Abstract
OBJECTIVE
Hyper-IgE recurrent infection syndrome (HIES or Job’s syndrome) is a rare disorder affecting the immune system and connective tissues. The purpose of this study is to describe the coronary abnormalities in genetically confirmed HIES patients as depicted by coronary MDCT angiography (MDCTA).
CONCLUSION
Coronary MDCTA has provided an opportunity for noninvasive evaluation of the coronary arteries in patients with HIES. These coronary abnormalities vary from tortuosity to ectatic dilation and focal aneurysms of the coronary arteries. Such an evaluation has potential value in identifying new aspects of this disease and thereby providing better understanding of the pathophysiology of the disorder.
Keywords: coronary abnormalities, coronary aneurysms, ectatic coronaries, hyper-IgE recurrent infection syndrome (HIES or Job’s syndrome), coronary MDCT angiography
Coronary MDCT angiography (MDCTA) is a valuable tool for the detection of coronary atherosclerotic disease (CAD) [1] and congenital anomalies [2] of the coronary arterial system. The use of this evolving technology potentially may allow noninvasive assessment of coronary arteries in many more disease processes not historically associated with CAD. Such a comprehensive evaluation of the coronary artery tree may contribute to the understanding of the pathophysiology and potential detection of new aspects of these diseases.
Individuals with hyper-IgE recurrent infection syndrome (HIES or Job’s syndrome), a rare disorder affecting the immune system and connective tissues, may benefit from a noninvasive method of coronary artery examination. HIES is characterized by eczema, recurrent infections of the skin and lungs, and elevated levels of serum IgE [3]. Connective tissue and skeletal abnormalities manifest as characteristic facial features, joint hyperextensibility, scoliosis, osteopenia, retained primary denture, and others [3]. This autosomal-dominant syndrome results primarily from mutations of the SH2 and DNA-binding domains of STAT3 [4, 5].
Coronary artery abnormalities in HIES were first described by Ling et al. [6] in two patients. The authors identified aneurysmal dilation involving the left anterior descending coronary artery (LAD) and long ectatic segments of the right coronary artery (RCA). Since that report, two other patients with HIES and coronary artery aneurysm have been described [7, 8]. However, these case reports have been limited in the number of patients, and correlation with STAT3 mutations was not performed. The purpose of this study is to examine and describe the coronary artery abnormalities in a larger number of patients with genetically confirmed HIES using coronary MCCTA.
Materials and Methods
A total of nine patients with HIES determined by an HIES clinical score that combines immunologic and nonimmunologic features [3, 9] and STAT3 mutations were included in the study. All patients gave signed informed consent for the study, which was approved by the local institutional review board and was compliant with HIPAA regulations. Patient histories were obtained for coronary artery disease risk factors including hypertension, hypercholesterolemia, tobacco use, and family history of atherosclerotic coronary artery disease as well as cardiac medications, including antihypertensives and cholesterol-lowering medications. All patients underwent genetic sequence analysis from isolated polymorphonuclear leukocytes and peripheral blood mononuclear cells from venous blood samples using methods described in prior studies [5].
Coronary MDCTA was performed on all patients using a 16-MDCT scanner (Brilliance 16, Philips Healthcare). The coronary MDCTA protocol was similar to previously described techniques using identical equipment [1, 2]. Briefly, 50–100 mg of oral metoprolol was given 30–60 minutes before coronary MDCTA when needed to lower the heart rate below 65 beats per minute [1] and was administered successfully in all patients. Nitroglycerin was not used. Coronary MDCTA was performed using a tube voltage of 120 kV and current of 400–500 mAs with a 220-mm field of view and retrospective ECG gating without dose modulation. Nonionic contrast material (120–130 mL of iopamidol, Isovue, Bracco Diagnostics) was injected through an 18- to 20-gauge peripheral venous access at a rate of 4–5 mL/s followed by 50 mL of normal saline at the same injection rate. Postprocessing, analysis, and interpretation of the axial and the multiplanner reformatted images were performed in consensus by two readers on a 3D software tool (Virtual Place, AZE). Both readers were blinded to the HIES clinical scores and genetic analyses. The readers had 7 and 3 years of coronary CT interpretative experience. Using the modified 17-segment model of the American Heart Association reporting system, analyses included the identification of coronary abnormalities such as atherosclerosis (calcified or noncalcified), coronary dilation (ectasia or aneurysm), and tortuosity. Ectasia was defined as enlargement of the coronary vessel diameter to more than 4 mm or a diameter of a segment measuring more than 1.5 times that of an adjacent segment [10]. An aneurysm was defined as focal enlargement of the coronary diameter of more than 5 mm [10]. Although more difficult to define, a vessel was considered tortuous when it formed an unusual S, C, or U configuration [11].
