Abstract
Background
Preclinical data suggest synergistic activity of bortezomib, gemcitabine, and liposomal doxorubicin. Because tolerance to therapy may be attenuated in elderly patients, we performed an age-stratified phase I trial of this combination.
Patients and Methods
Two parallel age-stratified arms (<65 and ≥65 years old) were accrued (3+3 design). Starting doses included bortezomib 0.7 mg/m2 (days 1 and 8), gemcitabine 500 mg/m2 (days 1 and 8) and liposomal doxorubicin 20 mg/m2 (day 1).
Results
In the <65-year old group, 65 patients were treated; the maximum tolerated dose was bortezomib 1.3 mg/m2, gemcitabine 800 mg/m2, and liposomal doxorubicin 35 mg/m2. In the ≥65-year old group, 28 patients were treated; the recommended phase II dose was bortezomib 1.0 mg/m2, gemcitabine 800 mg/m2, and liposomal doxorubicin 20 mg/m2. Dose-limiting toxicities included thrombocytopenia and neutropenia. The most common toxicities were mild cytopenias, fatigue, and neuropathy. Ten patients achieved partial responses (6 of 7 patients with cutaneous T-cell lymphoma; 4 of 16 patients with small cell carcinomas, including lung, prostate, ovarian, and nasopharyngeal).
Conclusion
Combination bortezomib, gemcitabine and liposomal doxorubicin is well-tolerated, but with a lower recommended phase II dose in elderly patients, and demonstrated antitumor activity, especially in T-cell and small cell histology malignancies.
Keywords: Bortezomib, gemcitabine, liposomal doxorubicin, cutaneous T-cell lymphoma, small cel carcinoma, phase I
INTRODUCTION
Bortezomib, is a specific and reversible proteasome inhibitor that is also a potent NF-κB inhibitor and potentiates the activity of chemotherapy in various tumors in vitro and in mouse models.1, 2 Preclinical studies suggest that through the prevention of IκB degradation, bortezomib may block chemotherapy-induced NF-kB activation and augment the apoptotic response to chemotherapeutic agents.3, 4
Gemcitabine is a nucleoside analog that exhibits cell phase specificity, primarily killing cells undergoing DNA synthesis (S phase) and also blocking the progression of cells through the G1/S phase boundary.5–7 Gemcitabine is metabolized to the active diphosphate (dFdCDP) and triphosphate (dFdCTP) nucleosides, which inhibit DNA synthesis, by nucleoside kinases inside the cell.8
The liposomal formulation of doxorubicin is characterized by a very long circulation half-life, favorable pharmacokinetic behavior, and specific accumulation in tumor tissues.9 Liposomal encapsulation of doxorubicin may reduce both the nonspecific drug delivery to normal tissues as well as the high peak plasma levels of free drug responsible for its toxicity.10 These features account for lower toxicity profile of liposomal doxorubicin, including differences in cardiotoxicity, vesicant effects, nausea, vomiting, alopecia, and myelosuppression.11
Bortezomib, a potent proteasome and NF-κB inhibitor, potentiates the activity of chemotherapy in diverse tumors in vitro and in mouse models, and clinical and preclinical data suggest that combinations of bortezomib, gemcitabine, and liposomal doxorubicin are synergistic, especially when liposomal doxorubicin is administered before bortezomib.10, 12–17 The therapeutic potential of this combination is especially attractive because these anti-neoplastic agents have different mechanisms of action.
In addition to the synergistic activity and good tolerance observed in the doublet combinations, this three-drug combination is attractive because each individual drug has known antitumor activity in multiple tumor types.10, 12–17 Bortezomib has antitumor activity in patients with multiple myeloma and mantle cell lymphoma. Gemcitabine has antitumor activity in patients with breast, pancreatic, bladder, ovarian, non-small cell lung cancer, and lymphoma. Doxorubicin has antitumor activity in patients with breast, bladder, ovarian, endometrial, thyroid, gastric, small cell lung, sarcomas, neuroblastoma, Wilm's tumor, lymphoma, leukemia, and multiple myeloma. The combination of these three agents offers the potential to overcome tumor resistance to each individual drug.
Herein we describe the first trial that combines these three agents. Phase I trials enroll a heterogeneous patient population, and determination of the maximum tolerated dose (MTD) for a new drug or drug combination may be influenced by characteristics of the patients enrolled. Since tolerance to combination therapy may be attenuated in elderly patients, we designed our phase I trial in an age-stratified fashion to evaluate the toxicity, safety, and preliminary antitumor activity of combination bortezomib, gemcitabine, and liposomal doxorubicin in patients with advanced malignancy.
METHODS
Inclusion and Exclusion Criteria
Patient eligibility criteria included patients with histologic proof of advanced cancer, who were not candidates for known regimens or protocol treatments of higher efficacy or priority, unless the standard therapy includes one or more of the drugs in this protocol; estimated life expectancy of at least 12 weeks; performance status of ≤ 2 (Zubrod scale); measurable disease, as defined by Response Evaluation Criteria in Solid Tumors 1.0 (RECIST), by the World Health Organization (WHO) for lymphomas, or by the modified Severity-Weighted Assessment Tool (mSWAT) for cutaneous T-cell lymphomas; adequate function of bone marrow (absolute neutrophil count > 1,500, platelets > 100,000), liver (bilirubin ≤ 1.5 mg/dL, serum glutamic pyruvic transaminase (SGPT) < 3x normal), kidney (creatinine ≤ 1.5 mg/dL), and heart (ejection fraction ≥ 50%). Patients must have been off all previous chemotherapy or radiotherapy for 3 weeks. All patients signed consent in accordance with the guidelines of the MD Anderson Cancer Center Institutional Review Board.
