Abstract
Purpose
Static cold storage (SCS) of the liver for transplantation is limited by time. Continuation of metabolic activity leads to depletion of energy stores and loss of cellular function, which results in poor post-transplant function. Machine perfusion (MP) applied at the end of preservation may improve the viability of marginal liver grafts and provides information on the quality of the organ. We attempt to define the limits to SCS in terms of easily measurable perfusion parameters and investigate whether MP can improve liver viability.
Methods
Rat livers were cold-stored for 0, 24, 48, 72, and 120 hours, after which they were treated with subnormothermic machine perfusion (SNMP). Livers cold-stored for 48 and 72 hours were transplanted orthotopically with or without SNMP. During SNMP easily measurable parameters were monitored and adenosine triphosphate (ATP) content was measured following preservation and SNMP.
Results
ATP increased significantly during SNMP, but the recovered ATP content deteriorated with increased duration of SCS, with minimal improvement after 72 hours of SCS. Vascular resistance during SNMP increased with extended preservation. After 48 hours of SCS, orthotopic transplantation survival increased significantly from 50% to 100% with SNMP, but did not improve after 72 hours.
Conclusions
Vascular resistance and ATP recovery suggest a decrease in viability after 48 hours of SCS. Survival data confirms the loss of post-transplant graft function and supports the use of ATP and vascular resistance as useful indicators. Further, we show that the recoverability of a liver using SNMP is limited to 48 hours of SCS.
Keywords: Liver, Transplantation, Machine Perfusion, Preservation, Cold Ischemia, Adenosine Triphosphate
Introduction
Reduction of metabolic activity by lowering the temperature facilitates the preservation of solid organs and provides enough time for the logistics of liver transplantation. In combination with specialized preservation solutions, static cold storage (SCS) of the liver slows the depletion of energy stores and loss of cellular function, and renders sufficiently high organ quality up to approximately 24 hours of preservation (1,2). However, SCS is limited, as evidenced by decreasing transplant survival with a longer duration of preservation (3,4). Improvement of the quality of livers after preservation would extend the limitations of SCS to allow better allocation of organs and result in a reduction in graft failure and retransplantation.
Machine perfusion (MP) has been employed at the end of ischemia to recover organs prior to transplantation, improving both the quality of organs after preservation and transplant outcome (5–7). In addition, MP allows the real-time measurement of parameters that correlate with graft quality, which facilitates more informed and objective decision making in the organ allocation process (8). As a result, MP potentially reduces the unnecessary discarding of viable livers.
MP has been shown to improve various metabolic functions after a limited duration of SCS (9), after WI (6) and following sequential warm (WI) and cold ischemia (CI) (5,10). It is commonly accepted that WI and CI do not affect all cell types equally, with hepatocytes being particularly susceptible to WI, while CI has a greater affect on the sinusoidal endothelium (11,12). However, numerous studies suggest interplay between the two and an overlap in their injury mechanisms. In a model of donation after cardiac death (DCD) Qing et al. found that the limits to CI - reflected in survival, histological and biochemical injury - are impacted by the duration of WI (13). They report that the limit for CI is reduced from 20 to 6 hours if WI is increased from 10 to 30 minutes.
The limitations of MP in recovering cold ischemically injured livers have not yet been examined. Moreover, it has not yet been attempted to define the limit of SCS in context of perfusion-preservation. Here, we expose livers to extended durations of SCS while monitoring various metabolic and hemodynamic parameters during subnormothermic machine perfusion (SNMP). In an orthotopic transplantation model we examine the effect of perfusion on survival and determine whether perfusion parameters reflect transplant outcome and thus define the limits to SCS with and without MP.
Materials and methods
Animals and experimental groups
Male and female Lewis rats (180–250g) were used for all experiments (Charles River Laboratories, Wilmington, MA). The animals were maintained in accordance with National Research Council guidelines and the experimental protocols were approved by the IACUC of Massachusetts General Hospital (Boston, MA, USA). The liver was procured and subjected to SCS for 0 (fresh), 24, 48, 72, and 120 hours after which the liver underwent 3 hours of subnormothermic machine perfusion (SNMP) the tissue was analyzed for adenosine triphosphate (ATP) content and compared to preservation without SNMP. For two groups selected, livers were orthotopically transplanted after 48 and 72 hours, with or without SNMP treatment (n=4 per group).
