Abstract
Galectins released by tumor cells and macrophages can bind surface glycoproteins of tumor-infiltrating lymphocytes (TILs), forming glycoprotein-galectin lattices with immunosuppressive activities. Specifically, TILs covered by galectin-3 are unable to secrete cytokines after stimulation. Treating TILs ex vivo with galectin antagonists for a few hours boosts their functions. Several galectin antagonists are currently available for clinical trials.
Keywords: galectin, tumor-infiltrating lymphocyte, tumor, immunosuppression, human, dysfunction, glycobiology
Tumor-Infiltrating Lymphocytes (TILs) Are Dysfunctional
Many cancer patients mount a spontaneous antitumor T-cell response. However, evidence that the tumor microenvironment is immunosuppressive is accumulating. Tumor-infiltrating lymphocytes (TILs) freshly isolated from a variety of human tumor samples have often proven defective in lysing relevant target cells and secreting interferon-γ (IFNγ). TILs usually express immune checkpoint receptors, such as cytotoxic T lymphocyte associated protein 4 (CTLA4) and programmed cell death-1 (PDCD1, better known as PD-1), molecular features of exhausted lymphocytes. Blocking these receptors with antibodies has been shown to prolong survival of T cells and boost their proliferation upon activation in vitro. Such antibodies have shown their efficacy in a trial with advanced melanoma patients.1
Galectin Antagonists Boost Human TIL Functions
Galectins are lectins and thus recognize sugar moieties. By oligomerizing and crosslinking glycoproteins at the surface of T cells, galectins form glycoprotein-galectin lattices that impede the motility of receptors important for T-cell functions. We proposed several years ago that extracellular galectin-3 is responsible for deficiencies in TIL functions.2,3 Galectin-3 is secreted by cancer cells, macrophages, and activated T cells, and can accumulate in the tumor microenvironment. Treating CD8+ or CD4+ TILs freshly isolated from solid tumors or carcinoma ascites with galectin antagonists or with an anti-galectin-3 antibody, even for just a few hours, markedly increased their IFNγ secretion and cytotoxicity.2,3
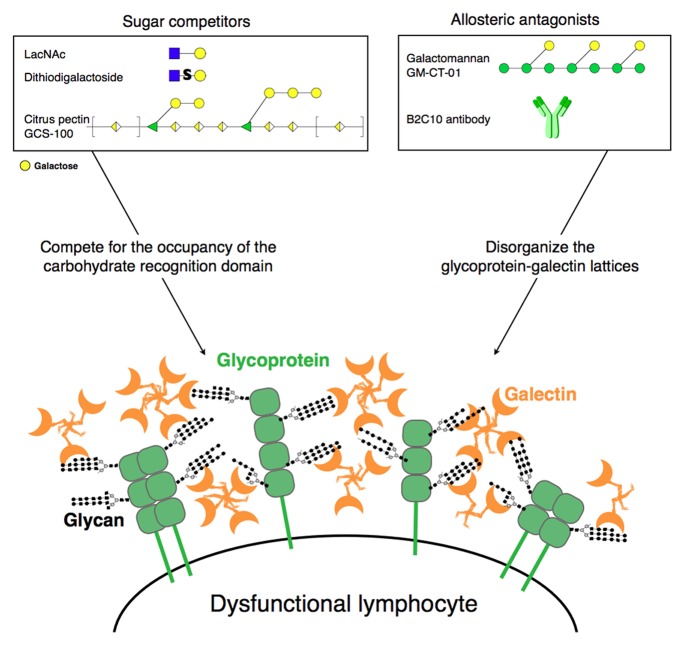
Galectin-1 and galectin-3 recognize N-acetyl-lactosamine (LacNAc) motifs. Detaching galectin-1 and galectin-3 from cells can be achieved with competing sugars such as lactose, LacNAc, some dithiodigalactosides designed by Ulf Nilsson (Lund University) and developed by Galecto Biotech, or clinical-grade citrus pectin named GCS-100 developed by La Jolla Pharmaceuticals (Fig. 1). Antibody B2C10 that is directed against the N-terminal part of galectin-3, at the opposite site of the carbohydrate recognition domain, can also detach galectin-3 from cells, most probably by disassembling galectin-3 oligomers, thus keeping it in a lower affinity form. We recently described that another clinical-grade product developed by Galectin Therapeutics, GM-CT-01, is also able to boost human TIL functions via disorganizing glycoprotein-galectin lattices, without actually detaching galectins from cells.4 GM-CT-01 is a galactomannan of about 50 kDa obtained by hydrolysis of guar gum, extracted from guar beans. Its half-life in Cynomolgus monkeys is between 12 to 18 h. GM-CT-01 interacts with a site in galectin-1, also present in galectin-3, opposite to the carbohydrate-binding domain, acting as an allosteric antagonist.