Study Design, Toxicity Assessment, Treatment Plan, and Determination of Maximum Tolerated Dose / Recommended Phase II Dose
Two parallel age-stratified arms (< 65 and ≥ 65 years old) were accrued, with at least three patients per dose level per arm. All three patients were evaluated for at least three weeks before starting additional patients in that arm on the next dose level. Patients in the ≥ 65-year old group were begun on a dose level only after three patients in the < 65-year old group had been evaluated at that dose level for at least three weeks. The < 65-year old arm and the ≥ 65-year old arm followed the same rules for dose level expansion, determining dose-limiting toxicities (DLTs), and establishing the maximum tolerated dose (MTD).
Toxicity was assessed with the Common Terminology Criteria for Adverse Events v3.0 (CTCAE). DLT was defined as any non-hematologic toxicity grade 3 or greater except for alopecia or grade 4 nausea adequately treated with a well-tolerated antiemetic; grade 2 motor neuropathy; grade 4 neutropenia in the presence of granulocyte colony-stimulating factor (G-CSF); grade 3 neutropenia accompanied by infection; grade 4 thrombocytopenia lasting more than two weeks; grade 4 thrombocytopenia accompanied by bleeding; failure to recover absolute neutrophil count (ANC) to ≥1500/uL or platelets to ≥ 100,000/uL by week 5, even after G-CSF is given; persistent grade 3 nausea, vomiting, or diarrhea that is adequately prophylaxed with antiemetics and/or antidiarrheals. DLT was assessed during the first cycle.
Dose levels were escalated in cohorts of three untreated patients as long as no DLT was observed. If one patient was observed to suffer a DLT at a particular dose level, this cohort was expanded. If no additional patients in the expanded cohort of six patients experienced a DLT, dose escalation resumed. If a second patient enrolled at the same dose level in a cohort of up to six patients experienced a DLT, the MTD was considered to have been exceeded, and dose escalations were ceased. The next lower dose level was considered the MTD and an additional six patients were treated at the MTD dose level for each arm to determine a more accurate toxicity profile for that age group. Dose escalation was not permitted for individual patients.
Patients with grade 3 non-hematologic toxicity (except for alopecia or medication-controlled nausea) had treatment held until resolution of toxicity to ≤ grade 1 and then resumed treatment at the next lower dose level. If ≥ grade 3 thrombocytopenia and/or grade 4 neutropenia occurred on day 8, the day 8 gemcitabine dose was held during that course and the bortezomib doses on days 8 and 11 were held during that course. If in the presence of G-CSF, grade 4 neutropenia recurred and was accompanied by infection, then subsequent courses of gemcitabine were reduced by 50%.
If palmar/plantar dysesthesia occurred, the next liposomal doxorubicin dose was delayed until resolution to ≤ grade 1. If grade 3 palmar/plantar dysesthesia occurred, the liposomal doxorubicin dose was decreased by 25% when treatment resumed. Liposomal doxorubicin dose was adjusted for toxicity as follows: bilirubin 1.6 – 3 mg/dL, give 50% of normal dose; bilirubin > 3 mg/dL, give 25% of normal dose. Recovery to ANC ≥ 1500/uL and platelet count ≥ 100,000/uL was required before starting the next cycle. Patients with neuropathy had a dose adjustment of bortezomib as per prespecified guidelines.
The starting doses were bortezomib 0.7 mg/m2 (days 1 and 8), gemcitabine 500 mg/m2 (days 1 and 8) and liposomal doxorubicin 20 mg/m2 (day 1). Subsequent dose levels escalated the dose of the individual drugs in a stair-step fashion, as shown in Table 1. Liposomal doxorubicin was administered before gemcitabine, and bortezomib was given after gemcitabine on the same day. Liposomal doxorubicin was infused over 2 hours, gemcitabine was infused over 30 minutes, and bortezomib was given as a 3 – 5 second IV push. Each treatment cycle was 21 days. Additional patients were permitted to enroll on dose levels deemed to be safe, if antitumor activity had been observed for that cancer type. All patients were included in the safety analysis.
Table 1.