Liver procurement
Following anesthesia with isoflurane (Baxter, Deerfield, IL) the abdomen was opened by transverse incision. The right phrenic vein was ligated, and the liver was freed of its surrounding ligaments. The infrahepatic inferior vena cava (IHIVC) was mobilized, by ligating the adrenal vein, lumbar plexus and right renal vein. The bile duct was cannulated (Surflo 28-gauge polyethylene stent; Terumo Medical Corp, Somerset, NJ) and dissected. The gastroduodenal and splenic vein branches of the portal vein (PV) were ligated and dissected. The PV and IHIVC were cross-clamped, and the liver was flushed with cold University of Wisconsin (UW) solution (CoStorSol, Preservation Solutions, Inc, Elkhorn, WI). Cuffs fashioned from 16G and 14G catheters (Becton Dickinson, Franklin Lakes, NJ) were applied to the PV and IHIVC. The suprahepatic inferior vena cava (SHIVC) was tailored for a sutured anastomosis. Livers were then stored in UW solution in a temperature-controlled cold room (4 °C).
Sub-normothermic machine perfusion (SNMP)
After SCS, the liver was flushed with 10 mL of room temperature Williams medium E (WE, Sigma-Aldrich, St Louis, MO, USA) and placed in the organ chamber. The liver is perfused in a recirculating system that consists of a peristaltic Masterflex L/S pump (Cole Palmer, Vernon Hills, IL), a membrane oxygenator (Radnoti, Monrovia, CA) and a bubble trap (Radnoti). After the system was primed with WE, the liver was connected to the system by insertion of an 18G IV catheter (BD, Franklin Lakes, NJ) into the 14G portal vein cuff. Outflow flowed freely into the organ chamber. The total perfusate volume was 500 mL and consisted of WE supplemented with insulin (2 U/L Humulin; Eli Lilly & Co, Indianapolis, IN), penicillin (40,000 U/L)/streptomycin (40,000 μg/L) (Gibco/Invitrogen, Camarillo, CA), L-glutamine (0.292 g/L; Gibco/Invitrogen), hydrocortisone (10 mg/L, Pharmacia & Upjohn/Pfizer, New York, NY). Livers were perfused for 3 hours, followed by ATP analysis or orthotopic liver transplantation.
Liver transplantation
Recipients were anesthetized using isoflurane, 5% for induction, tapered off to 0.5% at the end of the procedure. Recipient hepatectomy commenced 20 minutes prior to the end of SNMP or SCS, after which the donor liver was flushed with sterile PBS containing 10 U/mL of heparin (APP pharmaceuticals, Schaumberg, IL). Implantation of the donor liver began with a sutured anastomosis of the SHVC using 7-0 prolene suture (Ethicon, Inc, Somerville, NJ, USA). Anastomosis of the PV and IHVC was achieved by inserting the cuffs into the recipient vessels as described by Kamada (14), allowing for an anhepatic period of 13–17 minutes. After completing the anastomosis of the bile duct the recipient was administered 1–2 mL of sterile PBS through the penile vein, the abdomen was irrigated and closed and finally the skin was closed and the animal was allowed to recover under a heat lamp.
Liver function, viability and metabolic assessment
Perfusate samples were taken from the outflow every 30 minutes and were analyzed for alanine aminotransferase using Infinity ALT liquid stable reagent (Thermo Electron, Victoria, Australia). Blood gas analysis was performed on PV inflow and IHIVC outflow every 30 minutes using a Rapidlab 845 blood gas analyzer (Bayer, Pittsburgh, PA) to determine the hepatic oxygen consumption using the perfusion flow (V), the concentration of oxygen in the inflow (O2-IN), outflow (O2-OUT), and the liver weight. Oxygen concentration (nM) was calculated as 0.0031 · pO2 · V. Vascular resistance over the portal vein was calculated by dividing the hydrostatic pressure on the vein by the flow rate. For determination of tissue ATP content, the liver was flash-frozen in liquid nitrogen and stored at -80 °C. Tissue was then crushed in liquid nitrogen and analyzed using an luminescence-based cell viability assay (Biovision, Milpitas, CA). ATP was normalized to protein content using a Coomassie brilliant blue (Bradford) assay (Fisher Scientific, Pittsburgh, PA, USA). Bile was collected throughout and quantified gravimetrically.
