Abstract
The neuroepithelium is a germinal epithelium containing progenitor cells that produce almost all of the central nervous system cells, including the ependyma. The neuroepithelium and ependyma constitute barriers containing polarized cells covering the embryonic or mature brain ventricles, respectively; therefore, they separate the cerebrospinal fluid that fills cavities from the developing or mature brain parenchyma. As barriers, the neuroepithelium and ependyma play key roles in the central nervous system development processes and physiology. These roles depend on mechanisms related to cell polarity, sensory primary cilia, motile cilia, tight junctions, adherens junctions and gap junctions, machinery for endocytosis and molecule secretion, and water channels. Here, the role of both barriers related to the development of diseases, such as neural tube defects, ciliary dyskinesia, and hydrocephalus, is reviewed.
Keywords: Ependyma, hydrocephalus, neural tube defects, cell junctions, cilia, development, aquaporin 4, astrocyte reaction
Purpose of the review
The ependyma constitute a ciliated epithelium that derives from the neuroepithelium during development and is located at the interface between the brain parenchyma and ventricles in the central nervous system (CNS). After neurulation, the neural plate forms the neural tube, which undergoes stereotypical constrictions by bending and expanding to form the embryonic vesicles, and becomes the forebrain, midbrain, and hindbrain. Therefore, the original cavity of the neural tube forms the embryonic ventricles, constituting a series of connected cavities lying deep in the brain that are filled with cerebrospinal fluid (CSF).1 Later during development, the forebrain ventricle develops massive expansion and splitting to form the lateral and third ventricles. In the midbrain, the ventricle remains as a narrow aqueduct connecting the third and fourth ventricles, with the latter located in the hindbrain. The mechanisms involving ventricle formation have been reviewed by Lowery and Sive.1 The neuroepithelium and ependyma constitute barriers lining a ventricular lumen in the developing and mature CNS, respectively, and perform important functions related to the development, morphogenesis, and physiology of the brain. Detailed reviews exist in the literature regarding the ependyma.2-7 This review is focused on the role of the neuroepithelium/ependyma on the origin and etiology of hydrocephalus and other related pathologies.
Hydrocephalus is not a single disease but a pathophysiological condition of CSF dynamics comprising fetal- and adult-onset forms.8 Hydrocephalus has been considered a CNS condition consisting of a net accumulation of intraventricular or extraventricular CSF independent of hydrostatic or barometric pressure. The increase in CSF volume causes an enlargement of the ventricular cavities, i.e., ventriculomegaly.9 Regarding the circulation of CSF, different forms of hydrocephalus have been grouped as non-communicating or communicating.10 The former entails forms presenting an obstruction in the intracerebroventricular CSF circulation, mainly in the aqueduct. In the case of the communicating hydrocephalus CSF circulates between the ventricles, but CSF absortion in some cases could be impaired by structural blockage or reduced physiological transport at the arachnoid membrane and its granulations, cranial nerve lymphatics, and capillaires and microvessels.11 In addition, there is not always a very high intraventricular pressure associated with hydrocephalus, as is the case of the so-called normal pressure hydrocephalus. It is estimated that a very small increase in the gradient of pressure between the ventricle and the subarachnoid space is sufficient to produce ventricular dilatation, which would occur at the expense of the brain’s interstitial fluid.12,13 Recently, a unifying classification has been proposed considering hydrocephalus with multiple points of intraventricular and extraventricular CSF circulation obstruction, resulting in CSF accumulation.14 The hydrocephalus origin can be congenital or acquired. Genetic factors are involved in congenital hydrocephalus formation, but other factors underlie its development, such as congenital malformations, intracerebral hemorrhages, maternal alcohol abuse, infection, and X-radiation during pregnancy.15 Alterations in the ependyma that are present in some cases of hydrocephalus, consisting on in its flattening or loss, have been considered to be a consequence of the high intracerebroventricular pressure and ventricle surface stretching, tissue compression, cerebral ischemia and hypoxia and neuroinflammation.2,5 In cases of adult-onset normal pressure hydrocephalus there is not detailed information on how the ependyma reacts. In this review it will be argued that the disruption of the ependyma could be due to problems in the neuroepithelium development in cases of fetal-onset hydrocephalus. However, the possibility of secondary changes due ventricle enlargement and tissue compression, cerebral ischemia and hypoxia, and neuroinflammation cannot be discarded.
Cilia-related diseases that are associated with hydrocephalus are considered consequences of defects in neuroepithelium/ependyma development and are related to primary cilia or motile water-propelling cilia functions. Cilia-related diseases include ciliary dyskinesia and situs inversus.16 Hydrocephalus can also involve problems in neurogenesis or corticogenesis, most likely sharing the same cellular origin.17
The role of the neuroepithelium in CNS development
During CNS development the germinal zone is constituted by a pseudostratified neuroepithelium. Radial glial cells originate in the neuroepithelium; these cells are neural multipotent stem cells that also guide migrating neurons, thus determining the neuronal fate and position in the developing brain.18 Therefore, the neuroepithelium performs neurogenesis at the brain-cerebrospinal fluid interface.19 Radial glial cells display an apical-basal polarization, and they stretch from the luminal surface to the basement membrane at the pial surface (Fig. 1A). They are joined with tight junctions apically (zonula occludens) and with adherens (zonula adherens) junctions and gap junctions in their lateral plasma membrane domains.19,20 The existence of an apical-basal polarity in the progenitors in the ventricular zone suggests that they receive extrinsic growth factor from the CSF, likely via their sensory apical primary cilium.19
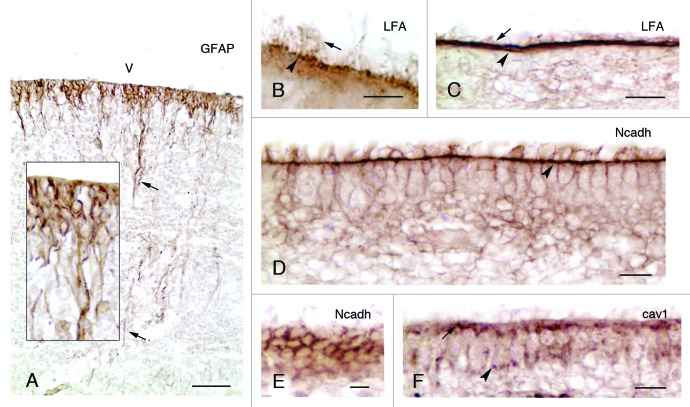
Figure 1. Development and properties of the multiciliated ependyma in the lateral ventricle of human fetuses. (A, insert) In a fetus at 23 wk of gestation, radial glial cells are observed that display long basal processes (arrows) and express GFAP. (B and C) In fetuses at 25 and 36 wk of gestation, multiciliated ependymal cells are already appreciated (arrows point to cilia) and present sialic acid at their apical pole (arrowheads), which is detected with an antibody against the Limax flavus agglutinin (LFA). (D-F) Mature multiciliated ependymal cells in a fetus at 30 wk of gestation. N-cadherin (Ncadh) is observed in transversal (D) and tangential sections (E) arranged in cell junctions (arrowhead in D). (F) Caveolin (cav1) is present in the apical pole (arrow) and basal processes (arrowhead) of mature multiciliated ependymal cells in a fetus at 30 wk of gestation. Abbreviations, v, ventricle lumen. Bars: A, 50 µm; B, 5 µm; C, E, F, 10 µm; D, 20 µm.
In addition to the aforementioned germinal function, the neuroepithelium is involved in normal brain morphogenesis, performing a temporal occlusion of the neural tube at a moment when the posterior neuropore is still open and the anterior pore is already closed, thus allowing for the expansion of the cranial ventricular system and brain growth.21 This expansion is induced by an increase in intraventricular pressure at a moment when the choroid plexuses are not developed; therefore, they do not yet produce CSF. The neuroepithelium has been suggested as the source of the particular chemical composition of embryonic intraventricular CSF. The cellular mechanism underlying such expansion in chick embryos has been reported to require calmodulin. This mechanism is dependent on extracellular Ca2+, mediated by cAMP and may involve N-cadherin.22 Recent studies in zebrafish development have shed further light regarding the role of neuroepithelial cells in this mechanism. Neuroepithelial tight junctions containing claudin5a create an early cerebral-ventricular barrier, thus allowing for ventricular lumen expansion driven by hydrostatic pressure.23 However, the neuroepithelium actively transports Na+ and secretes proteins and proteoglycans into the ventricle, contributing to an increase in the intraventricular hydrostatic pressure.21 Furthermore, neuroepithelial relaxation allows for lumen expansion through the regulation of myosin contractibility.24 Zebrafish mutant analyses indicate that the heartbeat and blood circulation also contributes to ventricle expansion.25 Additionally, the pressure created in the ventricle appears to play another important role in stimulating progenitor cell proliferation and brain morphogenesis.21 This stimulation explains the higher rate of ependymal cell production occurring in several ventricles during the ventriculomegaly process in congenital hydrocephalus, which has been described in the hyh mouse model.26
In the immature brain, the neuroepithelial cells present in their apical poles the so-called strap junctions, which have been considered different from tight junctions. These junctions would form a physical barrier restricting the movement of molecules such as proteins.27-29 These strap junctions might restrict the entry of protein from CSF, which present very high concentration in the early brain development, into brain interstitial fluid. The existence of embryonic CSF with a certain chemical composition supports morphogenetic processes and regionalization in the neural tube during development, playing a key role in promoting the survival and proliferation of neuroepithelial cells.30 Problems in the composition and circulation of the CSF have been hypothesized to underlie abnormal corticogenesis in the H-Tx rat model of congenital hydrocephalus.31,32 Lehtnen et al. recently reviewed the role of embryonic CSF in neurogenesis through growth- and survival-promoting factors, such as insulin and insulin-like growth factors (IGF) 1 and 2, fibroblast growth factor 2 (FGF2), sonic hedgehog, and retinoic acid.19
In addition to the role of the neuroepithelium derived from the presence of stem cells in several regions, in some locations the cells become specialized in secreting molecules and morphogens that govern CNS development.4 Thus, the dorsal and ventral lines of the roof and floor plates contain specialized epithelial cells that act as organizers guiding neuronal development, providing morphogens and signals, such as Netrin-1, SLIT, Sonic Hedgehog, and members of the TGFβ superfamily.33 The ultrastructural analysis of the midbrain floor plate has revealed the presence of secretory cell machinery most likely involved in the secretion of molecules toward the ventricle CSF; the functions of these molecules remain unclear.34-36
Finally, during development, a subpopulation of radial glial cells produces the ependymal cells, which will become differentiated cells that are unable to proliferate under normal conditions.37 In mice and humans, the differentiation process of the ependymal cells follows a precise temporospatial pattern throughout the ventricular system, which has been extensively reported and reviewed by Bruni et al., Bruni, and Sarnat.2,4,5,38 The homeobox gene Six3 controls the late maturation of the ependyma during late development, which suppresses radial glial cell properties.39
In the lateral ventricles of mature animals and humans, stem cells derived from the neuroepithelium are retained between the ependymal cells, constituting a neurogenic niche in the subventricular zone.40,41 In addition to supporting stem cells, ependymal cells also promote neurogenesis in the niches secreting Noggin, a bone morphogenetic protein (BMP) antagonist.42 In adult rats, the induced disruption of the mature multiciliated ependyma of the lateral ventricles with subventricular zone niches affects neurogenic and gliogenic activity.43 The mature ependyma presents limited repair in the lateral ventricles arising from stem cell niches in the subventricular zone.44,45 However, in hydrocephalus, the ependyma is massively disrupted and not regenerated. Then, in most ventricle surfaces, the ependyma is replaced by a particular layer of reactive astrocytes whose functions are explained in the last section of this review. In the DLg5 knockout mouse and the hyh mutant mouse,46,47 the neuroepithelium is disrupted in the ventricular areas with postnatal neurogenesis and they present an impairment of the subventricular zone niches.
