may present with dramatic onset of respiratory failure and circulatory collapse, along with copious pink frothy secretions.[1] Utmost vigilance is required for early recognition and optimal management of REPE, as symptoms worsen rapidly for first 24-48 h. If mortality is prevented in the first 48 h, recovery is usually complete.[10]
In REPE, the affected lung has poor compliance, but is overperfused, whereas the unaffected lung has better compliance, yet hypoperfused. This creates ventilation-perfusion mismatch, which is further exacerbated by conventional ventilation.[7]
Though continuous positive airway pressure, asynchronous DLV, high frequency jet ventilation and extracorporeal membrane oxygenation have proven beneficial in the treatment of REPE, a definitive treatment modality has not yet been described.[5,11,12,13]
As conventional mode of ventilation proved detrimental in our patient, DLV was initiated. A salvage strategy of ventilation was used for the affected lung with a high PEEP and low TV goals, whereas a lung protective strategy was adopted for the right lung with minimisation of FiO2, PEEP, peak inspiratory pressure and TV. This helped in improvements in compliance and ventilation-perfusion in the affected lung, and hence we were able to gradually wean off the ventilatory support and extubate successfully. DLT prevents the unaffected lung from being flooded with the oedema fluid from the contralateral lung, and facilitates DLV, which not only protects the affected lung with poor compliance, but also prevents the volutrauma and barotrauma to normal compliant lung.
Prevention of development of REPE post thoracocentesis include the use of low negative pressure (<−20 cmH2O) for suction during tube thoracostomy and restricting the drainage to about 1.5 L of pleural fluid. However, larger volumes can be safely drained if pleural pressures are monitored.[14]
CONCLUSION
Utmost sagacity is required in the management of REPE. Though REPE has a high mortality rate, if fatality is avoided at an early stage, patient recovery is usually complete. DLV may be the initial ventilatory treatment strategy, and we should not wait for deterioration to occur by starting conventional lung ventilation as the pathophysiology of REPE necessitates DLV.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Janmeja AK, Mohapatra PR, Saini MS, Khurana A. Re-expansion pulmonary edema – A case report. Lung India. 2007;24:72–4. [Google Scholar]
- 2.Echevarria C, Twomey D, Dunning J, Chanda B. Does re-expansion pulmonary oedema exist? Interact Cardiovasc Thorac Surg. 2008;7:485–9. doi: 10.1510/icvts.2008.178087. [DOI] [PubMed] [Google Scholar]
- 3.Komatsu T, Shibata S, Seo R, Tomii K, Ishihara K, Hayashi T, et al. Unilateral re-expansion pulmonary edema following treatment of pneumothorax with exceptionally massive sputum production, followed by circulatory collapse. Can Respir J. 2010;17:53–5. doi: 10.1155/2010/259195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherman SC. Reexpansion pulmonary edema: A case report and review of the current literature. J Emerg Med. 2003;24:23–7. doi: 10.1016/s0736-4679(02)00663-7. [DOI] [PubMed] [Google Scholar]
- 5.Cho SR, Lee JS, Kim MS. New treatment method for reexpansion pulmonary edema: Differential lung ventilation. Ann Thorac Surg. 2005;80:1933–4. doi: 10.1016/j.athoracsur.2004.06.029. [DOI] [PubMed] [Google Scholar]
- 6.Inaba K, Snider J, Holliday RL. Re-expansion pulmonary edema after repair of a missed diaphragmatic hernia. Can J Surg. 2001;44:295–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Paut O, Gaillat F, Delarve A, Bonneru JJ, Chauvet V, Camboulives J. Acute pulmonary oedema following repair of cangenital diaphragmatic hernia. Paediatr Anaesth. 1992;2:339–42. [Google Scholar]
- 8.El-Dawlatly A, Abdullah K, Al-Dohayan A. Unilateral pulmonary edema following surgical repair of diaphragmatic hernia: A case report. Internet J Anaesthesiol. 2002;6:about 3. Available from: http://www.ispub.com/IJA/6/2/12394 . [Google Scholar]
- 9.Matsumiya N, Dohi S, Kimura T, Naito H. Reexpansion pulmonary edema after mediastinal tumor removal. Anesth Analg. 1991;73:646–8. doi: 10.1213/00000539-199111000-00024. [DOI] [PubMed] [Google Scholar]
- 10.Light RW. Thoracentesis and pleural biopsy. In: Light RW, editor. Pleural Disease. 4th ed. Baltimore: Williams and Wilkins; 2003. pp. 358–77. [Google Scholar]
- 11.Wake M, Sanagawa Y, Okamoto Y. A case of anesthetic management for re-expansion pulmonary edema of the dependent lung saved by superimposed HFJV during one lung ventilation for the thoracoscopic operation associated with bilateral pneumothorax. Masui. 2000;49:643–5. [PubMed] [Google Scholar]
- 12.Tung YW, Lin F, Yang MS, Wu CW, Cheung KS. Bilateral developing reexpansion pulmonary edema treated with extracorporeal membrane oxygenation. Ann Thorac Surg. 2010;89:1268–71. doi: 10.1016/j.athoracsur.2009.07.064. [DOI] [PubMed] [Google Scholar]
- 13.Conen A, Joos L, Bingisser R. Ipsilateral reexpansion pulmonary edema after drainage of a spontaneous pneumothorax: A case report. J Med Case Rep. 2007;1:107. doi: 10.1186/1752-1947-1-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feller-Kopman D, Parker MJ, Schwartzstein RM. Assessment of pleural pressure in the evaluation of pleural effusions. Chest. 2009;135:201–9. doi: 10.1378/chest.08-1788. [DOI] [PubMed] [Google Scholar]