For the first week post-transplantation, daily blood samples (80 μL) were taken from surviving transplant recipients and every 5 days thereafter until 30 days post-transplantation. A metabolic panel was used in a Piccolo E?xpress chemistry analyzer (Abaxis, Union City, CA) to analyze aspartate aminotransferase (AST), ALT, total bilirubin, glucose, blood urea nitrogen (BUN) and albumin. Animals weight was monitored with the same interval as the blood sampling.
Statistical analysis
Linear regression and Pearson correlation coefficient (R) were used to analyze the relationship between ATP and SCS time. A two-tailed T-test was used to analyze the increase of ATP after perfusion and for change in vascular resistance from baseline. ATP content after different preservation times was analyzed using a one-way analysis of variance (1-way ANOVA). A log-rank (Mantel-Cox) test was used to analyze survival data between preservation groups and livers with high and low mean vascular resistance (>1.6 cm.H2O.min/mL). Vascular resistance and ALT release between groups over time was analyzed using a two-way analysis of variance (2-way ANOVA) with post-hoc Bonferroni corrections.
Results
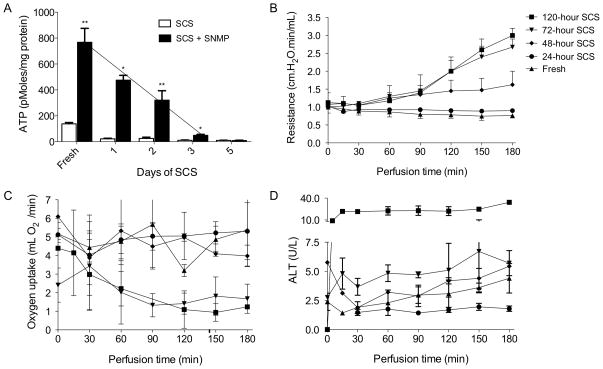
To determine easily measurable indicators of graft quality, perfusion parameters and post-perfusion energy status in the form of ATP concentration were determined after various durations of SCS. Confirming previous work, ATP content decreased with increased duration of preservation (Figure 1a). Moreover, a linear correlation between ATP content after perfusion and SCS time was observed up to 72 hours of preservation (r2=.99). SNMP was able to increase the ATP content of livers cold-stored for all durations except 120 hours, after which no significant improvement could be made (p < 3.2 x10−4). After 72 hours of storage, SNMP was no longer able to recover ATP to levels comparable of a fresh liver.
Figure 1. ATP and parameters during SNMP.
ATP content of fresh livers, after static cold storage (SCS; white bars) and after SCS with subnormothermic machine perfusion (SNMP; black bars)(A). Vascular resistance measured during SNMP of livers after SCS and fresh (B). Oxygen uptake rate from perfusate (C) and alanine transaminase release in to the perfusate (D). Error bars = SEM, * P<0.05 between SCS and SCS + SNMP **P<0.05 between SCS and SCS + SNMP and between duration of SCS and next measurement of longer duration of SCS.
While vascular resistance decreased slightly during perfusion of fresh livers and livers preserved for 24 hours, a gradual increase could be observed after longer SCS (Figure 1b). When livers were cold-stored for 72 or 120 hours a significant increase in resistance was seen from the start of perfusion (p<0.03). Compared to 48 hours of SCS, resistance was also higher after 72 and 120 hours (p<0.01). A high resistance profile during perfusion (mean vascular resistance >1.6 cm.H2O.min/mL) was associated with decreased survival after transplantation (p=0.025). Oxygen consumption showed a similar pattern with reduced oxygen uptake from the perfusate after 72 and 120 hours of SCS (Figure 1C). The release of ALT to the perfusate did not differ significantly between livers preserved for up to 72 hours; however, release of ALT was significantly increased after 120 hours (p=0.015; Figure 1D). Bile production during SNMP was negligible in all but the fresh group.