Dose Schema for Bortezomib, Gemcitabine, and Liposomal Doxorubicin
| Dose Level | Bortezomib | Gemcitabine | Liposomal doxorubicin |
|---|---|---|---|
| 1 | 0.7 mg/m2 Days 1 and 8 | 500 mg/m2 Days 1 and 8 | 20 mg/m2 Day 1 |
| 2 | 0.7 mg/m2 Days 1, 4, 8 and 11 | 500 mg/m2 Days 1 and 8 | 20 mg/m2 Day 1 |
| 3 | 0.7 mg/m2 Days 1, 4, 8 and 11 | 800 mg/m2 Days 1 and 8 | 20 mg/m2 Day 1 |
| 4 | 1.0 mg/m2 Days 1, 4, 8 and 11 | 800 mg/m2 Days 1 and 8 | 20 mg/m2 Day 1 |
| 5 | 1.0 mg/m2 Days 1, 4, 8 and 11 | 800 mg/m2 Days 1 and 8 | 24 mg/m2 Day 1 |
| 6 | 1.3 mg/m2 Days 1, 4, 8 and 11 | 800 mg/m2 Days 1 and 8 | 24 mg/m2 Day 1 |
| 7 | 1.3 mg/m2 Days 1, 4, 8 and 11 | 800 mg/m2 Days 1 and 8 | 30 mg/m2 Day 1 |
| 8 | 1.3mg/m2 Days 1, 4, 8 and 11 | 800 mg/m2 Days 1 and 8 | 35 mg/m2 Day 1 |
| 9 | 1.3 mg/m2 Days 1, 4, 8 and 11 | 800 mg/m2 Days 1 and 8 | 40 mg/m2 Day 1 |
Dose level 8 was identified as the recommended phase II dose for patients < 65 years old
Dose level 4 was identified as the recommended phase II dose for patients ≥ 65 years old
Treatment continued until disease progression, intercurrent illness that prevented further administration of treatment, unacceptable adverse event(s), patient decision to withdraw from the study, or total cumulative dose of doxorubicin plus liposomal doxorubicin exceeded 550 mg/m2 (or 400 mg/m2 in patients who received radiotherapy to the mediastinum).
Baseline and Treatment Assessments
At baseline, patients were assessed with a complete history and physical, CBC, SMA-12, cardiac scan (MUGA) or echocardiogram; electrocardiogram (ECG); serum β-hCG for premenopausal women; appropriate radiologic tests; bone marrow for patients with lymphoid malignancies. Radiologic tests were obtained within one month and all other tests within two weeks before initiating treatment.
During the first two cycles of treatment, patients were assessed with history and physical exam at least once per cycle, CBC prior to each dose of bortezomib, SMA-12 weekly, ECG after two cycles, and appropriate radiologic and tumor marker studies after two cycles.
During subsequent cycles, patients were assessed with history and physical at least once per cycle, CBC and SMA-12 at least every two weeks, ECG and appropriate radiologic and tumor markers every two months, cardiac scan (MUGA) or echocardiogram after every 100 mg/m2 increments of liposomal doxorubicin whenever total past anthracycline dose is ≥ 300 mg/m2. For patients with no prior anthracycline exposure, cardiac scan was obtained when cumulative liposomal doxorubicin dose = 300 mg/m2 and every 100 mg/m2 thereafter. If during treatment the LVEF fell to ≤ 45% or by 20% from the baseline level, liposomal doxorubicin was discontinued.
RESULTS
Patient Characteristics
Ninety-three patients were enrolled (65 patients in the ≥ 65-year old group and 28 in the < 65-year old group) (Table 2). The mean number of prior systemic treatments was 3.7 in the < 65-year old group and 4.0 in the ≥ 65-year old group. The majority of patients in both age groups had Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1. The most common tumor types enrolled were small cell lung carcinoma, colorectal carcinoma, breast adenocarcinoma, and cutaneous T-cell lymphoma.
Table 2.
Patient Characteristics (n=93)
| Characteristic | Arm < 65 years (N = 65) | Arm ≥ 65 years (N = 28) |
|---|---|---|
| Sex | ||
| Male | 27 (42%) | 14 (50%) |
| Female | 38 (58%) | 14 (50%) |
|
| ||
| Age | ||
| Median (Range) | 54 (2 – 64) | 69 (65 – 82) |
|
| ||
| ECOG Performance status | ||
| 0 | 12 (18%) | 5 (18%) |
| 1 | 42 (65%) | 18 (64%) |
| 2 | 11 (17%) | 4 (14%) |
|
| ||
| Prior treatment | ||
| Systemic therapy | 65 (100%) | 27 (96%) |
| Phase I trial enrollment | 13 (20%) | 6 (21%) |
| Radiotherapy | 39 (60%) | 14 (50%) |
| Surgery | 44 (68%) | 18 (64%) |
|
| ||
| Number of prior systemic regimens | ||
| Mean (Range) | 3.7 (1 – 9) | 4 (1 – 9) |
|
| ||
| Primary tumor type | ||
| Appendiceal adenocarcinoma | 1 | 0 |
| Bladder / renal pelvis (TCC) | 1 | 0 |
| Breast | 8 | 0 |
| Cervical | 1 | 0 |
| Colorectal | 6 | 6 |
| Endometrial | 1 | 1 |
| Esophageal / gastric | 2 | 1 |
| Head and neck | ||
| Other | 3 | 1 |
| Small cell sinonasal | 1 | 0 |
| Squamous cell | 4 | 1 |
| Thyroid, papillary | 1 | 0 |
| Lung | ||
| Non-small cell | 4 | 3 |
| Small cell | 11 | 2 |
| Lymphoma | ||
| B cell | 2 | 1 |
| Cutaneous T-cell | 4 | 3 |
| Hodgkins | 1 | 0 |
| T cell other | 1 | 0 |
| Melanoma | 3 | 2 |
| Multiple myeloma | 1 | 1 |
| Neuroendocrine | 1 | 1 |
| Ovarian | ||
| Epithelial | 1 | 0 |
| Small cell | 1 | 0 |
| Pancreatic adenocarcinoma | 1 | 0 |
| Primitive neuroectodermal | 1 | 0 |
| Prostate: | ||
| Adenocarcinoma | 0 | 2 |
| Small cell | 0 | 1 |
| Renal cell | 0 | 2 |
| Sarcoma | 2 | 0 |
| Unknown primary | 2 | 0 |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; TCC, transitional cell carcinoma
Toxicity and Recommended Dose
All patients treated were evaluated for toxicity (Table 3). The DLTs in the < 65-year old group included grade 4 thrombocytopenia with bleeding (2 patients), grade 4 neutropenia with sepsis (1 patient), grade 4 neutropenia with wound infection (1 patient), and grade 3 neutropenia with grade 4 mucositis (1 patient). The DLTs in the ≥ 65-year old group included grade 4 thrombocytopenia with bleeding (1 patient), grade 3 hyperbilirubinemia (1 patient), grade 3 elevated AST (1 patient), and grade 3 fatigue (1 patient).