Importance of ependymal cilia development in health and disease
In the ependymal cells, the beating of cilia is important for propelling CSF, and thus, the cilia must display an orientation that is tightly coupled to the anterior-posterior neuroaxis. CSF accumulation and hydrocephalus occur when the flow is disturbed. This orientation is defined by an ependymal planar polarity, which is acquired during development in a multi-step process involving two independent mechanisms of the movement of the cilia basal bodies: translational and rotational.48,49 Planar polarity during development is also important for the closure of the spinal neural tube.50 Thus, the consequences of failure in planar polarity include neural tube defects, including spina bifida and hydrocephalus. In the radial glial cells, the precursors of ependymal cells, primary cilia appear to play a key role in the development of planar polarity. Basal body translational position movement occurring in radial glial progenitors depends on the primary cilium, thus orchestrating the planar architecture of radial glial cells and translating the planar polarization to their progeny of ependymal cells.48 The movements of the basal bodies occur in connection with microtubules, actin, non-muscle myosin II, and cytokeratin and most likely also in relation with apical junctions.51,52 For rotational movement, an independent signaling pathway is involved that includes Dishevelled2, Vangl2, Celsr2 and Celsr3, which are required for ependymal motile cilia to establish the polarized fluid flow.49,52-54 Additionally, the passive flow of the CSF plays a refining role in the rotational orientation of the basal bodies during ependymal differentiation,49,55 orientation that is locked when the ependyma matures.53
Primary ciliary dyskinesia, also known as immotile cilia syndrome, results as a defect in ciliary and flagellar motility, and hydrocephalus is present along with other pathologies, such as situs inversus, that affect left-right asymmetry and cortical maldevelopment.16 Thus, the disturbed expression of several genes in mice models has been found to cause primary ciliary dyskinesia and hydrocephalus.16 Mouse strains that present differential susceptibility to hydrocephalus are associated with primary ciliary dyskinesia, which is higher than in humans.16 This difference may be explained by the segregation of genetic modifiers encoding proteins involved in ciliary function, brain development, and physiology.16 Hydin is one of the proteins involved in primary cilia dyskinesia and is present in the central pair of microtubules of the 9+2 axoneme present in motile cilia, where it is implicated in the regulation of the dynein arm activity.56 Mutations in hydin create cilia that are incapable of beating and generating fluid flow. Hydin has been found to be mutated in the hy3 mouse that develops hydrocephalus.57 Defects in hydin and in the central pair of microtubules are also associated with hydrocephalus in humans.58 The mutation in the spindle-like microcephaly-associated protein, which shares some homology with hydin, is also associated with cerebral cortex maldevelopment that promotes microcephaly.59 Motile ciliogenesis and maturation of the ependyma depends on the forkhead transcription factor Foxj1 through the regulation of the transport of γ tubulin-containing basal bodies toward the apical surface, most likely through the kinesin motor proteins kif9 and kif27.60 Foxj1 knockout mice present hydrocephalus but not aberrant left/right asymmetry, and their ependyma is devoid of cilia.60 Foxj1 in implicated in the control of the expression of ankyrin-3, an adaptor molecule that organizes membrane domains in the radial glial cell progenitors of ependymal cells.61 Ciliary function is also regulated by polycystin-1, a very large, highly glycosylated plasma receptor present in intercellular junctions and in the cilia of ependymal cells.62 In mice, knocking in this gene causes hydrocephalus with ependyma displaying morphologically normal cilia.62
Cell junctions in the development of the ependyma are involved in hydrocephalus and associated malformations
Currently, there is a growing body of evidence regarding the involvement of cell junctions during ependymal development in the triggering and the evolution of hydrocephalus, which has recently been reviewed by Rodríguez et al.17 This evidence includes results from animal models and studies in humans. A consequence of the alteration in cell junctions is the disruption of the natural barriers between the CSF and brain with developing or mature parenchyma, which leads to developmental and physiological abnormalities associated with hydrocephalus. A common sign of the maldevelopment of the neuroepithelial/ependyma cell junctions is the presence of anomalous ependymal organizations forming rosettes.63 In human fetuses, ependymal rosettes are commonly present in cases with hydrocephalus (unpublished observations of the authors),5 including cases with fetal spina bifida aperta,64 and defects in CNS development, such as lissencephaly and pachygyria.65 Abnormalities in neurogenesis and corticogenesis are also suggested to involve the disruption of the germinal ventricular and subventricular zones.17 A third consequence of the neuroepithelial/ependymal disruption is the manifestation of obstructions of narrow ventricles, such as the aqueduct, which occurs in the form of non-communicating hydrocephalus.
The disruption in the regulation of apical polarity affects the organization of cell junctions in the neuroepithelium/ependyma and leads to hydrocephalus, as observed in the inactivation of the atypical protein Kinase C (aPKC),66 and the Drosophila Lethal giant larvae Lgl1 homolog.67 Rosettes in the neuroepithelium are also present in the latter model.67
Cadherins, the primary calcium-dependent cell junction molecules present in the CNS, appear to play key roles in the adherens junctions in both the neuroepithelium and ependyma (Fig. 1D, E; Fig. 2A-C).68,69 In respect to the neuroepithelium, blocking N-cadherin in chick embryos gives rise to its disruption with the consequent formation of periventricular anomalous development of the ependyma that forms rosettes.70 The non-muscle myosin II-B interacts with N-cadherin containing junctions in the neuroepithelium. When the function of myosin II-B is ablated in mice, the neuroepithelial cells lose their cell adhesion ability, and in all the experimental mice hydrocephalus is developed71 Myosin IXa presenting a Rho GTPase-activating domain is expressed and needed during the maturation of ependymal cells.72 The experimental inhibition of myosin IXa in mice leads to the alteration in morphology and junctions of the ependyma and in their differentiation.72 N-cadherin is present in the cell junctions of mature multiciliated ependymal cells in mice and humans (Fig. 1D, E; Fig. 2C).64,73 These junctions are essential for the integrity of the cell layer, and massive death of the ependymal cells occurs via apoptosis when N-cadherin is experimentally blocked.74 E-cadherin is also present at the cell border of mature ependyma cells, presumably regulated by Numb and implicated in maintaining ependymal integrity.44 The levels of E-cadherin are found to be altered in rats infused with vascular endothelial growth factor (VEGF).75 In these rats, VEGF appears to phosphorylate and activate the receptor VEGFR2 in the ependyma, which exhibits alterations and partial denudation.75 This evidence has been linked to the origin of hydrocephalus in human cases where higher levels of VEGF have been recorded.75
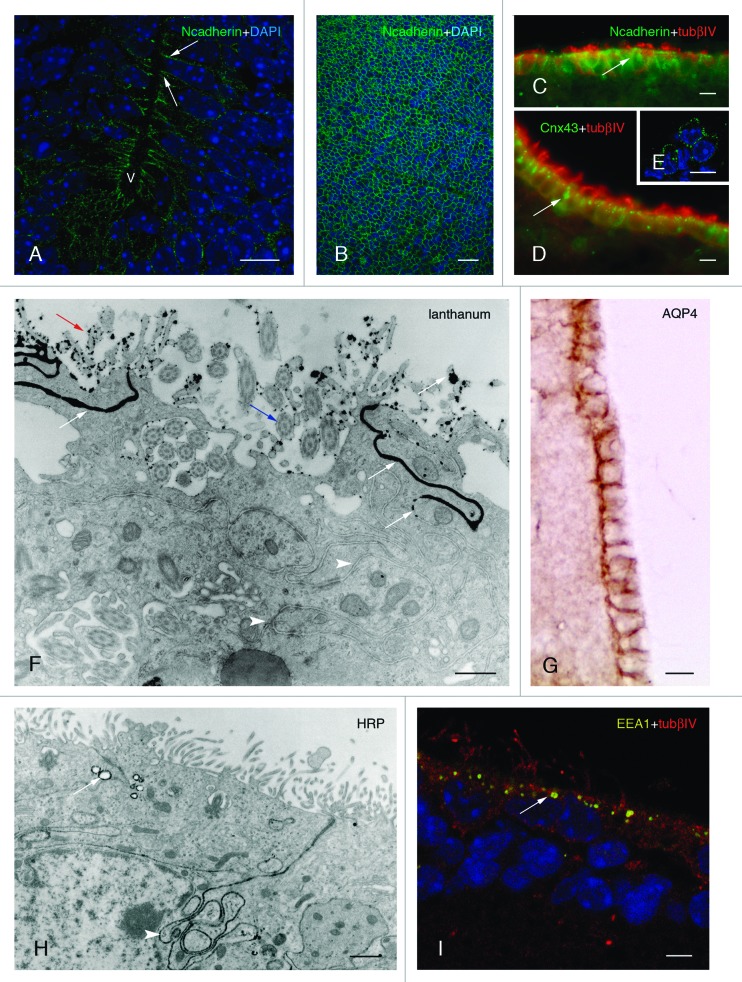
Figure 2. Development and properties of the multiciliated ependyma in the ventricle of the mouse. (A, B, and C). The neuroepithelial (A and B) and ependymal (C) cells express N-cadherin-containing junctions (in green, arrows) in their lateral plasma membrane domains, which are detected in transversal (A) and tangential views (B). (D and E) Multiciliated ependymal cells are joined with connexin43-containing (Cnx43) gap junctions (in green, arrow) that are appreciated in transversal (D) and tangential (E) views. (F) Multiciliated ependymal cells lack tight junctions, as shown with lanthanum nitrate applied to the ventricle and observed under transmission electron microscopy. The tracer (with black electrodensity, white arrows) is present passing through the lateral winding extracellular spaces (white arrowheads), proving the absence of functional tight junctions. Motile cilia (blue arrow) and microvilli (yellow arrow) are appreciated in the luminal pole of ependymocytes. (G) Aquaporin 4 (AQP4) is present in the laterobasal domain of multiciliated ependyma. (H) At the transmission electron microscope, multiciliated ependyma takes HRP applied in vivo into the ventricle, and the tracer is incorporated into the pynocytic vesicles and early endosomes (in black electrodense reaction, white arrow). The tracer is also observed in the lateral winding extracellular spaces (white arrowhead). (I) Early endosomes (detected with EEA1 in yellow, white arrow) are detected at the apical pole of multiciliated ependyma. (A and B) Micrographs represent Z-plane projections under confocal microscopy in 40-µm-thick frozen sections. (E and I) Micrographs represent 1 µm thick planes under confocal microscopy. (C and D) Micrographs are taken under fluorescent microscopy in a 10-µm-thick paraffin sections. Tubulin βIV (tubβIV) immunofluorescence is shown (in red) in cilia labeling in C and D. (A, B, E, and I) Micrographs present DAPI nuclear immunostaining (in blue). Abbreviations: v, ventricle lumen. Bars: A, C-E, G, 10 µm; B, 40 µm; F, 50 nm; H, 1 µm, I, 5 µm.