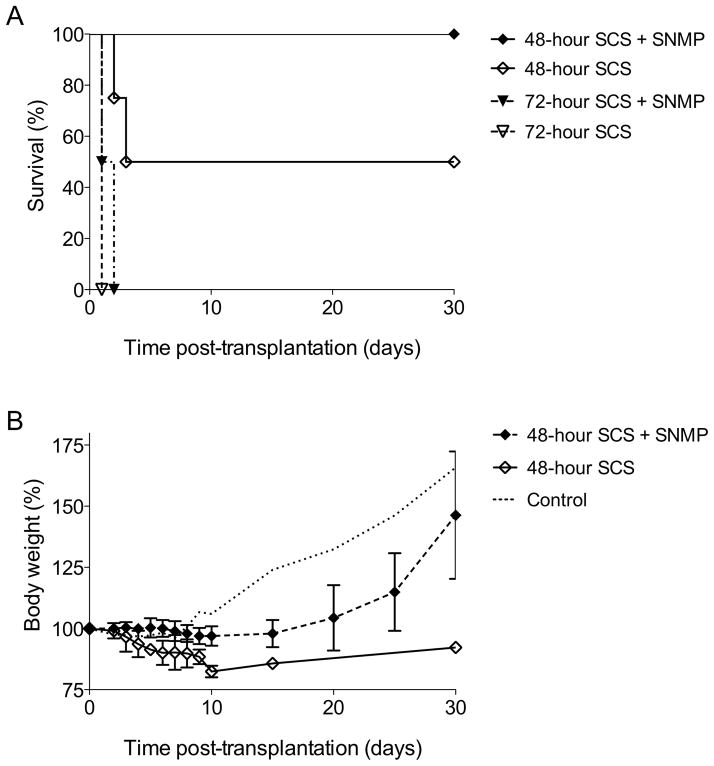
Livers were subsequently transplanted after 48 and 72 hours of SCS, with or without SNMP at the end of preservation. MP significantly improved 30-day survival after 48 hours of SCS (p=0.02), but not after 72 hours (Figure 2A). Long-term survival was 100% after 48 hours of SCS if recovered by 3 hours of SNMP, while 50% of the recipients did not survive the first week if the liver was not machine perfused. After 72 hours of SCS no recipients survived more than 2 days, regardless of treatment with SNMP. While all survivors lost weight in the first week after transplantation, recipients of machine perfused livers showed recovery and an increase in body weight beginning in the second week post-transplantation (Figure 2B). Survivors in the group without SNMP did not recover their body weight during the 30 days of follow-up. For comparison, the weight progression of recipients of livers cold-stored for 3 hours in UW solution are shown as a control group. Due to poor survival in most groups, there were no noteworthy significant differences in AST, ALT, total bilirubin, glucose, blood urea nitrogen (BUN) and albumin post-transplantation (not shown).
Figure 2. Post-transplantation survival and weight.
Kaplan-Meier survival curve of livers after transplantation. Livers were cold-stored (SCS) for 48 or 72 hours and underwent either subnormothermic machine perfusion (SNMP) or direct transplantation (A). Curve of weight progression after transplantation, normalized to percentage of weight at transplantation. The control curve shows the weight progression of recipients of livers cold-stored for 3 hours in UW solution (B). Error bars = SEM.
Discussion
In this study we examined several easily measureable parameters during subnormothermic machine perfusion after increasing duration of static cold storage to determine whether these parameters were able to reflect a limitation to cold preservation in a transplantation model. Moreover, in this model we determined whether applying SNMP could improve survival after extended SCS. We conclude that the parameters measured during SNMP, particularly ATP and vascular resistance, adequately reflect a significant loss in viability after more than 48 hours of storage, which was confirmed in the transplant survival results after 48 and 72 hours of SCS.
The most relevant indicator of viability appears to be ATP, which, while still recoverable after 48 hours, shows only minor improvement during SNMP after 72 hours of SCS. Hence, the minor ATP recovery after 72 hours of SCS corresponds to the lack of survival of these grafts after transplantation. While SNMP significantly recovers ATP after 48 hours and leads to a significant gain in survival, this is not seen after 72 hours, suggesting that CI injury has surpassed a limit of recoverability.