Table 3.
DLTs and Grade 3 or 4 Toxicities at Least Possibly Druq-Related
| Dose Level | No. of patients treated | No. of patients with G3/4 toxicity | No. of patients with DLTs | Grade 3 and 4 Toxicities |
|---|---|---|---|---|
| < 65 vearold qroup | ||||
| 1 | 3 | 3 | 0 | G3-4 thrombocytopenia (2) |
| G4 infection (1) | ||||
| G3 neutropenia (1) | ||||
| G3 anemia (2) | ||||
|
| ||||
| 2 | 4 | 2 | 0 | G4 thrombocytopenia (1) |
| G3 non-neutropenic fever (1) | ||||
| G3 gastrointestinal bleeding (1) | ||||
| G3 anemia (2) | ||||
| G3 bleeding skin lesion (1) | ||||
|
| ||||
| 3 | 3 | 1 | 0 | G3 infection / urosepsis (1) |
| G3 CNS ischemia (TIA after catheter removal) (1) | ||||
|
| ||||
| 4 | 3 | 1 | 0 | G3 thrombocytopenia (1) |
|
| ||||
| 5 | 6 | 3 | 1 | G4 thrombocytopenia with bleeding (1) (DLT) |
| G4 foot infection (1) | ||||
| G3 non-neutropenic fever (1) | ||||
| G3 chest pain (1) | ||||
|
| ||||
| 6 | 4 | 2 | 0 | G3/4 elevated ALT/AST(1) |
| G3 lower abdominal pain (1) | ||||
| G3 lipase(1) | ||||
|
| ||||
| 7 | 6 | 3 | 0 | G3/4 thrombocytopenia (4) |
| G4 neutropenia (1) | ||||
| G3 nausea/vomiting (1) | ||||
| G3 anemia (1) | ||||
|
| ||||
| 8 | 30 | 8 | 1 | G4 thrombocytopenia with bleeding (1) (DLT) |
| G3/4 thrombocytopenia (6) | ||||
| G4 non-neutropenic infection (1) | ||||
| G3 elevated ALT/AST (2) | ||||
|
| ||||
| 9 | 6 | 4 | 3 | G3/4 neutropenia with infection or mucositis (3) (DLT) |
| G3/4 thrombocytopenia (4) | ||||
| G3 anemia (1) | ||||
| G3 mucositis (1) | ||||
| G3 fatigue (1) | ||||
| G3 neuropathy (1) | ||||
|
| ||||
| ≥ 65 vear old qroup | ||||
|
| ||||
| 1 | 6 | 6 | 1 | G4 thrombocytopenia with bleeding (1) (DLT) |
| G3 hyperbilirubinemia (1) (DLT) | ||||
| G4 pericardial effusion (1) | ||||
| G4CNS ischemia (1) | ||||
| G4 neutropenia (1) | ||||
| G3 non-neutropenic fever (1) | ||||
| G3 thrombocytopenia (2) | ||||
| G3 anemia (1) | ||||
|
| ||||
| 2 | 3 | 3 | 0 | G3/4 thrombocytopenia (3) |
| G3 CHF (1) | ||||
| G3 anemia (1) | ||||
| G3 UTI (1) | ||||
|
| ||||
| 3 | 3 | 3 | 0 | G4 gastrointestinal bleeding (1) |
| G4 non-neutropenic fever (1) | ||||
| G3 neutropenia (1) | ||||
| G3 anemia (2) | ||||
| G3 thrombocytopenia (1) | ||||
|
| ||||
| 4 | 8 | 4 | 1 | G3 fatigue (1) (DLT) |
| G3 neutropenia (1) | ||||
|
| ||||
| 5 | 3 | 0 | 0 | |
|
| ||||
| 6 | 3 | 3 | 0 | G4 thrombocytopenia (2) |
| G3 fatigue (1) | ||||
| G3 anemia (2) | ||||
|
| ||||
| 7 | 2 | 2 | 1 | G3 elevated ALT/AST (1) (DLT) |
| G4 thrombocytopenia (1) | ||||
| G3 neuropathy (1) | ||||
Abbreviations: ALT, alanine transaminase; AST, aspartate aminotransferase; CHF, congestive heart failure; CNS, central nervous system; DLT, Dose limiting toxicity; DVT, deep vein thrombosis; G1, grade 1; G2, grade 2; G3, grade 3; G3/4, grade 3 or 4; G4, grade 4; TIA, transient ischemic attack; UTI, urinary tract infection
In the < 65-year old group, the MTD was identified as dose level 8, which included bortezomib 1.3 mg/m2 (days 1, 4, 8, 11), gemcitabine 800 mg/m2 (days 1, 8), and liposomal doxorubicin 35 mg/m2 (day 1) (Table 3). At higher doses, the incidence of DLTs was > 33%.