Results
The patients ranged in age from 30 to 55 years (mean, 44 years) and five of nine were men. STAT3 mutations of the SH2 domain were identified in six of the nine patients and of the DNA-binding domain in the remaining three patients (Table 1). None of the patients had the same STAT3 mutation. The HIES clinical scores ranged from 58 to 96, with scores of at least 40 considered consistent with the disease [3, 9].
TABLE 1.
Characteristics of Nine Patients With Hyper-IgE Recurrent Infection Syndrome (HIES)
| Patient No. | STAT3 Mutation (Domain) | HIES Clinical Scorea | Age (y) | Sex | Coronary Artery Disease Risk Factors |
|---|---|---|---|---|---|
|
| |||||
| 1 | 1939 A–G N647D (SH2) | 62 | 30 | M | Hypertension |
| 2 | 1861 T–G F621 V (SH2) | 96 | 36 | F | Hypertension |
| 3 | 1909 G–A V637 M (SH2) | 93 | 37 | F | Hypertension |
| 4 | 1145 G–A R382Q (DNA) | 79 | 45 | M | Hypertension, hypercholesterolemia, smoking |
| 5 | 1437 G–T W479C (DNA) | 58 | 45 | F | Smoking |
| 6 | 1387 delGTG V463del (DNA) | 69 | 45 | M | Hypercholesterolemia, hypertension |
| 7 | 1970 A–G Y657C (SH2) | 79 | 50 | M | Hypercholesterolemia, hypertension |
| 8 | 1954 G–A E652 K (SH2) | 69 | 53 | F | Hypercholesterolemia, smoking |
| 9 | 1865 C–T T622I (SH2) | 92 | 55 | M | Hypercholesterolemia, hypertension |
HIES clinical scores ranged from 58 to 96, with scores of at least 40 being considered consistent with the disease [3, 9]. Clinical findings included in the score are height of IgE, degree of eosinophilia, skin abscesses, pneumonias, parenchymal lung abnormalities, serious and fatal infections, newborn rash, eczema, sinusitis or otitis, candidiasis, retained primary teeth, scoliosis, minimal trauma fractures, hyperextensibility, characteristic facial appearance, and lymphoma.
Six of the nine patients showed some form of coronary arterial abnormality. All six patients with coronary abnormalities showed involvement of the RCA. The RCA was tortuous in one patient and both tortuous and ectatic in the remaining five patients. As the degree of dilation of the RCA increased, the degree of tortuosity decreased (Figs. 1 and 2). The ectatic segments of the RCA were smooth dilations without focal aneurysms. Segment 1 was involved in all six patients who showed these findings, and additional involvement of segment 2 was seen in four patients.
Fig. 1.
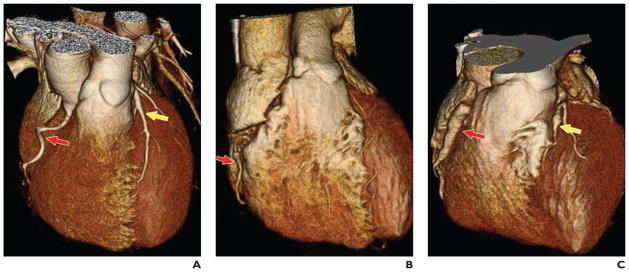
3D shaded surface display images of whole heart and coronary arteries in three patients with hyper-IgE recurrent infection syndrome.