Improving the energy status of the liver before implantation has been reported as an important mechanism of MP (5,15). A reduction in energy status, most frequently ATP content, has often been correlated to the duration of ischemia (16,17). Clinically, the energy status after preservation has been demonstrated to correlate with transplant outcome, (18) however, this has been negated by others that have suggested that the recovery of ATP during reperfusion is a more important indicator (19). Additional studies have shown that the extent of ischemic injury directly affects the ability of the liver to recover ATP during oxygenated reperfusion, which is in turn linked to post-transplant survival (17,20) (21).
Since, ATP measurement requires a biopsy, less invasive alternatives have been sought as a surrogate index of viability. Bile flow rates a have been shown to correlate to ATP content and duration of ischemia and may be useful as a prognostic indicator (22–24). MP has been shown to improve bile secretion during reperfusion (25,26) and bile flow during MP may predict transplant success (27,28). In light of the extended preservation times we applied in this study, bile production was insufficient for reliable analysis. This is in part an effect of SNMP, as at room temperature bile production is reduced compared to normothermic sanguineous perfusion.
This study demonstrates focuses on improvement of the graft as a whole using SNMP. However, it has been shown that sinusoidal endothelial cells are more severely affected by cold conditions (12) and has also been shown to recover more slowly than hepatocytes following CI (29). In part, the increased vascular resistance that we observe during MP after prolonged SCS is likely to stem from a degree of endothelial dysfunction. Hepatic resistance should therefore be considered as an indicator of endothelial function rather than hepatocyte viability. Vascular resistance during MP is also associated with poor transplant outcome, with increased resistance predicting primary non-function post-transplantation (28). We confirm this association here through the observation of high vascular resistance with prolonged ischemic injury and a correlation between high resistance during SNMP and poor transplant outcome.
Conclusions
The goal of this study was to determine whether easily measurable machine perfusion parameters could be used to identify a time limit to cold liver preservation and whether MP could improve liver viability after extended preservation. Vascular resistance over the portal vein and ATP recovery after perfusion indicate that the ability of SNMP to recover cold ischemic livers greatly decreases after 48 hours of preservation. This limit is confirmed by transplantation, which is successful after 48, but not 72 hours of cold preservation. Moreover, this study demonstrates that SNMP can significantly improve the transplant outcome of livers after extended preservation.
Acknowledgments
Funding from the National Institutes of Health (R00DK080942, R01DK096075, R01EB008678), and the Shriners Hospitals for Children are gratefully acknowledged.
Footnotes
The Authors state that this manuscript has not been published previously and is not currently being assessed for publication by any journal other than the International Journal of Artificial Organs.
Each Author has contributed substantially to the research, preparation and production of the paper and approves of its submission to the Journal.
The Authors have not conflict of interest to declare.
The data presented has been presented at The Liver Meeting 2012 of the AASLD in poster session “Cellular Immunobiology, Preservation and Cell Transplantation” on November 10th 2012.