In the hyh mutant mouse, a defect in the vesicular traffic mediated by αSNAP can explain the alteration of the fate of molecules that are present in adherens junctions.17,76 This defect in the formation of adherens junctions can trigger the disruption of the neuroepithelium, which has been shown to be associated with a prenatal and early postnatal mild ventriculomegaly and communicant hydrocephalus.77-79 In the hyh mouse, later in the postnatal development, the obstruction of the narrow aqueduct due to the absence of neuroepithelium/ependyma leads to a severe non-communicant hydrocephalus.80 The brain parenchyma astrocytes can mediate the fusion of the ventricle walls devoid of the ependymal barrier. In another mouse model with DLg5 knocked out, the migration of cadherin-containing vesicles and their delivery of cell junction molecules appear to be altered.46 Similar to the hyh mouse, a disruption in the formation of the ependyma occurred and was followed by aqueduct stenosis, leading to severe hydrocephalus.46
In human fetuses with hydrocephalus, observations in the last decade point in the same direction as the aforementioned animal models.64,81,82 In fetuses with spina bifida aperta and hydrocephalus, N-cadherin cell junctions are abnormally located preceding their disruption in the cells of the ventricular zone of ventricle walls including the aqueduct.64 These observations strongly suggest that defects in the ependymal lineage are implicated in the origin of hydrocephalus and in the obliteration of the aqueduct in human.
Interestingly, secondary forms of hydrocephalus that appear in the intraventricular hemorrhage can be explained based on a neuroepithelial disruption. Thus, hydrocephalus defects in the ependyma have also been described in a posthemorrhagic rat model.83 Recent evidence provided by Yung et al.84 demonstrated that lysophosphatidic acid (LPA) present in the blood delivered in intracerebroventricular hemorrhages mediates the disruption of the neuroepithelium. In addition, the presence of LPA explains defects in neurogenesis that commonly are associated with fetal hydrocephalus.84
In addition to the aforementioned role of the ependyma on the CSF composition and circulation,31,32 in the case of hydrocephalus, the disruption of the ventricular zone in hydrocephalus can also be implied in the existence of abnormal neurogenesis and corticogenesis in animal models and humans, which has been recently reviewed by Rodríguez et al.17 Some of these alterations could be explained by an abnormal proliferation of neural progenitors in the ventricular zone and by abnormal radial migrations caused by an absent scaffold of the basal processes of radial glial cells. The consequences of these alterations could include the displacement of progenitors into the ventricle lacking a neuroepithelial/ependymal barrier described in animal models and human,17,47,77,79,81 affectations of corticogenesis and the presence of periventricular heterotopias,78 which are clusters of neuroblasts/neurons ectopically displaced near the lateral ventricles.85
Experimental studies in mice have shown that alcohol exposure, which is sometimes associated with hydrocephalus and defects in the cortical development, alters the development of the neuroepithelium in the midline during the neural tube formation, thus originating an enlargement and perforation of the ventricles.86 Periventricular heterotopies are also present in rats with congenital hydrocephalus produced by the administration of etanol during their development, thus indicating problems in the formation and migration of neuroblasts.87
Ependyma as a polarized cell barrier between the ventricular CSF and brain parenchyma
The presence of an ependymal barrier between the ventricular CSF and brain parenchyma is implicated in the flow of substances through both sides of the interface, which is disturbed after ependymal disruption in the presence of hydrocephalus. Mature ependymal cells are polygonal cells, cuboidal to the columnar depending on the ventricle location, which display polarized structural and functional organizations. At the basal pole, ependymal cells present basal lamina labyrinths that are remnants of basal lamina from embryonic capillaries. Apically, their luminal pole is in contact with the ventricular CSF, and the cells display microvilli and an average of 16 motile cilia (9+2 axoneme) per cell with a length of approximately 13 µm.88,89 Throughout this review, these cells will be cited hereafter as multiciliated ependymal cells to differentiate them from other specialized ependymal cells, such as tanycytes, which are monociliated, and choroid plexus epithelial cells, which also present cilia.
Multiciliated ependymal cells are joined with adherens junctions. As explained above, the expression of cell junction molecules, such as cadherins, is important for the integrity of the neuroepithelium and ependyma, and the expression of these molecules changes during development. Thus, protocadherins 2A and 15 are present at stages most likely corresponding to radial glial cells.90,91
The existence of abundant gap junctions has been widely demonstrated in ependymal cells.88,92 In particular, the mRNAs coding connexin43, connexin26, and connexin30 have been reported to be present in the mouse and rat ependyma.93,94 The connexin43 protein has been detected in the ependyma of mice (Fig. 2D, E) and humans.64,73,95 Connexins in the ependymal cells have been suggested to be regulated by the basic fibroblast growth factor (bFGF).95 Gap junctions in ependymal cells are involved in electrical and metabolic couplings integrating the functioning of the cell layer. Gap junctions play a role in the synchronization of cilia beating and in CSF circulation.64,96 However, the presence of abundant gap junctions in specialized ependymal cells, such as monociliated tanycytes and non-ciliated α1-tanyctes, suggests that in these cell populations, coupling performs an unknown function different from beating cilia.97,98 Gap junctions have also been proposed to play a role in integrating ependymal function with underlying astroglia and the formation of panglial syncytium that regulates water and ion transport.99
The arrangement of the cytoplasmatic organelles in the ependymal cells also displays polarization.73,88 The mature ependyma in mammals presents a cytoskeleton with a reduced presence of the glial fibrillary acidic protein (GFAP) compared with radial glial cells and immature ependyma. Instead of expressing GFAP, ependymocytes express vimentin.38 In the ependyma, a highly ordered F-actin network forms part of the apical terminal bar complex, whose presence is suggested to be related with the organization of microvilli and with the reaction to different forces, including the factors derived from the CSF movement.100 The presence of actin and myosin near the basal bodies is also related to the movement of cilia.6
The presence of tight junctions appears as a transient characteristic of the neuroepithelium that will give rise to the multiciliated ependyma but remains in specialized ependyma present in circumventricular organs. The early study of Brightman and Palay described the multiciliated ependyma presenting tight junctions (zonula occludens).88 Nevertheless, immunolocalizations of molecules associated with tight junctions have revealed that if tight junctions are present, they are incomplete.101,102 In the apical pole, ependymal cells also present abundant gap junctions allowing for 2–4 nm extracellular spaces, which make extracellular spaces permeable to large molecules, including proteins and tracers (Fig. 2F).73,103 Recent studies have experimentally proven the presence of such extracellular permeable spaces between the ependymal cells that allow the passage of tracers, such as lanthanum nitrate and peptides.73,74,104 Proteins with dissimilar molecular weights, such as horseradish peroxidase and ferritin, which have molecular weights of 43 and 560 kDa, respectively, also pass through the ependymal extracellular spaces.105,106 The injection of labeled metabolites, such as [14C]sucrose and [14C]inulin, in the ventricles of rats has proven that the transependymal diffusion in the different ventricle walls depend on CSF circulation.107,108
However, despite the presence of permeable extracellular spaces, experimental approaches have shown that multiciliated ependyma also represent a regulated barrier to the diffusion between the ventricle CSF and brain parenchyma. Thus, phorbol ester induces changes in the adherens junctions of ependymal cells,101 which are also altered by a mannose-dependent recognition system.109 Currently, regardless of the absence of occluded pathways in their extracellular spaces, multiciliated ependyma is recognized as a partial barrier that regulates the transport and metabolism of some molecules in both directions through the ventricle-brain parenchyma interface.6
Ependyma presents regional structural and high functional specializations in such regions as the circumventricular organs, constituting the epithelial cells of the choroid plexus or the hypothalamic tanycytes. In such organs, tight junctions are absent in the endothelium vessels, which are fenestrated, and thus, these tight junctions they lack a blood-brain barrier. Therefore, circumventricular organs are neurohemal organs considered to be brain windows.110 The functional blood-CSF barrier consisting in hermetic apical tight junctions is in circumventricular organs displaced to the epithelial cells and tanycytes.110 Tanycytes present radially directed basal processes that extend for a variable distance into subadjacent neuropile, enwrapping blood vessels or terminating on neurons, glial, or in the external glial limitans.38 Choroid plexus epithelial cells produce secretions and the main proportion of CSF, which fills the ventricles, spinal cord canal, and subarachnoid spaces. Choroid plexus constitutes an open window to systemic circulation that results as a location of penetration for toxicants and pathogens in the CNS.103
Consequently, three barriers with different properties are considered in the CNS, a blood-brain barrier at the blood vessels, a blood-CSF barrier at the choroid plexus and the arachnoid membrane, and a CSF-brain parenchyma barrier at the ependyma. Only the two first barriers involve the sealing of extracellular spaces with tight junctions. The ependyma would constitute a partial barrier, depending on the substances transported.