In the ≥ 65-year old group, the MTD was more difficult to define. There were initially three patients treated at dose level 4 and among these patients there was one DLT (fatigue). A dose expansion was then performed to six patients at dose level 4, with no further DLTs. Dose escalation continued to dose level 7, with one DLT at level 7 (elevated AST). However, review of the data subsequently revealed that patients on dose levels 5, 6, and 7 virtually always needed to miss doses or have dose reductions, albeit outside the DLT window (cycle 1). Therefore, it was felt that safely administering this regimen to elderly patients was possible only at dose levels up to dose level 4. An additional two patients (total = 8) were then added to dose level 4, for a total of eight patients on that dose level. These patients were added as part of the dose expansion that included patients with responsive diseases, and also to obtain further safety data in this elderly group. No further DLTs were encountered in this group. Therefore, dose level 4 (bortezomib 1.0 mg/m2, gemcitabine 800 mg/m2, liposomal doxorubicin 20 mg/m2) was determined to be the recommended phase II dose for patients ≥ 65-year old.
Overall, the most common drug-related toxicities observed in the < 65-year old group included fatigue (43%), infection (38%), thrombocytopenia (32%), anemia (23%), and nausea (22%). The most common drug-related toxicities observed in the ≥ 65-year old group included anemia (50%), fatigue (50%), thrombocytopenia (40%), neuropathy (29%), elevated AST (29%), dyspnea (28%), nausea (25%), constipation (21%). Fifty-one patients (55%) experienced no drug-related toxicities greater than grade 2.
The ≥ 65-year old group experienced a higher percentage of grade 3 or 4 anemia (29% vs. 9%), elevated AST (18% vs. 6%), fatigue (11% vs. 2%), and neutropenia (11% vs.5%). Each group had similar rates of grade 3 or 4 thrombocytopenia (25% vs. 23%) and non-neutropenic fever (11% vs. 8%).
Antitumor Activity
As mentioned, enrollment of additional patients at the highest dose levels deemed safe was permitted if antitumor activity had been observed for that disease type. Overall, 92 of 93 patients (99%) were evaluable for response. (Figure 1) One patient withdrew early due to financial issues. In addition, six patients came off treatment early for toxicity and were considered treatment failures.
Figure 1.
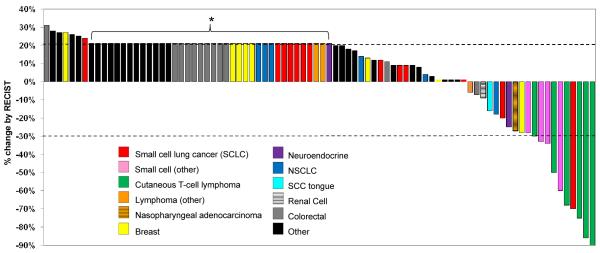
Best response in 86 of 93 patients treated. Patients with new lesions or clinical progression were designated in the figure as 21% progression (asterisk). 7 patients are not included because of early withdrawal from the study, either due to toxicity (n=6) or financial inconvenience (n=1).
Eighty-six patients are therefore shown in Figure 1, which includes all patients who were restaged at once, as well as patients who progressed before restaging. Of the 92 evaluable patients, ten (11%) had a partial response (PR), including six of seven patients (86%) with cutaneous T-cell lymphoma, and four of 16 patients (25%) with small cell histology carcinomas (lung, prostate, ovarian, nasopharyngeal), (Table 4; Figure 2a, 2b, 3a). The median duration of PRs was 6 cycles (range, 3 to 10 cycles). The median number of prior systemic therapies among patients with PRs was 3 regimens (range, 1 to 9 regimens). Stable disease (SD) lasting ≥ 6 cycles was achieved in an additional 12 patients (13%) (Table 4).
Table 4.
Patients with Complete/Partial Responses or Stable Disease ≥ 6 Cycles
| Pt # | Dose level | Age (yrs) | Diagnosis | Best Response | Cycles received* | # of prior systemic therapies |
|---|---|---|---|---|---|---|
| < 65 arm | ||||||
| 23 | 5 | 53 | CTCL | −90% (PR) | 6 | 4 |
| 29 | 5 | 60 | CTCL | −75% (PR) | 4 | 1 |
| 16 | 3 | 64 | Small cell lung | −70% (PR) | 10 | 3 |
| 8 | 1 | 32 | CTCL | −68% (PR) | 3 | 5 |
| 104 | 8 | 45 | Small cell nasopharyngeal | −34% (PR) | 6 | 2 |
| 93 | 8 | 24 | Small cell ovarian | −33% (PR) | 8 | 1 |
| 92 | 8 | 50 | Small cell sinonasal | −28% (SD) | 6 | 9 |
| 81 | 8 | 64 | Breast | −28% (SD) | 7 | 8 |
| 21 | 4 | 64 | NSCLC | −18% (SD) | 12 | 3 |
| 57 | 8 | 59 | Squamous cell tongue | −16% (SD) | 7 | 4 |
| 50 | 9 | 47 | Breast | +1%(SD) | 6 | 6 |
| 103 | 8 | 62 | NSCLC | +4% (SD) | 12 | 4 |
| 18 | 4 | 53 | TCC bladder | +12% (SD) | 10 | 5 |
| ≥ 65 arm | ||||||
| 4 | 1 | 71 | CTCL | −86% (PR) | 4 | 9 |
| 79 | 4 | 69 | Small cell prostate | −58% (PR) | 9 | 3 |
| 26 | 3 | 72 | CTCL | −50% (PR) | 8 | 2 |
| 33 | 4 | 73 | CTCL | −30% (PR) | 6 | 4 |
| 94 | 6 | 69 | Neuroendocrine | −25% (SD) | 15 | 0 |
| 17 | 2 | 81 | RCC | −9% (SD) | 12 | 3 |
| 25 | 3 | 76 | Colorectal | −7% (SD) | 9 | 4 |
| 28 | 3 | 67 | Adenoid cystic | +9% (SD) | 6 | 1 |
| 2 | 1 | 76 | TCC renal pelvis | +18% (SD) | 10 | 4 |
Abbreviations: CTCL, cutaneous T-cell lymphoma; NSCLC, non-small cell lung carcinoma; PR, partial response; Pt, patient; yrs, years; RCC, renal cell carcinoma; SD, stable disease
1 cycle = 3 weeks
Figure 2a.