Figure 1. Galectin antagonists that could boost the functions of human tumor-infiltrating lymphocytes. Sugars in the boxes are depicted following the Consortium for Functional Glycomics nomenclature. Sugars decorating the surface glycoproteins are drawn in black and gray for simplification. Molecules and structures are not to scale.
Is TIL Immunosuppression a Story About Sugars and Galectins?
We have reported that freshly isolated CD8+ TILs that are heavily covered by galectin-3 are the CD8+ TILs that harbor a glycome rich in lactosamine motifs and thus strongly stained with Lycopersicon esculentum lectin (LEL).4 These TILs were totally blocked for IFNγ secretion upon stimulation. Addition of galectin antagonists disorganizes galectin-glycoprotein lattices and retrieves the ability of the LELhigh galectin-3high CD8+ TILs to secrete IFNγ after stimulation. This is in agreement with our working hypothesis: a high percentage of TILs are activated lymphocytes, which therefore harbor many LacNAc motifs, the natural ligands of galectin-1 and -3.5 The abundance of LacNAc motifs and galectins would favor the formation of galectin-glycoprotein lattices at the TIL surface and result in a decreased surface motility of the receptors involved in T-cell functions.
Are Galectin Antagonists Candidate Treatments for Clinical Trials?
Remarkably, briefly treating human TILs ex vivo with galectin antagonists is sufficient to strongly increase IFNγ secretion and cytolytic ability, a reactivation that appears unique to galectin antagonists.4 In contrast, other groups have treated T cells with antibodies specific for inhibitory receptors, such as those aforementioned. This treatment does not provide an immediate functional correction but instead results in an enhanced proliferation of T cells, yielding a higher number of functional T cells a few days later.6 We have observed that two clinical-grade galectin antagonists, GCS-100 and GM-CT-01, boost IFNγ secretion upon ex vivo stimulation among ~80% of CD8+ and ~50% of CD4+ patient TIL samples. These TIL samples were obtained from patients bearing tumors of distinct histological origins, including those arising from melanocyte, biliary tract, prostate, esophagus, liver, colon, pancreas, and ovary. Galectin antagonists had no effect on the IFNγ secretory responses of stimulated blood T lymphocytes from donors without cancer. These two compounds have already been injected intravenously in cancer patients without severe side effects.
In addition to its straightforward effect on TIL functions, inhibition of extracellular galectins may have other beneficial antitumoral benefits. Upregulated galectin-3 expression and secretion is a feature of alternative macrophage activation.7 Galectin antagonists could interrupt the galectin-3 feedback loop that enhances alternative macrophage polarization and activation, dampening chronic inflammation.7 Moreover, in murine models, extracellular galectin-3 seems to favor breast8 and melanoma metastases9 by supporting tumor cell adhesion. Resistance of galectin-3 knock-out mice to melanoma metastasis also correlates with a higher NK cytotoxicity.9
Cautionary Remarks
Considering the pleiotropic roles of galectin-1 and galectin-3 and the fact that the galectin antagonists mentioned above have a broad specificity for several galectins, care must be exercised when using these compounds for human clinical therapy. Indeed, we cannot exclude that galectins cover activated T cells in autoimmune diseases where galectins are abundant and may restrain T-cell functions. Systemic injections of galectin antagonists may thus, in theory, exacerbate some autoimmune diseases. Such systemic injections could also disturb leukocyte migration across vascular and lymphatic endothelium, as it is regulated by galectins.10