A, Image in 30-year-old man shows tortuous right coronary artery (RCA) (red arrow) and ectatic left anterior descending coronary artery (LAD) (yellow arrow).
B, Image in 37-year-old woman shows less tortuous but ectatic RCA (arrow).
C, Image in 45-year-old man shows relatively straightened RCA (red arrow) in comparison with A and B and aneurysm of LAD (yellow arrow).
Fig. 2.
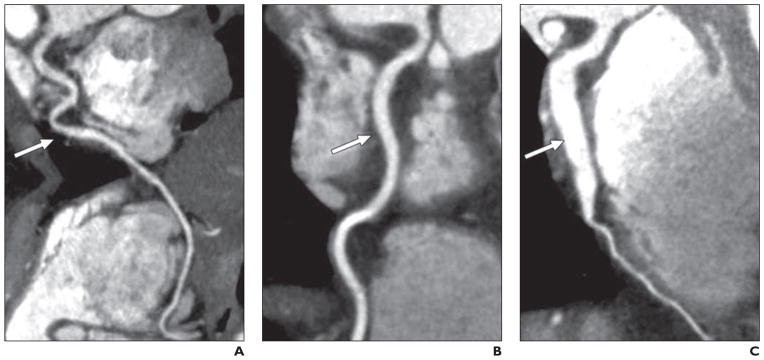
Multiplanar reconstruction stretch views of right coronary artery (RCA) in patients with hyper-IgE recurrent infection syndrome.
A, Image in 37-year-old woman shows tortuous RCA (arrow).
B, Image in 53-year-old woman shows RCA (arrow) that is less tortuous but more dilated than A, measuring up to 5 mm in diameter.
C, Image in 45-year-old man shows severely dilated RCA (arrow) with minimal tortuosity, measuring up to 8 mm in diameter.
The proximal–mid LAD was abnormally dilated in these six patients (Figs. 1 and 3). The LAD findings were smooth ectatic short segments in three patients or a focal aneurysm seen in two patients. The proximal–mid LAD of one patient was ectatic with a small focal aneurysm. The rest of the distal LAD was normal in the six patients without unusual tortuosity. In one of these patients, the second diagonal branch of the LAD showed a small focal aneurysm. The left main coronary artery and left circumflex coronary artery were normal in all patients. These findings were seen in segment 6 in six patients, and additional involvement of segment 7 and the proximal aspect of segment 8 was seen in two patients with additional segment 10 dilation in one patient. The mean diameters of the dilated vessels were (± SD) 5.6 ± 1.3 mm for the RCA and 5.7 ± 1.3 mm for the LAD.
Fig. 3.
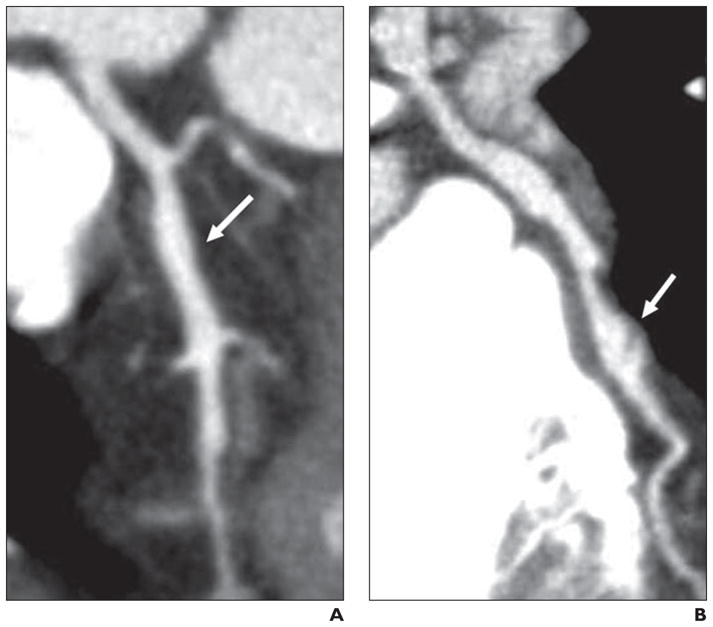
Multiplanar reconstruction stretch views of left anterior descending coronary artery (LAD) in patients with hyper-IgE recurrent infection syndrome.