Role of ependyma in ventricle CSF production and circulation
The choroid plexus produces most of the CSF. The CSF and blood plasma compositions are very similar, with the only major difference being the significantly lower concentration of proteins in the CSF. The highly specialized ependymal cells of the choroid plexus produce CSF by appropriate machineries for ionic transport and the secretion of different molecules. A source of extrachoroidal CSF exists that ranges between 30% and 60% of the total CSF.111 This CSF originates from the interstitial brain parenchyma that reaches the ventricle through the permeable barrier of the multiciliated ependyma.
The CSF plays important roles in CNS physiology, including absorbing mechanical and thermal stress, removing waste products that form in the CNS (sink action), creating an appropriate extracellular molecular composition, and transporting humoral mediators and nutrients.103 Thus, it forms the third circulatory system, in which the CSF is simultaneously a source and sink for distributing molecules. The multiciliated ependyma, as a permeable partial barrier at the CSF-brain parenchyma, also plays an important role in the balance of molecule transport between the ventricular CSF and interstitial fluid.103
CSF circulation is driven by its production rate (volume) and the vasculature pulsatile kinetics.112-114 The directed and coordinated beating of ependymal cilia into the ventricle also appears to contribute to CSF circulation.89 The cilia beating in the mammalian ependyma ranges between 28 and 40.7 Hz and is inhibited by serotonin,16,115 which is most likely released from a supraependymal axon network arising from the raphe nuclei.116 Furthermore, ATP and the pituitary adenylate cyclase-activating polypeptide (PACAP) modulate ciliary activity.89,115,117 The melanin-concentrating hormone exerts control in the third ventricle.118 Ependymal cells express D1 and D2 dopamine receptor subtypes, and dopamine can be released from subependymal axons to modulate ciliary functions.119
CSF circulation is disturbed and slowed when motile cilia development is altered in mice.54 Ciliary beating is particularly important for circulation through narrow canals, such as the cerebral aqueduct, allowing for laminar flow near the ventricle wall surface.10 Mutations in genes implicated in the assembly or structure of ependymal motile cilia, such as Mdnah, Ift88, hy3, Celsr2, and Celsr3, have been found to alter the CSF dynamics and result in hydrocephalus.54,96,120-122 Nevertheless, hydrocephalus should be considered because ciliated epithelial cells of choroid plexus are also affected and implicated in CSF production. The beating direction of cilia in the ependymal cells of the lateral ventricles is also important for the migration of young neurons generated in neurogenic niches in the subventricular zone.123
Sialic acid is a negatively charged sugar present in glycoproteins or glycolipids that constitute the glycocalix of ependymal cells at their luminal pole (Fig. 1B, C). Sialic acid has been suggested as a regulator for cellular and molecular interactions, cell masking in innate immunity, recognition and signaling, molecular protection against proteolytic attack, and providing a charged filtration barrier and a protective electrical shield for repulsion.124 The existence of abundant sialic acid in glycocalix at the luminal pole of the multiciliated ependyma suggests a role of this barrier in CSF circulation and likely in transependymal permeability. Thus, the presence of sialic acid maintains a hydrated film to allow for a laminar regimen of CSF circulation near the ventricle wall.10,124 Sialic acid could also exert repulsive forces between the ventricle walls in narrow ventricles, such as the aqueduct or spinal cord central canal (Fig. 3A).10,124 Sialic acid is also abundant in the glycoproteins of the Reissner’s fiber, a structure that is secreted by the specialized secretory ependyma of the subcommissural organ at the entrance of the aqueduct.10 The Reissner’s fiber runs caudally, reaching the end of the narrow spinal cord central canal.10 The potential functions of Reissner’s fiber include the facilitation of CSF circulation through narrow conduits and restraining the closing of the aqueduct.10,125-127 However, it remains intriguing that in humans, the subcommissural organ is only present in the fetus and children, secreting glycoproteins that do not form a Reissner’s fiber.128 Experimental results support the hypothetical functions suggested for sialic acid present in both the ependyma and Reissner’s fiber in relation to CSF circulation and hydrocephalus. The removal of sialic acid in the ependyma of rats with bacterial and viral neuraminidases leads to multiciliated ependyma disruption,43,129 indicating that the presence of sialic acid is important for the ependymal integrity, as in other barriers, such as the pulmonary endothelium.130 The intracerebroventricular administration of neuraminidase in rats also disrupts the molecular assembly of the highly sialylated glycoproteins that form the Reissner’s fiber.129 In these rats, a consequent fusion of the ventricle walls exists in the aqueduct and a non-communicant hydrocephalus.126
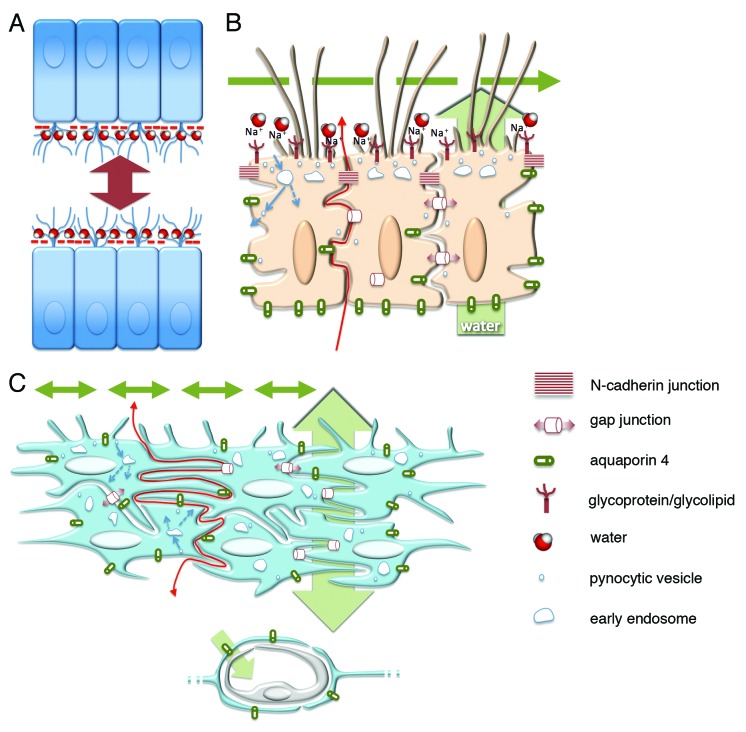
Figure 3. Schematic representation of the probable barrier roles played by the ependyma and the astrocyte reaction in health and hydrocephalus. (A) Sialic acid negative charges (- symbol in red) are present at the apical surface of multiciliated ependyma, which allows for repulsive forces and CSF circulation in narrow ventricle cavities and for a water film that facilitates the laminar flow of CSF. (B) The ependyma lacks tight junctions that allow the diffusion of molecules and ions toward the CSF (red arrow) through winding extracellular spaces. The polarized distribution of aquaporin 4 drives water transport in the same direction (green arrow behind the ependyma). Motile cilia mediate CSF transport at the luminal side (green broken arrow). Probable pynocytosis and transcytosis are represented at the apical pole of ependymal cells taking substances from the ventricle (blue arrows). Gap and adherens junctions are represented. (C) The hypothetical role of the layer containing reactive astrocytes covering ventricle walls with ependymal disruption. The mechanisms operating that attempt to reestablish homeostasis at both sides of the barrier are shown lacking the polarization present in the ependyma. Transport of water driven by astrocyte endfeet (asterisk) and non-polarized transports of CSF through the ependyma (double-end green arrow behind the reactive astrocyte cell layer) and into the ventricle (double-end green arrows into the ventricle lumen) are represented.