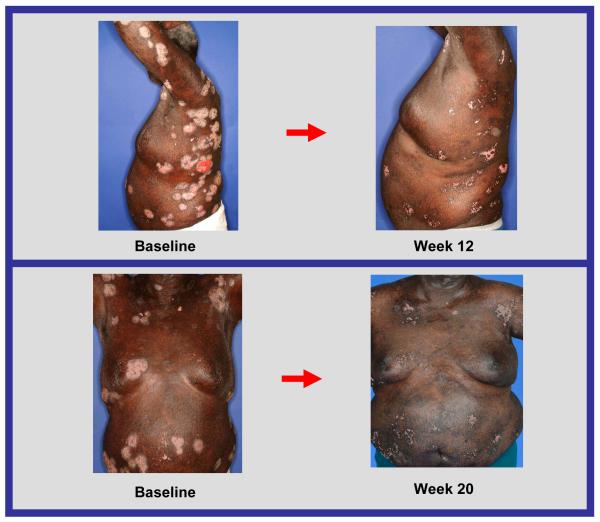
Response to treatment in a patient with cutaneous T-cell lymphoma
Figure 2b.
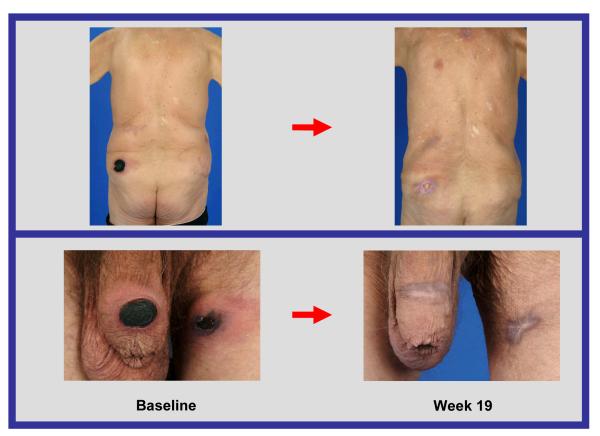
Response to treatment in a patient with cutaneous T-cell lymphoma
DISCUSSION
The combination regimen of bortezomib, gemcitabine, and liposomal doxorubicin was well tolerated. Only one patient, in the ≥ 65-year old group, developed clinically significant heart failure. No other cardiac toxicity was observed. Cytopenias, especially thrombocytopenia, were the most prominent laboratory toxicity, which resulted in frequent dose delays or dose reductions, especially at doses higher than dose level 4, in the older patient group.
Six out of the seven patients (86%) with cutaneous T-cell lymphoma achieved partial responses, despite receiving extensive prior systemic therapy (median number of prior systemic therapies was 4), and these responses were achieved even at low dose levels. In some cases, remarkable improvement in skin lesions was observed (Figure 2a, 2b).
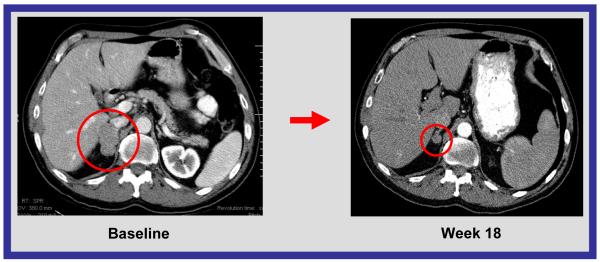
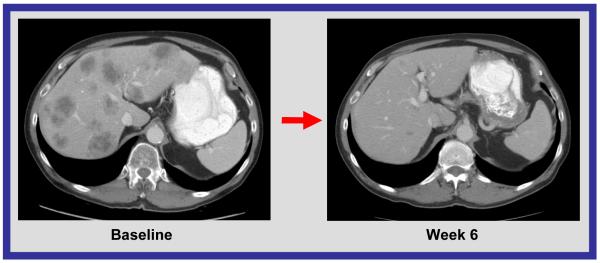
Significant responses in small cell histology solid tumor malignancies (4 of 16 patients) were also observed, including a 70% decrease in a patient with small cell lung carcinoma, a 58% decrease in a patient with small cell carcinoma of the prostate, 33% decrease in a patient with small cell carcinoma of the ovary, and a 34% decrease in a patient with small cell carcinoma of the nasopharynx (Figure 2c, 2d). The role of bortezomib in these responses should be considered. Preclinical studies demonstrated that bortezomib reduced Bcl-2 levels and induced apoptosis in small cell lung cancer cell lines.18 Bcl-2 is reported to be overexpressed in up to 90% of small cell lung cancer (SCLC) tumors and is associated with chemotherapy resistance.19 The ability of bortezomib to overcome Bcl-2-mediated resistance to apoptosis and to stabilize the proapoptotic Bax, a binding partner of Bcl-2, are two of the proposed mechanisms by which bortezomib is thought to be of potential therapeutic benefit in SCLC19 (21). However, bortezomib as a single agent has minimal activity in SCLC, suggesting that the combination may have been key. Extrapulmonary small cell carcinoma is rare, with approximately 1,000 cases diagnosed each year in the United States.20 Irrespective of their site of origin, small cell cancers share distinctive histochemical and electron microscopic features and aggressive clinical behavior.20 Because few effective treatments are available for patients with metastatic disease, and because of the responses observed in heavily pretreated patients, further evaluation may be warranted.