A, Image in 53-year-old woman shows ectatic proximal–mid segment of LAD (arrow) measuring up to 4.5 mm in diameter compared with more proximal portion of vessel, which measures 2.5 mm.
B, Image in 45-year-old man shows ectatic segment of proximal LAD and aneurysm of mid LAD (arrow) measuring up to 7.5 mm in diameter with filling defect, suggestive of clot.
No coronary calcification or significant atherosclerosis was identified on coronary MDCTA in any of the patients despite the presence of at least one risk factor for CAD in all. Only one patient had cardiac-related symptoms. This patient had a history of myocardial infarction leading to diagnostic imaging by coronary MDCTA and conventional coronary angiography. Both diagnostic techniques showed severe dilation of segments 1 and 2 of the RCA, ectasia of segments 6 and 7, and aneurysm of the proximal aspect of segments 8 and 10 (second diagonal) of the LAD. This patient also had a small filling defect in the LAD aneurysm, suggestive of a clot.
Discussion
This study identifies and describes the various coronary arterial abnormalities seen in patients with HIES. To the best of our knowledge, this study describes the findings on coronary MDCTA in the largest group of genetically confirmed HIES patients. These coronary abnormalities, which were seen in six of nine patients, vary from tortuosity of the RCA to ectatic dilation and focal aneurysms of both the RCA and LAD. Despite the presence of at least one risk factor for CAD in all patients, none showed coronary calcifications or significant atherosclerotic coronary artery disease on coronary MDCTA. This finding virtually excludes atherosclerosis, the most common culprit, as a cause of the coronary abnormalities [12].
Additionally, the appearance of the RCA findings that include tortuosity and smooth ectatic dilations hints more toward a connective tissue abnormality as a cause. However, this study was not designed to determine the specificity of coronary artery tortuosity in HIES or correlation with the various aspects of connective tissue abnormalities, such as joint hyperextensibility and severe scoliosis, that are exhibited in patients with HIES [3, 5]. These characteristics are also identified in heritable connective tissue disorders such as Ehlers-Danlos [13] and Marfan [14] syndromes, which exhibit various degrees of vascular aneurysms. Although these patients are prone to multiple infections, the fusiform rather than saccular appearance of these aneurysms suggests a noninfectious cause. That the patients were largely asymptomatic from the abnormalities also suggests a noninfectious cause. However, pathologic assessment of these coronary arteries would be required to verify this suggestion and to determine the definite cause, the lack of which is a limitation of this study.
Other limitations of our study are the relatively small number of patients and the lack of matched controls to truly identify the incidence and clinical significance of these findings in HIES patients. Despite these limitations, the presence of coronary abnormalities in six of nine patients with STAT3 mutations is clearly more common than previously expected or described. Future work into the function of STAT3 in vascular remodeling as well as longitudinal studies including younger patients will assist in determining the natural history of these abnormalities and consideration of possible therapies. Another limitation is the lack of conventional coronary angiographic correlation. However, one patient who suffered a myocardial infarction did undergo diagnostic conventional angiography, which showed findings similar to those seen on coronary MDCTA.
Coronary MDCTA has provided an opportunity for the noninvasive evaluation of the coronary arteries in patients with HIES. Such an evaluation has potential value in identifying new aspects of this disease and thereby providing better understanding of the pathophysiology of the disorder. Such developments are important for better patient management and care.
Acknowledgments
This project was funded in part by the National Cancer Institute, National Institutes of Health, under contracts N01-CO-12400 and HHSN26120080000. The project was also supported in part by the National Institute of Allergy and Infectious Diseases and the National Institute of Diabetes and Digestive and Kidney Diseases.