The possibility that alterations of the subcommissural play a role in the pathogenesis of hydrocephalus has been suggested in animal models and humans and reviewed by Meiniel.131 However, it remains obscure whether the alterations in the subcommissural organ in human fetuses are primary or epiphenomenal,132 which may also occur in the case of rats deficient in folic acid or B12 vitamin.133 It is difficult to claim the basis of the relationship between an altered subcommissural organ and the occlusion of the cerebral aqueduct and hydrocephalus. In several cases, the subcommissural organ, the ciliogenesis of multiciliated ependyma, and the choroid plexus epithelial cells are all affected, as occurs in mice deficient in the Regulatory factor X (RFX).121,134 Concurrence occurs in the subcommissural organ defects, ependymal alterations, and hydrocephalus with aqueductal stenosis/obliteration in mice overexpressing the gene for the pituitary adenylate cyclase-activating polypeptide receptor PAC1,135 displaying the inactivation of the huntingtin homolog Hdh and deficient in the homeodomain transcription factor Msx1.136,137 Nevertheless, in several cases, strong evidence supports the implication of the subcommissural organ in the origin of non-communicant hydrocephalus. First, the experimental immunological blockage of the Reissner’s fiber of maternally delivered antibodies during the development in rats produces aqueduct stenosis.138 Second, a tight association of the impairment in the subcommissural organ secretion with the aqueduct stenosis occurs in the H-Tx rat, which is a well-characterized model of congenital hydrocephalus.139
Role of the ependyma in water transport
Aquaporins constitute a family of water channel proteins that plays relevant roles in brain water physiology through barriers, including the ependyma. Aquaporins are tetramers of proteins surrounding a water pore that transport water in both directions. One of the members of the family, aquaporin 1, is located in the apical membrane of the specialized ependymal cells the choroid plexus.140 In the choroid plexus, aquaporin 1 is involved in the water transport, following osmotic gradient, from the blood to the ventricles for the CSF formation.141 Accordingly, aquaporin 1 null mice present a reduced CSF production and, consequently, a lower intracranial pressure.142 Owler et al. 2010 have recently reviewed the responses of aquaporins in changes of CSF pressure in cases of hydrocephalus.143 Aquaporin 4 is the principal member of the family present in the mammalian brain, located primarily at the borders between brain parenchyma and major fluid compartments, with a particular prevalence in periventricular areas.144 Thus, it is present in astrocyte perivascular endfeet, in the glia limitans at the border with the subarachnoid CSF, in periventricular astrocytes, and in the ependyma.145 Aquaporin 4 is regulated by reversible protein phosphorylation and protein-protein interactions and is likely to play a key role in the transduction or amplification of signals involved in the osmosensory feedback control of systemic salts and water balance.146-148 Aquaporin 4 regulates water flow through the brain parenchyma-ventricle CSF interface and at the blood brain barrier.149 In the case of the astrocytes perivascular and pial endfeet, water flow outward along with K+ passing through Kir4.1 potassium channels may occur.148 Aquaporin 4 could participates in intracranial pressure adjustements enhancing interstitial fluid reabsorption into brain capillaries.141,150Similar to other epithelia, in multiciliated ependymal cells, aquaporin 4 is located in the basolateral plasma membranes (Fig. 2G),73,145 and this location results in a directed water flow. Aquaporin 4 in the multiciliated ependyma is also most likely involved in maintaining their structural and functional integrity and also in the arrangement of the connexin43-containing gap junctions.151,152
Aquaporin 4 appears to play a key role in the presence of edemas and CSF accumulations occurring in hydrocephalus.153 In hydrocephalus, edema is considered caused by CSF extravasation through ependyma reacing interstitial fluid. In agreement with a protective role in hydrocephalus, aquaporin 4 is upregulated in rats with induced hydrocephalus,154 and a correlation occurs between aquaporin 4 expression and the apparent diffusion coefficient in periventricular edema, calculated with magnetic resonance imaging (MRI).155 In rats with induced hydrocephalus, aquaporin 4 facilitates the clearance of CSF into the parenchymal vasculature.156 A higher expression of aquaporin 4 in the periventricular astrocytes has been correlated with the severity of hydrocephalus in animal models.150 Similarly, evidence supports a role of aquaporin 4 in humans with hydrocephalus.157
Therefore, aquaporins can be considered potential therapeutic targets that act either on the regulation of the CSF production in the choroid plexus, on the interstitial CSF reabsortion, and/or on the edema formation in the different forms of hydrocephalus.141,143,153
Multiciliated ependyma as an immunological barrier
The multiciliated ependyma has been suggested to contribute to immunological processes. The specialized ciliated ependyma of the choroid plexus forming the blood-CSF barrier is considered one of the main routes of cellular infiltration into the CNS during normal conditions. However, in infectious and inflammatory conditions, the damaged barrier of multiciliated ependyma has been reported to be the predominant source of leukocyte infiltration reaching the ventricle.102 Thus, the multiciliated ependyma is an immunologically active site that, upon activation, produces effector molecules, which would support leukocyte transmigration.158 The presence of the intercellular adhesion molecule ICAM-1 and the vascular adhesion molecule VCAM-1 on the ependymal microvilli supports this function.159 In endothelial cells, vimentin has been found to play an important role in regulating this barrier in lymphocyte diapedesis.160 A similar role may be postulated for the presence of vimentin being expressed by the ependyma.
Role of glial reactions after the disruption of the ependyma
During development, the appearance of hydrocephalus and ventriculomegaly in animal models and humans is associated with important damages in the cortical myelin, which triggers astroglial and microglial reactions.64,78,81,161,162 Reactions of astrocytes and microglia can be induced by increased intracranial pressures. Accordingly, intraventricular CSF drainage through shunting in H-Tx rats with congenital hydrocephalus and in feline with experimental hydrocephalus has been shown partially preventing or reducing the astrocyte reaction.163,164 In the hyh mouse model, part of reactive astrocytes form a new cell layer that covers the ventricular walls denuded of multiciliated ependyma.73,78 This particular reaction also occurs in other animal models with hydrocephalus, such as the H-Tx rat (unpublished observations by the authors), after the induced disruption of the ependyma in rats using intracerebroventricular injections of neuroaminidase,43 and in human fetuses presenting primary hydrocephalus and spina bifida aperta.64,81 Several morphological and functional characteristics of reactive astrocytes partially resemble the ependyma, such as presenting microvilli in contact with the ventricle lumen, aquaporin 4, caveolae, paracellular permeability, and the cellular mechanism that is implicated in the endocytosis or transcytosis of molecules through the new barrier (Fig. 1F; Fig. 2G, H, I; Fig. 3B, C).73 In contrast to the ependyma, the reactive astrocytes would not function displaying the same functional polarization; however, they are proposed as an attempt to reestablish homeostasis at both sides of the ventricle interface.73 Similar to the hyh mouse, this astrocyte barrier is present in human cases with congenital hydrocephalus, where the barrier may also play similar functions.73,81
Conclusion
The ependyma has been widely considered as a barrier with poorly defined functions. However, the number of investigations that recognize the important roles for the neuroepithelium and mature ependyma in the development and physiology of the CNS is expanding. The knowledge of these roles furthers the understanding of the etiology of developmental and related diseases, such as hydrocephalus, and is useful for the design of new therapeutic approaches.
Disclosure of potential conflicts of interest
The authors declare no conflicts of interest.
Acknowledgments
Supported by grants SAF2010–19087 (Ministerio de Educación Cultura y Deporte, Spain) and P-11-CVI-07637 (Junta de Andalucía, Consejería de Economía Innovación y Ciencia, Spain) to PF-LL, and PI12/0631 (Instituto de Salud Carlos III, Spain) to AJJ.
References
- 1.Lowery LA, Sive H. Totally tubular: the mystery behind function and origin of the brain ventricular system. Bioessays. 2009;31:446–58. doi: 10.1002/bies.200800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bruni JE, Del Bigio MR, Clattenburg RE. Ependyma: normal and pathological. A review of the literature. Brain Res. 1985;356:1–19. doi: 10.1016/0165-0173(85)90016-5. [DOI] [PubMed] [Google Scholar]
- 3.Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 4.Sarnat HB. Role of human fetal ependyma. Pediatr Neurol. 1992;8:163–78. doi: 10.1016/0887-8994(92)90063-5. [DOI] [PubMed] [Google Scholar]
- 5.Sarnat HB. Ependymal reactions to injury. A review. J Neuropathol Exp Neurol. 1995;54:1–15. doi: 10.1097/00005072-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Del Bigio MR. The ependyma: a protective barrier between brain and cerebrospinal fluid. Glia. 1995;14:1–13. doi: 10.1002/glia.440140102. [DOI] [PubMed] [Google Scholar]
- 7.Del Bigio MR. Ependymal cells: biology and pathology. Acta Neuropathol. 2010;119:55–73. doi: 10.1007/s00401-009-0624-y. [DOI] [PubMed] [Google Scholar]
- 8.Oi S. Hydrocephalus research update--controversies in definition and classification of hydrocephalus. Neurol Med Chir (Tokyo) 2010;50:859–69. doi: 10.2176/nmc.50.859. [DOI] [PubMed] [Google Scholar]