References
- 1.Todo S, Nery J, Yanaga K, Podesta L, Gordon RD, Starzl TE. Extended preservation of human liver grafts with UW solution. JAMA. 1989 Feb 3;261(5):711–4. [PMC free article] [PubMed] [Google Scholar]
- 2.Jamieson NV, Sundberg R, Lindell S, Claesson K, Moen J, Vreugdenhil PK, et al. Preservation of the canine liver for 24–48 hours using simple cold storage with UW solution. Transplantation. 1988 Oct;46(4):517–22. doi: 10.1097/00007890-198810000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Adam R, Bismuth H, Diamond T, Ducot B, Morino M, Astarcioglu I, et al. Effect of extended cold ischaemia with UW solution on graft function after liver transplantation. Lancet. 1992 Dec 5;340(8832):1373–6. doi: 10.1016/0140-6736(92)92559-x. [DOI] [PubMed] [Google Scholar]
- 4.Moore DE, Feurer ID, Speroff T, Gorden DL, Wright JK, Chari RS, Pinson CW. Impact of donor, technical, and recipient risk factors on survival and quality of life after liver transplantation. Arch Surg. 2005 Mar;140(3):273–7. doi: 10.1001/archsurg.140.3.273. [DOI] [PubMed] [Google Scholar]
- 5.Xu H, Berendsen T, Kim K, Soto-Gutiérrez A, Bertheium F, Yarmush ML, Hertl M. Excorporeal normothermic machine perfusion resuscitates pig DCD livers with extended warm ischemia. J Surg Res. 2011 Oct 24; doi: 10.1016/j.jss.2011.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berendsen TA, Bruinsma BG, Lee J, D’Andrea V, Qiang L, Izamis M, et al. A simplified subnormothermic machine perfusion model restores ischemically damaged liver grafts in a rat model of orthotopic liver transplantation. Transplant Res. 2012 May 9;1(6) doi: 10.1186/2047-1440-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dutkowski P, Furrer K, Tian Y, Graf R, Clavien PA. Novel short-term hypothermic oxygenated perfusion (HOPE) system prevents injury in rat liver graft from non-heart beating donor. Ann Surg. 2006 Dec;244(6):968–76. doi: 10.1097/01.sla.0000247056.85590.6b. discussion 976–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perk S, Izamis ML, Tolboom H, Uygun B, Yarmush ML, Uygun K. A fitness index for transplantation of machine-perfused cadaveric rat livers. BMC Res Notes. 2012;5:325. doi: 10.1186/1756-0500-5-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutkowski P, Graf R, Clavien PA. Rescue of the cold preserved rat liver by hypothermic oxygenated machine perfusion. Am J Transplant. 2006 May;6(5 Pt 1):903–12. doi: 10.1111/j.1600-6143.2006.01264.x. [DOI] [PubMed] [Google Scholar]
- 10.Tolboom H, Milwid JM, Izamis ML, Uygun K, Berthiaume F, Yarmush ML. Sequential cold storage and normothermic perfusion of the ischemic rat liver. Transplant Proc. 2008 Jun;40(5):1306–9. doi: 10.1016/j.transproceed.2008.03.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schön MR, Kollmar O, Akkoc N, Matthes M, Wolf S, Schrem H, et al. Cold ischemia affects sinusoidal endothelial cells while warm ischemia affects hepatocytes in liver transplantation. Transplant Proc. 1998 Aug;30(5):2318–20. doi: 10.1016/s0041-1345(98)00638-1. [DOI] [PubMed] [Google Scholar]
- 12.Ikeda T, Yanaga K, Kishikawa K, Kakizoe S, Shimada M, Sugimachi K. Ischemic injury in liver transplantation: Difference in injury sites between warm and cold ischemia in rats. Hepatology. 1992 Aug;16(2):454–61. doi: 10.1002/hep.1840160226. [DOI] [PubMed] [Google Scholar]
- 13.Qing DK. Prolonging warm ischemia reduces the cold preservation limits of liver grafts in swine. Hepatobiliary Pancreat Dis Int. 2006 Nov;5(4):515–20. [PubMed] [Google Scholar]
- 14.Kamada N, Calne RY. Orthotopic liver transplantation in the rat. Technique using cuff for portal vein anastomosis and biliary drainage. Transplantation. 1979 Jul;28(1):47–50. [PubMed] [Google Scholar]
- 15.de Rougemont O, Breitenstein S, Leskosek B, Weber A, Graf R, Clavien PA, Dutkowski P. One hour hypothermic oxygenated perfusion (HOPE) protects nonviable liver allografts donated after cardiac death. Ann Surg. 2009 Nov;250(5):674–83. doi: 10.1097/SLA.0b013e3181bcb1ee. [DOI] [PubMed] [Google Scholar]