Figure 2c.
CT scan of abdomen. Response to treatment in a patient with small cell lung carcinoma. 70% decrease by RECIST.
Figure 2d.
CT scan of abdomen. Response to treatment in liver metastases in a patient with small cell carcinoma of the prostate. 58% decrease by RECIST.
In most cases, the recommended phase II dose for a new drug or drug combination is based upon observation of toxicity in a limited number of patients. Even a cursory review of the methods employed to enroll patients on phase I trials yields the conclusion that more than one MTD must exist for a new drug or drug combination.
This study demonstrates preliminary evidence for the potential role of age stratification in the design of phase I clinical trials. As predicted, toxicities developed earlier in the elderly cohort. This phenomenon has not been extensively studied in previous phase I trials, although age-dependent dosing of standard chemotherapies has been investigated in recent studies. Elderly patients with breast cancer may benefit equally from standard chemotherapy regimens, but may suffer greater side effects than their younger counterparts.21 In contrast, a recent study demonstrated that selected elderly patients with colon cancer can receive the same benefit from fluorouracil-based adjuvant therapy as their younger counterparts, without a significant increase in toxic effects.22 Nevertheless, several lines of evidence suggest that older individuals metabolize drugs more slowly.23, 24 Therefore, it is plausible that even if lower doses of certain drugs are administered in older patients, the same downstream effect may be achieved.
Although the recommended phase II dose for the younger age group was dose level 8, and the recommended phase II dose for the older patients was dose level 4, the differences between these dose levels was relatively modest: bortezomib 1.3 mg/m2 vs. 1.0 mg/m2; liposomal doxorubicin, 35 mg/m2 vs. 20 mg/m2; and gemcitabine, 800 mg/m2 for both age groups.
SUMMARY
In summary, our results suggest that incorporation of age-stratification or other risk factor stratification strategies into early trials, with the goal of identification of alternative recommended phase II doses for various subpopulations, may be warranted to maximize patient safety. Antitumor activity in heavily pretreated patients with advanced malignancies (T-cell lymphomas, small cell histology malignancies) was observed with the combination regimen of bortezomib, gemcitabine, and liposomal doxorubicin, and further evaluation at the age-dependent recommended phase II doses identified in this trial may be worthwhile.
ACKNOWLEDGMENTS
We would like to thank Christine Eberle for assistance with manuscript preparation and Joann Aaron for editorial assistance.
FUNDING No funding was received for this work
Footnotes
The authors have no conflict of interests to disclose.
Prior presentation of results: American Society of Clinical Oncology Annual Meeting 2007, poster presentation
REFERENCES
- 1.Papandreou CN, Daliani DD, Nix D, Yang H, Madden T, Wang X, et al. Phase I trial of the proteasome inhibitor bortezomib in patients with advanced solid tumors with observations in androgen-independent prostate cancer. J Clin Oncol. 2004;22(11):2108–21. doi: 10.1200/JCO.2004.02.106. [DOI] [PubMed] [Google Scholar]
- 2.Hamilton AL, Eder JP, Pavlick AC, Clark JW, Liebes L, Garcia-Carbonero R, et al. Proteasome inhibition with bortezomib (PS-341): a phase I study with pharmacodynamic end points using a day 1 and day 4 schedule in a 14-day cycle. J Clin Oncol. 2005;23(25):6107–16. doi: 10.1200/JCO.2005.01.136. [DOI] [PubMed] [Google Scholar]
- 3.Heinz-Josef L. Clinical update: proteasome inhibitors in solid tumors. Cancer Treat Rev. 2003;29(Suppl 1):41–48. doi: 10.1016/s0305-7372(03)00082-3. [DOI] [PubMed] [Google Scholar]
- 4.Paramore A, Frantz S. Bortezomib. Nat Rev Drug Discov. 2003;2(8):611–2. doi: 10.1038/nrd1159. [DOI] [PubMed] [Google Scholar]
- 5.Huang P, Chubb S, Hertel LW, Grindey GB, Plunkett W. Action of 2',2'-difluorodeoxycytidine on DNA synthesis. Cancer Res. 1991;51(22):6110–7. [PubMed] [Google Scholar]
- 6.Plunkett W, Huang P, Gandhi V. Preclinical characteristics of gemcitabine. Anticancer Drugs. 1995;6(Suppl 6):7–13. doi: 10.1097/00001813-199512006-00002. [DOI] [PubMed] [Google Scholar]