- 9.McKechnie L, Vasudevan C, Levene M. Neonatal outcome of congenital ventriculomegaly. Semin Fetal Neonatal Med. 2012;17:301–7. doi: 10.1016/j.siny.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 10.Pérez-Fígares JM, Jimenez AJ, Rodríguez EM. Subcommissural organ, cerebrospinal fluid circulation, and hydrocephalus. Microsc Res Tech. 2001;52:591–607. doi: 10.1002/1097-0029(20010301)52:5<591::AID-JEMT1043>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 11.McAllister JP., 2nd Pathophysiology of congenital and neonatal hydrocephalus. Semin Fetal Neonatal Med. 2012;17:285–94. doi: 10.1016/j.siny.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Peña A, Harris NG, Bolton MD, Czosnyka M, Pickard JD. Communicating hydrocephalus: the biomechanics of progressive ventricular enlargement revisited. Acta Neurochir Suppl. 2002;81:59–63. doi: 10.1007/978-3-7091-6738-0_15. [DOI] [PubMed] [Google Scholar]
- 13.Levine DN. Intracranial pressure and ventricular expansion in hydrocephalus: have we been asking the wrong question? J Neurol Sci. 2008;269:1–11. doi: 10.1016/j.jns.2007.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Rekate HL. A consensus on the classification of hydrocephalus: its utility in the assessment of abnormalities of cerebrospinal fluid dynamics. Childs Nerv Syst. 2011;27:1535–41. doi: 10.1007/s00381-011-1558-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, Williams MA, Rigamonti D. Genetics of human hydrocephalus. J Neurol. 2006;253:1255–66. doi: 10.1007/s00415-006-0245-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee L. Riding the wave of ependymal cilia: genetic susceptibility to hydrocephalus in primary ciliary dyskinesia. J Neurosci Res. 2013;91:1117–32. doi: 10.1002/jnr.23238. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez EM, Guerra MM, Vío K, González C, Ortloff A, Bátiz LF, Rodríguez S, Jara MC, Muñoz RI, Ortega E, et al. A cell junction pathology of neural stem cells leads to abnormal neurogenesis and hydrocephalus. Biol Res. 2012;45:231–42. doi: 10.4067/S0716-97602012000300005. [DOI] [PubMed] [Google Scholar]
- 18.Rakic P. Developmental and evolutionary adaptations of cortical radial glia. Cereb Cortex N Y N 1991 2003; 13:541-9. [DOI] [PubMed]
- 19.Lehtinen MK, Walsh CA. Neurogenesis at the brain-cerebrospinal fluid interface. Annu Rev Cell Dev Biol. 2011;27:653–79. doi: 10.1146/annurev-cellbio-092910-154026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–88. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- 21.Gato A, Desmond ME. Why the embryo still matters: CSF and the neuroepithelium as interdependent regulators of embryonic brain growth, morphogenesis and histiogenesis. Dev Biol. 2009;327:263–72. doi: 10.1016/j.ydbio.2008.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Desmond ME, Duzy MJ, Federici BD. Second messenger regulation of occlusion of the spinal neurocoel in the chick embryo. Dev Dyn. 1993;197:291–306. doi: 10.1002/aja.1001970407. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Piontek J, Wolburg H, Piehl C, Liss M, Otten C, Christ A, Willnow TE, Blasig IE, Abdelilah-Seyfried S. Establishment of a neuroepithelial barrier by Claudin5a is essential for zebrafish brain ventricular lumen expansion. Proc Natl Acad Sci U S A. 2010;107:1425–30. doi: 10.1073/pnas.0911996107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gutzman JH, Sive H. Epithelial relaxation mediated by the myosin phosphatase regulator Mypt1 is required for brain ventricle lumen expansion and hindbrain morphogenesis. Development. 2010;137:795–804. doi: 10.1242/dev.042705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lowery LA, Sive H. Initial formation of zebrafish brain ventricles occurs independently of circulation and requires the nagie oko and snakehead/atp1a1a.1 gene products. Development. 2005;132:2057–67. doi: 10.1242/dev.01791. [DOI] [PubMed] [Google Scholar]
- 26.Bátiz LF, Jiménez AJ, Guerra M, Rodríguez-Pérez LM, Toledo CD, Vio K, Páez P, Pérez-Fígares JM, Rodríguez EM. New ependymal cells are born postnatally in two discrete regions of the mouse brain and support ventricular enlargement in hydrocephalus. Acta Neuropathol. 2011;121:721–35. doi: 10.1007/s00401-011-0799-x. [DOI] [PubMed] [Google Scholar]
- 27.Dziegielewska KM, Hinds LA, Møllgård K, Reynolds ML, Saunders NR. Blood-brain, blood-cerebrospinal fluid and cerebrospinal fluid-brain barriers in a marsupial (Macropus eugenii) during development. J Physiol. 1988;403:367–88. doi: 10.1113/jphysiol.1988.sp017254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR. Barriers in the developing brain and Neurotoxicology. Neurotoxicology. 2012;33:586–604. doi: 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 29.Saunders NR, Habgood MD, Dziegielewska KM. Barrier mechanisms in the brain, II. Immature brain. Clin Exp Pharmacol Physiol. 1999;26:85–91. doi: 10.1046/j.1440-1681.1999.02987.x. [DOI] [PubMed] [Google Scholar]
- 30.Martin C, Alonso MI, Santiago C, Moro JA, De la Mano A, Carretero R, Gato A. Early embryonic brain development in rats requires the trophic influence of cerebrospinal fluid. Int J Dev Neurosci. 2009;27:733–40. doi: 10.1016/j.ijdevneu.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 31.Mashayekhi F, Draper CE, Bannister CM, Pourghasem M, Owen-Lynch PJ, Miyan JA. Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain. 2002;125:1859–74. doi: 10.1093/brain/awf182. [DOI] [PubMed] [Google Scholar]
- 32.Owen-Lynch PJ, Draper CE, Mashayekhi F, Bannister CM, Miyan JA. Defective cell cycle control underlies abnormal cortical development in the hydrocephalic Texas rat. Brain. 2003;126:623–31. doi: 10.1093/brain/awg058. [DOI] [PubMed] [Google Scholar]
- 33.Sánchez-Camacho C, Rodríguez J, Ruiz JM, Trousse F, Bovolenta P. Morphogens as growth cone signalling molecules. Brain Res Brain Res Rev. 2005;49:242–52. doi: 10.1016/j.brainresrev.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 34.del Brío MA, Riera P, Muñoz RI, Montecinos H, Rodríguez EM. The metencephalic floor plate of chick embryos expresses two secretory glycoproteins homologous with the two glycoproteins secreted by the subcommissural organ. Histochem Cell Biol. 2000;113:415–26. doi: 10.1007/s004180000136. [DOI] [PubMed] [Google Scholar]
- 35.del Brio MA, Riera P, Peruzzo B, Rodríguez EM. Hindbrain floor plate of the rat: ultrastructural changes occurring during development. Microsc Res Tech. 2001;52:615–26. doi: 10.1002/1097-0029(20010301)52:5<615::AID-JEMT1045>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 36.Richter HG, Muñoz RI, Millán CS, Guiñazú MF, Yulis CR, Rodríguez EM. The floor plate cells from bovines express the mRNA encoding for SCO-spondin and its translation products. Brain Res Mol Brain Res. 2001;93:137–47. doi: 10.1016/S0169-328X(01)00181-4. [DOI] [PubMed] [Google Scholar]
- 37.Spassky N, Merkle FT, Flames N, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–8. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 39.Lavado A, Oliver G. Six3 is required for ependymal cell maturation. Development. 2011;138:5291–300. doi: 10.1242/dev.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tramontin AD, García-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex N Y N 1991 2003; 13:580-7. [DOI] [PubMed]
- 41.Sanai N, Nguyen T, Ihrie RA, Mirzadeh Z, Tsai H-H, Wong M, Gupta N, Berger MS, Huang E, Garcia-Verdugo J-M, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–6. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lim DA, Tramontin AD, Trevejo JM, Herrera DG, García-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28:713–26. doi: 10.1016/S0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 43.Del Carmen Gómez-Roldán M, Pérez-Martín M, Capilla-González V, Cifuentes M, Pérez J, García-Verdugo JM, Fernández-Llebrez P. Neuroblast proliferation on the surface of the adult rat striatal wall after focal ependymal loss by intracerebroventricular injection of neuraminidase. J Comp Neurol. 2008;507:1571–87. doi: 10.1002/cne.21618. [DOI] [PubMed] [Google Scholar]
- 44.Kuo CT, Mirzadeh Z, Soriano-Navarro M, Rasin M, Wang D, Shen J, Sestan N, Garcia-Verdugo J, Alvarez-Buylla A, Jan LY, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127:1253–64. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo J, Shook BA, Daniels SB, Conover JC. Subventricular zone-mediated ependyma repair in the adult mammalian brain. J Neurosci. 2008;28:3804–13. doi: 10.1523/JNEUROSCI.0224-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nechiporuk T, Fernandez TE, Vasioukhin V. Failure of epithelial tube maintenance causes hydrocephalus and renal cysts in Dlg5-/- mice. Dev Cell. 2007;13:338–50. doi: 10.1016/j.devcel.2007.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jiménez AJ, García-Verdugo JM, González CA, Bátiz LF, Rodríguez-Pérez LM, Páez P, Soriano-Navarro M, Roales-Buján R, Rivera P, Rodríguez S, et al. Disruption of the neurogenic niche in the subventricular zone of postnatal hydrocephalic hyh mice. J Neuropathol Exp Neurol. 2009;68:1006–20. doi: 10.1097/NEN.0b013e3181b44a5a. [DOI] [PubMed] [Google Scholar]
- 48.Mirzadeh Z, Han Y-G, Soriano-Navarro M, García-Verdugo JM, Alvarez-Buylla A. Cilia organize ependymal planar polarity. J Neurosci. 2010;30:2600–10. doi: 10.1523/JNEUROSCI.3744-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kishimoto N, Sawamoto K. Planar polarity of ependymal cilia. Differentiation. 2012;83:S86–90. doi: 10.1016/j.diff.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 50.Wallingford JB. Planar cell polarity, ciliogenesis and neural tube defects. Hum Mol Genet. 2006;15:R227–34. doi: 10.1093/hmg/ddl216. [DOI] [PubMed] [Google Scholar]
- 51.Hirota Y, Meunier A, Huang S, Shimozawa T, Yamada O, Kida YS, Inoue M, Ito T, Kato H, Sakaguchi M, et al. Planar polarity of multiciliated ependymal cells involves the anterior migration of basal bodies regulated by non-muscle myosin II. Development. 2010;137:3037–46. doi: 10.1242/dev.050120. [DOI] [PubMed] [Google Scholar]