The authors thank Joie Davis for her contribution in clinical scoring.
Footnotes
The content of this article does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the United States Government.
References
- 1.Garcia MJ, Lessick J, Hoffmann MH. Accuracy of 16-row multidetector computed tomography for the assessment of coronary artery stenosis. JAMA. 2006;296:403–411. doi: 10.1001/jama.296.4.403. [DOI] [PubMed] [Google Scholar]
- 2.Datta J, White CS, Gilkeson RC, et al. Anomalous coronary arteries in adults: depiction at multi-detector row CT angiography. Radiology. 2005;235:812–818. doi: 10.1148/radiol.2353040314. [DOI] [PubMed] [Google Scholar]
- 3.Grimbacher B, Holland SM, Gallin JI, et al. Hyper-IgE syndrome with recurrent infections: an autosomal dominant multisystem disorder. N Engl J Med. 1999;340:692–702. doi: 10.1056/NEJM199903043400904. [DOI] [PubMed] [Google Scholar]
- 4.Minegishi Y, Saito M, Tsuchiya S, et al. Dominant-negative mutations in the DNA-binding domain of STAT3 cause hyper-IgE syndrome. Nature. 2007;448:1058–1062. doi: 10.1038/nature06096. [DOI] [PubMed] [Google Scholar]
- 5.Holland SM, DeLeo FR, Elloumi HZ, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med. 2007;357:1608–1619. doi: 10.1056/NEJMoa073687. [DOI] [PubMed] [Google Scholar]
- 6.Ling JC, Freeman AF, Gharib AM, et al. Coronary artery aneurysms in patients with hyper IgE recurrent infection syndrome. Clin Immunol. 2007;122:255–258. doi: 10.1016/j.clim.2006.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alomar-Melero E, Martin TD, Janelle GM, Peng YG. An unusual giant right coronary artery aneurysm resembles an intracardiac mass. Anesth Analg. 2008;107:1161–1162. doi: 10.1213/ane.0b013e318181f74f. [DOI] [PubMed] [Google Scholar]
- 8.Young TY, Jerome D, Gupta S. Hyperimmunoglobulinemia E syndrome associated with coronary artery aneurysms: deficiency of central memory CD4+ T cells and expansion of effector memory CD4+ T cells. Ann Allergy Asthma Immunol. 2007;98:389–392. doi: 10.1016/S1081-1206(10)60887-3. [DOI] [PubMed] [Google Scholar]
- 9.Grimbacher B, Schaffer AA, Holland SM, et al. Genetic linkage of hyper-IgE syndrome to chromosome 4. Am J Hum Genet. 1999;65:735–744. doi: 10.1086/302547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arnold R, Ley S, Ley-Zaporozhan J, et al. Visualization of coronary arteries in patients after childhood Kawasaki syndrome: value of multidetector CT and MR imaging in comparison to conventional coronary catheterization. Pediatr Radiol. 2007;37:998–1006. doi: 10.1007/s00247-007-0566-2. [DOI] [PubMed] [Google Scholar]
- 11.Bullitt E, Gerig G, Pizer SM, Lin W, Aylward SR. Measuring tortuosity of the intracerebral vasculature from MRA images. IEEE Trans Med Imaging. 2003;22:1163–1171. doi: 10.1109/TMI.2003.816964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Manginas A, Cokkinos DV. Coronary artery ectasias: imaging, functional assessment and clinical implications. Eur Heart J. 2006;27:1026–1031. doi: 10.1093/eurheartj/ehi725. [DOI] [PubMed] [Google Scholar]
- 13.Germain DP. The vascular Ehlers-Danlos syndrome. Curr Treat Options Cardiovasc Med. 2006;8:121–127. doi: 10.1007/s11936-006-0004-z. [DOI] [PubMed] [Google Scholar]
- 14.Judge DP, Dietz HC. Marfan’s syndrome. Lancet. 2005;366:1965–1976. doi: 10.1016/S0140-6736(05)67789-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