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Glossary
Abbreviations:
- TIL
tumor-infiltrating lymphocyte
- LacNAc
N-acetyl-lactosamine
- LEL
Lycopersicon esculentum lectin
Citation: Gordon-Alonso M, Demotte N, van der Bruggen P. Sugars boost exhausted tumor-infiltrating lymphocytes by counteracting immunosuppressive activities of galectins. OncoImmunology 2014; 3:e28783; 10.4161/onci.28783
References
- 1.Wolchok JD, Kluger H, Callahan MK, Postow MA, Rizvi NA, Lesokhin AM, Segal NH, Ariyan CE, Gordon RA, Reed K, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369:122–33. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Demotte N, Wieërs G, Van Der Smissen P, Moser M, Schmidt C, Thielemans K, Squifflet J-L, Weynand B, Carrasco J, Lurquin C, et al. A galectin-3 ligand corrects the impaired function of human CD4 and CD8 tumor-infiltrating lymphocytes and favors tumor rejection in mice. Cancer Res. 2010;70:7476–88. doi: 10.1158/0008-5472.CAN-10-0761. [DOI] [PubMed] [Google Scholar]
- 3.Demotte N, Stroobant V, Courtoy PJ, Van Der Smissen P, Colau D, Luescher IF, Hivroz C, Nicaise J, Squifflet JL, Mourad M, et al. Restoring the association of the T cell receptor with CD8 reverses anergy in human tumor-infiltrating lymphocytes. Immunity. 2008;28:414–24. doi: 10.1016/j.immuni.2008.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Demotte N, Bigirimana R, Wieërs G, Stroobant V, Squifflet J-L, Carrasco J, Thielemans K, Baurain J-F, Van Der Smissen P, Courtoy PJ, et al. A short treatment with galactomannan GM-CT-01 corrects the functions of freshly isolated human tumor-infiltrating lymphocytes. Clin Cancer Res. 2014;20:1823–33. doi: 10.1158/1078-0432.CCR-13-2459. [DOI] [PubMed] [Google Scholar]
- 5.Antonopoulos A, Demotte N, Stroobant V, Haslam SM, van der Bruggen P, Dell A. Loss of effector function of human cytolytic T lymphocytes is accompanied by major alterations in N- and O-glycosylation. J Biol Chem. 2012;287:11240–51. doi: 10.1074/jbc.M111.320820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fourcade J, Sun Z, Pagliano O, Guillaume P, Luescher IF, Sander C, Kirkwood JM, Olive D, Kuchroo V, Zarour HM. CD8(+) T cells specific for tumor antigens can be rendered dysfunctional by the tumor microenvironment through upregulation of the inhibitory receptors BTLA and PD-1. Cancer Res. 2012;72:887–96. doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henderson NC, Mackinnon AC, Farnworth SL, Kipari T, Haslett C, Iredale JP, Liu FT, Hughes J, Sethi T. Galectin-3 expression and secretion links macrophages to the promotion of renal fibrosis. Am J Pathol. 2008;172:288–98. doi: 10.2353/ajpath.2008.070726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murugaesu N, Iravani M, van Weverwijk A, Ivetic A, Johnson DA, Antonopoulos A, Fearns A, Jamal-Hanjani M, Sims D, Fenwick K, et al. An in vivo functional screen identifies ST6GalNAc2 sialyltransferase as a breast cancer metastasis suppressor. Cancer Discov. 2014;4:304–17. doi: 10.1158/2159-8290.CD-13-0287. [DOI] [PubMed] [Google Scholar]
- 9.Radosavljevic G, Jovanovic I, Majstorovic I, Mitrovic M, Lisnic VJ, Arsenijevic N, Jonjic S, Lukic ML. Deletion of galectin-3 in the host attenuates metastasis of murine melanoma by modulating tumor adhesion and NK cell activity. Clin Exp Metastasis. 2011;28:451–62. doi: 10.1007/s10585-011-9383-y. [DOI] [PubMed] [Google Scholar]
- 10.Cooper D, Iqbal AJ, Gittens BR, Cervone C, Perretti M. The effect of galectins on leukocyte trafficking in inflammation: sweet or sour? Ann N Y Acad Sci. 2012;1253:181–92. doi: 10.1111/j.1749-6632.2011.06291.x. [DOI] [PubMed] [Google Scholar]