- 52.Wallingford JB. Planar cell polarity signaling, cilia and polarized ciliary beating. Curr Opin Cell Biol. 2010;22:597–604. doi: 10.1016/j.ceb.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guirao B, Meunier A, Mortaud S, Aguilar A, Corsi J-M, Strehl L, Hirota Y, Desoeuvre A, Boutin C, Han Y-G, et al. Coupling between hydrodynamic forces and planar cell polarity orients mammalian motile cilia. Nat Cell Biol. 2010;12:341–50. doi: 10.1038/ncb2040. [DOI] [PubMed] [Google Scholar]
- 54.Tissir F, Qu Y, Montcouquiol M, Zhou L, Komatsu K, Shi D, Fujimori T, Labeau J, Tyteca D, Courtoy P, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–7. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 55.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
- 56.Lechtreck K-F, Delmotte P, Robinson ML, Sanderson MJ, Witman GB. Mutations in Hydin impair ciliary motility in mice. J Cell Biol. 2008;180:633–43. doi: 10.1083/jcb.200710162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davy BE, Robinson ML. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum Mol Genet. 2003;12:1163–70. doi: 10.1093/hmg/ddg122. [DOI] [PubMed] [Google Scholar]
- 58.Doggett NA, Xie G, Meincke LJ, Sutherland RD, Mundt MO, Berbari NS, Davy BE, Robinson ML, Rudd MK, Weber JL, et al. A 360-kb interchromosomal duplication of the human HYDIN locus. Genomics. 2006;88:762–71. doi: 10.1016/j.ygeno.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Ponting CP. A novel domain suggests a ciliary function for ASPM, a brain size determining gene. Bioinformatics. 2006;22:1031–5. doi: 10.1093/bioinformatics/btl022. [DOI] [PubMed] [Google Scholar]
- 60.Jacquet BV, Salinas-Mondragon R, Liang H, Therit B, Buie JD, Dykstra M, Campbell K, Ostrowski LE, Brody SL, Ghashghaei HT. FoxJ1-dependent gene expression is required for differentiation of radial glia into ependymal cells and a subset of astrocytes in the postnatal brain. Development. 2009;136:4021–31. doi: 10.1242/dev.041129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paez-Gonzalez P, Abdi K, Luciano D, Liu Y, Soriano-Navarro M, Rawlins E, Bennett V, Garcia-Verdugo JM, Kuo CT. Ank3-dependent SVZ niche assembly is required for the continued production of new neurons. Neuron. 2011;71:61–75. doi: 10.1016/j.neuron.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wodarczyk C, Rowe I, Chiaravalli M, Pema M, Qian F, Boletta A. A novel mouse model reveals that polycystin-1 deficiency in ependyma and choroid plexus results in dysfunctional cilia and hydrocephalus. PLoS One. 2009;4:e7137. doi: 10.1371/journal.pone.0007137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johanson CE, Szmydynger-Chodobska J, Chodobski A, Baird A, McMillan P, Stopa EG. Altered formation and bulk absorption of cerebrospinal fluid in FGF-2-induced hydrocephalus. Am J Physiol. 1999;277:R263–71. doi: 10.1152/ajpregu.1999.277.1.R263. [DOI] [PubMed] [Google Scholar]
- 64.Sival DA, Guerra M, den Dunnen WFA, Bátiz LF, Alvial G, Castañeyra-Perdomo A, Rodríguez EM. Neuroependymal denudation is in progress in full-term human foetal spina bifida aperta. Brain Pathol. 2011;21:163–79. doi: 10.1111/j.1750-3639.2010.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sarnat HB, Darwish HZ, Barth PG, Trevenen CL, Pinto A, Kotagal S, Shishikura K, Osawa M, Korobkin R. Ependymal abnormalities in lissencephaly/pachygyria. J Neuropathol Exp Neurol. 1993;52:525–41. doi: 10.1097/00005072-199309000-00011. [DOI] [PubMed] [Google Scholar]
- 66.Imai F, Hirai S, Akimoto K, Koyama H, Miyata T, Ogawa M, Noguchi S, Sasaoka T, Noda T, Ohno S. Inactivation of aPKClambda results in the loss of adherens junctions in neuroepithelial cells without affecting neurogenesis in mouse neocortex. Development. 2006;133:1735–44. doi: 10.1242/dev.02330. [DOI] [PubMed] [Google Scholar]
- 67.Klezovitch O, Fernandez TE, Tapscott SJ, Vasioukhin V. Loss of cell polarity causes severe brain dysplasia in Lgl1 knockout mice. Genes Dev. 2004;18:559–71. doi: 10.1101/gad.1178004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987;120:215–27. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- 69.Chenn A, Zhang YA, Chang BT, McConnell SK. Intrinsic polarity of mammalian neuroepithelial cells. Mol Cell Neurosci. 1998;11:183–93. doi: 10.1006/mcne.1998.0680. [DOI] [PubMed] [Google Scholar]
- 70.Gänzler-Odenthal SI, Redies C. Blocking N-cadherin function disrupts the epithelial structure of differentiating neural tissue in the embryonic chicken brain. J Neurosci. 1998;18:5415–25. doi: 10.1523/JNEUROSCI.18-14-05415.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ma X, Bao J, Adelstein RS. Loss of cell adhesion causes hydrocephalus in nonmuscle myosin II-B-ablated and mutated mice. Mol Biol Cell. 2007;18:2305–12. doi: 10.1091/mbc.E07-01-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abouhamed M, Grobe K, San IVLC, Thelen S, Honnert U, Balda MS, Matter K, Bähler M. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol Biol Cell. 2009;20:5074–85. doi: 10.1091/mbc.E09-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Roales-Buján R, Páez P, Guerra M, Rodríguez S, Vío K, Ho-Plagaro A, García-Bonilla M, Rodríguez-Pérez L-M, Domínguez-Pinos M-D, Rodríguez E-M, et al. Astrocytes acquire morphological and functional characteristics of ependymal cells following disruption of ependyma in hydrocephalus. Acta Neuropathol. 2012;124:531–46. doi: 10.1007/s00401-012-0992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Oliver C, González CA, Alvial G, Flores CA, Rodríguez EM, Bátiz LF. Disruption of CDH2/N-cadherin-based adherens junctions leads to apoptosis of ependymal cells and denudation of brain ventricular walls. J Neuropathol Exp Neurol. 2013;72:846–60. doi: 10.1097/NEN.0b013e3182a2d5fe. [DOI] [PubMed] [Google Scholar]
- 75.Shim JW, Sandlund J, Han CH, Hameed MQ, Connors S, Klagsbrun M, Madsen JR, Irwin N. VEGF, which is elevated in the CSF of patients with hydrocephalus, causes ventriculomegaly and ependymal changes in rats. Exp Neurol. 2013;247:703–9. doi: 10.1016/j.expneurol.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 76.Chae TH, Kim S, Marz KE, Hanson PI, Walsh CA. The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet. 2004;36:264–70. doi: 10.1038/ng1302. [DOI] [PubMed] [Google Scholar]
- 77.Jiménez AJ, Tomé M, Páez P, Wagner C, Rodríguez S, Fernández-Llebrez P, Rodríguez EM, Pérez-Fígares JM. A programmed ependymal denudation precedes congenital hydrocephalus in the hyh mutant mouse. J Neuropathol Exp Neurol. 2001;60:1105–19. doi: 10.1093/jnen/60.11.1105. [DOI] [PubMed] [Google Scholar]
- 78.Páez P, Bátiz L-F, Roales-Buján R, Rodríguez-Pérez L-M, Rodríguez S, Jiménez AJ, Rodríguez EM, Pérez-Fígares JM. Patterned neuropathologic events occurring in hyh congenital hydrocephalic mutant mice. J Neuropathol Exp Neurol. 2007;66:1082–92. doi: 10.1097/nen.0b013e31815c1952. [DOI] [PubMed] [Google Scholar]
- 79.Bátiz LF, Roales-Buján R, Rodríguez-Pérez LM, Matas IM, Páez P, Roque M, Jiménez AJ, Ramos C, Pérez-Fígares JM. A simple PCR-based genotyping method for M105I mutation of alpha-SNAP enhances the study of early pathological changes in hyh phenotype. Mol Cell Probes. 2009;23:281–90. doi: 10.1016/j.mcp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 80.Wagner C, Batiz LF, Rodríguez S, Jiménez AJ, Páez P, Tomé M, Pérez-Fígares JM, Rodríguez EM. Cellular mechanisms involved in the stenosis and obliteration of the cerebral aqueduct of hyh mutant mice developing congenital hydrocephalus. J Neuropathol Exp Neurol. 2003;62:1019–40. doi: 10.1093/jnen/62.10.1019. [DOI] [PubMed] [Google Scholar]
- 81.Domínguez-Pinos MD, Páez P, Jiménez A-J, Weil B, Arráez M-A, Pérez-Fígares J-M, Rodríguez E-M. Ependymal denudation and alterations of the subventricular zone occur in human fetuses with a moderate communicating hydrocephalus. J Neuropathol Exp Neurol. 2005;64:595–604. doi: 10.1097/01.jnen.0000171648.86718.bb. [DOI] [PubMed] [Google Scholar]
- 82.de Wit OA, den Dunnen WF, Sollie KM, Muñoz RI, Meiners LC, Brouwer OF, Rodríguez EM, Sival DA. Pathogenesis of cerebral malformations in human fetuses with meningomyelocele. Cerebrospinal Fluid Res. 2008;5:4. doi: 10.1186/1743-8454-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cherian SS, Love S, Silver IA, Porter HJ, Whitelaw AGL, Thoresen M. Posthemorrhagic ventricular dilation in the neonate: development and characterization of a rat model. J Neuropathol Exp Neurol. 2003;62:292–303. doi: 10.1093/jnen/62.3.292. [DOI] [PubMed] [Google Scholar]
- 84.Yung YC, Mutoh T, Lin M-E, Noguchi K, Rivera RR, Choi JW, Kingsbury MA, Chun J. Lysophosphatidic Acid Signaling May Initiate Fetal Hydrocephalus. Sci Transl Med 2011; 3:99ra87-99ra87. [DOI] [PMC free article] [PubMed]
- 85.Ferland RJ, Batiz LF, Neal J, Lian G, Bundock E, Lu J, Hsiao Y-C, Diamond R, Mei D, Banham AH, et al. Disruption of neural progenitors along the ventricular and subventricular zones in periventricular heterotopia. Hum Mol Genet. 2009;18:497–516. doi: 10.1093/hmg/ddn377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou FC, Sari Y, Powrozek T, Goodlett CR, Li T-K. Moderate alcohol exposure compromises neural tube midline development in prenatal brain. Brain Res Dev Brain Res. 2003;144:43–55. doi: 10.1016/S0165-3806(03)00158-5. [DOI] [PubMed] [Google Scholar]
- 87.Sakata-Haga H, Sawada K, Ohnishi T, Fukui Y. Hydrocephalus following prenatal exposure to ethanol. Acta Neuropathol. 2004;108:393–8. doi: 10.1007/s00401-004-0901-8. [DOI] [PubMed] [Google Scholar]
- 88.Brightman MW, Palay SL. THE FINE STRUCTURE OF EPENDYMA IN THE BRAIN OF THE RAT. J Cell Biol. 1963;19:415–39. doi: 10.1083/jcb.19.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nelson DJ, Wright EM. The distribution, activity, and function of the cilia in the frog brain. J Physiol. 1974;243:63–78. doi: 10.1113/jphysiol.1974.sp010742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murcia CL, Woychik RP. Expression of Pcdh15 in the inner ear, nervous system and various epithelia of the developing embryo. Mech Dev. 2001;105:163–6. doi: 10.1016/S0925-4773(01)00388-4. [DOI] [PubMed] [Google Scholar]
- 91.Hirano S, Wang X, Suzuki ST. Restricted expression of protocadherin 2A in the developing mouse brain. Brain Res Mol Brain Res. 2002;98:119–23. doi: 10.1016/S0169-328X(01)00317-5. [DOI] [PubMed] [Google Scholar]