- 16.Berendsen TA, Izamis ML, Xu H, Liu Q, Hertl M, Berthiaume F, et al. Hepatocyte viability and adenosine triphosphate content decrease linearly over time during conventional cold storage of rat liver grafts. Transplant Proc. 2011 Jun;43(5):1484–8. doi: 10.1016/j.transproceed.2010.12.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ma Y, Wu LW, Wu JL, Liang YJ, Zhu ZY, Hu RD, He XS. Energy metabolism and survival of liver grafts from non-heart-beating donor rats with warm ischemia injury. Hepatobiliary Pancreat Dis Int. 2006 Nov;5(4):521–5. [PubMed] [Google Scholar]
- 18.Lanir A, Jenkins RL, Caldwell C, Lee RG, Khettry U, Clouse ME. Hepatic transplantation survival: Correlation with adenine nucleotide level in donor liver. Hepatology. 1988;8(3):471–5. doi: 10.1002/hep.1840080306. [DOI] [PubMed] [Google Scholar]
- 19.Kamiike W, Burdelski M, Steinhoff G, Ringe B, Lauchart W, Pichlmayr R. Adenine nucleotide metabolism and its relation to organ viability in human liver transplantation. Transplantation. 1988 Jan;45(1):138–43. doi: 10.1097/00007890-198801000-00030. [DOI] [PubMed] [Google Scholar]
- 20.Morimoto T, Kusumoto K, Isselhard W. Impairment of grafts by short-term warm ischemia in rat liver transplantation. Transplantation. 1991 Sep;52(3):424–31. doi: 10.1097/00007890-199109000-00006. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell SJ, Churchill TA, Winslet MC, Fuller BJ. Energy metabolism following prolonged hepatic cold preservation: Benefits of interrupted hypoxia on the adenine nucleotide pool in rat liver. Cryobiology. 1999 Sep;39(2):130–7. doi: 10.1006/cryo.1999.2191. [DOI] [PubMed] [Google Scholar]
- 22.Tolboom H, Pouw RE, Izamis ML, Milwid JM, Sharma N, Soto-Gutierrez A, et al. Recovery of warm ischemic rat liver grafts by normothermic extracorporeal perfusion. Transplantation. 2009 Jan 27;87(2):170–7. doi: 10.1097/TP.0b013e318192df6b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karwinski W, Husøy AM, Farstad M, Søreide O. Sixty minutes of normothermic ischemia in the rat liver: Correlation between adenine nucleotides and bile excretion. J Surg Res. 1989 Feb;46(2):99–103. doi: 10.1016/0022-4804(89)90210-2. [DOI] [PubMed] [Google Scholar]
- 24.Sumimoto K, Inagaki K, Yamada K, Kawasaki T, Dohi K. Reliable indices for the determination of viability of grafted liver immediately after orthotopic transplantation. Bile flow rate and cellular adenosine triphosphate level. Transplantation. 1988 Oct;46(4):506–9. doi: 10.1097/00007890-198810000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Imber CJ, St Peter SD, Lopez de Cenarruzabeitia I, Pigott D, James T, Taylor R, et al. Advantages of normothermic perfusion over cold storage in liver preservation. Transplantation. 2002 Mar 15;73(5):701–9. doi: 10.1097/00007890-200203150-00008. [DOI] [PubMed] [Google Scholar]
- 26.Ferrigno A, Rizzo V, Boncompagni E, Bianchi A, Gringeri E, Neri D, et al. Machine perfusion at 20°C reduces preservation damage to livers from non-heart beating donors. Cryobiology. 2011 Apr;62(2):152–8. doi: 10.1016/j.cryobiol.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 27.Rojas A, Chen L, Bartlett RH, Arenas JD. Assessment of liver function during extracorporeal membrane oxygenation in the non-heart beating donor swine. Transplant Proc. 2004 Jun;36(5):1268–70. doi: 10.1016/j.transproceed.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 28.Brockmann J, Reddy S, Coussios C, Pigott D, Guirriero D, Hughes D, et al. Normothermic perfusion: A new paradigm for organ preservation. Ann Surg. 2009 Jul;250(1):1–6. doi: 10.1097/SLA.0b013e3181a63c10. [DOI] [PubMed] [Google Scholar]
- 29.Wang L, Florman S, Roayaie S, Basile J, Zhang ZY, Machac J, et al. Differential in vivo recovery of sinusoidal endothelial cells, hepatocytes, and kupffer cells after cold preservation and liver transplantation in rats. Transplantation. 1998 Sep 15;66(5):573–8. doi: 10.1097/00007890-199809150-00004. [DOI] [PubMed] [Google Scholar]