- 7.Plunkett W, Huang P, Xu YZ, Heinemann V, Grunewald R, Gandhi V. Gemcitabine: metabolism, mechanisms of action, and self-potentiation. Semin Oncol. 1995;22(4 Suppl 11):3–10. [PubMed] [Google Scholar]
- 8.Risbood PA, Kane CT, Jr., Hossain MT, Vadapalli S, Chadda SK. Synthesis of gemcitabine triphosphate (dFdCTP) as a tris(triethylammonium) salt. Bioorg Med Chem Lett. 2008;18(9):2957–8. doi: 10.1016/j.bmcl.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siegal T, Horowitz A, Gabizon A. Doxorubicin encapsulated in sterically stabilized liposomes for the treatment of a brain tumor model: biodistribution and therapeutic efficacy. J Neurosurg. 1995;83(6):1029–37. doi: 10.3171/jns.1995.83.6.1029. [DOI] [PubMed] [Google Scholar]
- 10.Rivera E, Valero V, Syrewicz L, Rahman Z, Esteva FJ, Theriault RL, et al. Phase I study of stealth liposomal doxorubicin in combination with gemcitabine in the treatment of patients with metastatic breast cancer. J Clin Oncol. 2001;19(6):1716–22. doi: 10.1200/JCO.2001.19.6.1716. [DOI] [PubMed] [Google Scholar]
- 11.Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal Doxorubicin: review of animal and human studies. Clin Pharmacokinet. 2003;42(5):419–36. doi: 10.2165/00003088-200342050-00002. [DOI] [PubMed] [Google Scholar]
- 12.Mitsiades N, Mitsiades CS, Richardson PG, Poulaki V, Tai YT, Chauhan D, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101(6):2377–80. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 13.Kamat AM, Karashima T, Davis DW, Lashinger L, Bar-Eli M, Millikan R, et al. The proteasome inhibitor bortezomib synergizes with gemcitabine to block the growth of human 253JB-V bladder tumors in vivo. Mol Cancer Ther. 2004;3(3):279–90. [PubMed] [Google Scholar]
- 14.Ryan DP, Appleman LJ, Lynch T, Supko JG, Fidias P, Clark JW, et al. Phase I clinical trial of bortezomib in combination with gemcitabine in patients with advanced solid tumors. Cancer. 2006;107(10):2482–9. doi: 10.1002/cncr.22264. [DOI] [PubMed] [Google Scholar]
- 15.Luu TCW, Lim D, et al. Phase I trial of fixed-dose rate gemcitabine in combination with bortezomib in advanced solid tumors. Anticancer Res. 2010 Jan;30(1):167–74. [PubMed] [Google Scholar]
- 16.Dees EC, O'Neil BH, Lindley CM, Collichio F, Carey LA, Collins J, et al. A phase I and pharmacologic study of the combination of bortezomib and pegylated liposomal doxorubicin in patients with refractory solid tumors. Cancer Chemother Pharmacol. 2008;63(1):99–107. doi: 10.1007/s00280-008-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera E, Valero V, Arun B, Royce M, Adinin R, Hoelzer K, et al. Phase II study of pegylated liposomal doxorubicin in combination with gemcitabine in patients with metastatic breast cancer. J Clin Oncol. 2003;21(17):3249–54. doi: 10.1200/JCO.2003.03.111. [DOI] [PubMed] [Google Scholar]
- 18.Mortenson MM, Schlieman MG, Virudachalam S, Lara PN, Gandara DG, Davies AM, et al. Reduction in BCL-2 levels by 26S proteasome inhibition with bortezomib is associated with induction of apoptosis in small cell lung cancer. Lung Cancer. 2005;49(2):163–70. doi: 10.1016/j.lungcan.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 19.Davies AM, Lara PN, Jr., Mack PC, Gandara DR. Incorporating bortezomib into the treatment of lung cancer. Clin Cancer Res. 2007;13(15 Pt 2):s4647–51. doi: 10.1158/1078-0432.CCR-07-0334. [DOI] [PubMed] [Google Scholar]
- 20.Walenkamp AMSG, Sleijfer DT. Clinical and therapeutic aspects of extrapulmonary small cell carcinoma. Cancer Treat Rev. 2009;35(3):228–36. doi: 10.1016/j.ctrv.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Muss HB, Woolf S, Berry D, Cirrincione C, Weiss RB, Budman D, et al. Adjuvant chemotherapy in older and younger women with lymph node-positive breast cancer. JAMA. 2005;293(9):1073–81. doi: 10.1001/jama.293.9.1073. [DOI] [PubMed] [Google Scholar]
- 22.Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, et al. A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med. 2001;345(15):1091–7. doi: 10.1056/NEJMoa010957. [DOI] [PubMed] [Google Scholar]
- 23.O'Malley K, Crooks J, Duke E, Stevenson IH. Effect of age and sex on human drug metabolism. Br Med J. 1971;3(5775):607–9. doi: 10.1136/bmj.3.5775.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lipshitz DAGS, Reis R, et al. Cancer in the Elderly: Basic Science and Clinical Aspects. Ann Intern Med. 1985;(102):218–28. doi: 10.7326/0003-4819-102-2-218. [DOI] [PubMed] [Google Scholar]