- 92.Bouillé C, Mesnil M, Barriere H, Gabrion J. Gap junctional intercellular communication between cultured ependymal cells, revealed by lucifer yellow CH transfer and freeze-fracture. Glia. 1991;4:25–36. doi: 10.1002/glia.440040104. [DOI] [PubMed] [Google Scholar]
- 93.Condorelli DF, Trovato-Salinaro A, Mudò G, Mirone MB, Belluardo N. Cellular expression of connexins in the rat brain: neuronal localization, effects of kainate-induced seizures and expression in apoptotic neuronal cells. Eur J Neurosci. 2003;18:1807–27. doi: 10.1046/j.1460-9568.2003.02910.x. [DOI] [PubMed] [Google Scholar]
- 94.Söhl G, Odermatt B, Maxeiner S, Degen J, Willecke K. New insights into the expression and function of neural connexins with transgenic mouse mutants. Brain Res Brain Res Rev. 2004;47:245–59. doi: 10.1016/j.brainresrev.2004.05.006. [DOI] [PubMed] [Google Scholar]
- 95.Yamamoto T, Kardami E, Nagy JI. Basic fibroblast growth factor in rat brain: localization to glial gap junctions correlates with connexin43 distribution. Brain Res. 1991;554:336–43. doi: 10.1016/0006-8993(91)90213-F. [DOI] [PubMed] [Google Scholar]
- 96.Banizs B, Pike MM, Millican CL, Ferguson WB, Komlosi P, Sheetz J, Bell PD, Schwiebert EM, Yoder BK. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–39. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- 97.Jarvis CR, Andrew RD. Correlated electrophysiology and morphology of the ependyma in rat hypothalamus. J Neurosci. 1988;8:3691–702. doi: 10.1523/JNEUROSCI.08-10-03691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Guerra M, Blázquez JL, Peruzzo B, Peláez B, Rodríguez S, Toranzo D, Pastor F, Rodríguez EM. Cell organization of the rat pars tuberalis. Evidence for open communication between pars tuberalis cells, cerebrospinal fluid and tanycytes. Cell Tissue Res. 2010;339:359–81. doi: 10.1007/s00441-009-0885-8. [DOI] [PubMed] [Google Scholar]
- 99.Rash JE, Duffy HS, Dudek FE, Bilhartz BL, Whalen LR, Yasumura T. Grid-mapped freeze-fracture analysis of gap junctions in gray and white matter of adult rat central nervous system, with evidence for a “panglial syncytium” that is not coupled to neurons. J Comp Neurol. 1997;388:265–92. doi: 10.1002/(SICI)1096-9861(19971117)388:2<265::AID-CNE6>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 100.Li Y-C, Bai W-Z, Hashikawa T. Regionally varying F-actin network in the apical cytoplasm of ependymocytes. Neurosci Res. 2007;57:522–30. doi: 10.1016/j.neures.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 101.Lippoldt A, Jansson A, Kniesel U, Andbjer B, Andersson A, Wolburg H, Fuxe K, Haller H. Phorbol ester induced changes in tight and adherens junctions in the choroid plexus epithelium and in the ependyma. Brain Res. 2000;854:197–206. doi: 10.1016/S0006-8993(99)02355-0. [DOI] [PubMed] [Google Scholar]
- 102.Alvarez JI, Teale JM. Differential changes in junctional complex proteins suggest the ependymal lining as the main source of leukocyte infiltration into ventricles in murine neurocysticercosis. J Neuroimmunol. 2007;187:102–13. doi: 10.1016/j.jneuroim.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. doi: 10.1177/0192623310394214. [DOI] [PubMed] [Google Scholar]
- 104.Zhang J, Liss M, Wolburg H, Blasig IE, Abdelilah-Seyfried S. Involvement of claudins in zebrafish brain ventricle morphogenesis. Ann N Y Acad Sci. 2012;1257:193–8. doi: 10.1111/j.1749-6632.2012.06507.x. [DOI] [PubMed] [Google Scholar]
- 105.Cifuentes M, Fernández-LLebrez P, Pérez J, Pérez-Fígares JM, Rodríguez EM. Distribution of intraventricularly injected horseradish peroxidase in cerebrospinal fluid compartments of the rat spinal cord. Cell Tissue Res. 1992;270:485–94. doi: 10.1007/BF00645050. [DOI] [PubMed] [Google Scholar]
- 106.Nicholson C. Signals that go with the flow. Trends Neurosci. 1999;22:143–5. doi: 10.1016/S0166-2236(98)01388-5. [DOI] [PubMed] [Google Scholar]
- 107.Ghersi-Egea JF, Finnegan W, Chen JL, Fenstermacher JD. Rapid distribution of intraventricularly administered sucrose into cerebrospinal fluid cisterns via subarachnoid velae in rat. Neuroscience. 1996;75:1271–88. doi: 10.1016/0306-4522(96)00281-3. [DOI] [PubMed] [Google Scholar]
- 108.Proescholdt MG, Hutto B, Brady LS, Herkenham M. Studies of cerebrospinal fluid flow and penetration into brain following lateral ventricle and cisterna magna injections of the tracer [14C]inulin in rat. Neuroscience. 2000;95:577–92. doi: 10.1016/S0306-4522(99)00417-0. [DOI] [PubMed] [Google Scholar]
- 109.Kuchler S, Graff MN, Gobaille S, Vincendon G, Roche AC, Delaunoy JP, Monsigny M, Zanetta JP. Mannose dependent tightening of the rat ependymal cell barrier. In vivo and in vitro study using neoglycoproteins. Neurochem Int. 1994;24:43–55. doi: 10.1016/0197-0186(94)90128-7. [DOI] [PubMed] [Google Scholar]
- 110.Langlet F, Mullier A, Bouret SG, Prevot V, Dehouck B. Tanycyte-like cells form a blood-cerebrospinal fluid barrier in the circumventricular organs of the mouse brain. J Comp Neurol. 2013;521:3389–405. doi: 10.1002/cne.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Rosenberg GA, Kyner WT, Estrada E. Bulk flow of brain interstitial fluid under normal and hyperosmolar conditions. Am J Physiol. 1980;238:F42–9. doi: 10.1152/ajprenal.1980.238.1.F42. [DOI] [PubMed] [Google Scholar]
- 112.Wagshul ME, Eide PK, Madsen JR. The pulsating brain: A review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS. 2011;8:5. doi: 10.1186/2045-8118-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sweetman B, Linninger AA. Cerebrospinal fluid flow dynamics in the central nervous system. Ann Biomed Eng. 2011;39:484–96. doi: 10.1007/s10439-010-0141-0. [DOI] [PubMed] [Google Scholar]
- 114.Yamada S, Miyazaki M, Kanazawa H, Higashi M, Morohoshi Y, Bluml S, McComb JG. Visualization of cerebrospinal fluid movement with spin labeling at MR imaging: preliminary results in normal and pathophysiologic conditions. Radiology. 2008;249:644–52. doi: 10.1148/radiol.2492071985. [DOI] [PubMed] [Google Scholar]
- 115.Nguyen T, Chin WC, O’Brien JA, Verdugo P, Berger AJ. Intracellular pathways regulating ciliary beating of rat brain ependymal cells. J Physiol. 2001;531:131–40. doi: 10.1111/j.1469-7793.2001.0131j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Aghajanian GK, Gallager DW. Raphe origin of serotonergic nerves terminating in the cerebral ventricles. Brain Res. 1975;88:221–31. doi: 10.1016/0006-8993(75)90386-8. [DOI] [PubMed] [Google Scholar]
- 117.Mönkkönen KS, Hirst RA, Laitinen JT, O’Callaghan C. PACAP27 regulates ciliary function in primary cultures of rat brain ependymal cells. Neuropeptides. 2008;42:633–40. doi: 10.1016/j.npep.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 118.Conductier G, Brau F, Viola A, Langlet F, Ramkumar N, Dehouck B, Lemaire T, Chapot R, Lucas L, Rovère C, et al. Melanin-concentrating hormone regulates beat frequency of ependymal cilia and ventricular volume. Nat Neurosci. 2013;16:845–7. doi: 10.1038/nn.3401. [DOI] [PubMed] [Google Scholar]
- 119.Tomé M, Moreira E, Pérez-Fígares J-M, Jiménez AJ. Presence of D1- and D2-like dopamine receptors in the rat, mouse and bovine multiciliated ependyma. J Neural Transm. 2007;114:983–94. doi: 10.1007/s00702-007-0666-z. [DOI] [PubMed] [Google Scholar]
- 120.Davy BE, Robinson ML. Congenital hydrocephalus in hy3 mice is caused by a frameshift mutation in Hydin, a large novel gene. Hum Mol Genet. 2003;12:1163–70. doi: 10.1093/hmg/ddg122. [DOI] [PubMed] [Google Scholar]
- 121.Baas D, Meiniel A, Benadiba C, Bonnafe E, Meiniel O, Reith W, Durand B. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci. 2006;24:1020–30. doi: 10.1111/j.1460-9568.2006.05002.x. [DOI] [PubMed] [Google Scholar]
- 122.Ibañez-Tallon I, Pagenstecher A, Fliegauf M, Olbrich H, Kispert A, Ketelsen U-P, North A, Heintz N, Omran H. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet. 2004;13:2133–41. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- 123.Sawamoto K, Wichterle H, Gonzalez-Perez O, Cholfin JA, Yamada M, Spassky N, Murcia NS, Garcia-Verdugo JM, Marin O, Rubenstein JLR, et al. New neurons follow the flow of cerebrospinal fluid in the adult brain. Science. 2006;311:629–32. doi: 10.1126/science.1119133. [DOI] [PubMed] [Google Scholar]
- 124.Kelm S, Schauer R. Sialic acids in molecular and cellular interactions. Int Rev Cytol. 1997;175:137–240. doi: 10.1016/S0074-7696(08)62127-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cifuentes M, Rodríguez S, Pérez J, Grondona JM, Rodríguez EM, Fernández-Llebrez P. Decreased cerebrospinal fluid flow through the central canal of the spinal cord of rats immunologically deprived of Reissner’s fibre. Exp Brain Res. 1994;98:431–40. doi: 10.1007/BF00233981. [DOI] [PubMed] [Google Scholar]
- 126.Grondona JM, Pérez-Martín M, Cifuentes M, Pérez J, Estivill-Torrús G, Pérez-Fígares JM, Fernández-Llebrez P, Rodríguez EM. Neuraminidase injected into the cerebrospinal fluid impairs the assembly of the glycoproteins secreted by the subcommissural organ preventing the formation of Reissner’s fiber. Histochem Cell Biol. 1998;109:391–8. doi: 10.1007/s004180050240. [DOI] [PubMed] [Google Scholar]
- 127.Picketts DJ. Neuropeptide signaling and hydrocephalus: SCO with the flow. J Clin Invest. 2006;116:1828–32. doi: 10.1172/JCI29148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rodríguez EM, Oksche A, Montecinos H. Human subcommissural organ, with particular emphasis on its secretory activity during the fetal life. Microsc Res Tech. 2001;52:573–90. doi: 10.1002/1097-0029(20010301)52:5<573::AID-JEMT1042>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 129.Grondona JM, Pérez-Martín M, Cifuentes M, Pérez J, Jiménez AJ, Pérez-Fígares JM, Fernández-Llebrez P. Ependymal denudation, aqueductal obliteration and hydrocephalus after a single injection of neuraminidase into the lateral ventricle of adult rats. J Neuropathol Exp Neurol. 1996;55:999–1008. doi: 10.1097/00005072-199609000-00007. [DOI] [PubMed] [Google Scholar]
- 130.Cioffi DL, Pandey S, Alvarez DF, Cioffi EA. Terminal sialic acids are an important determinant of pulmonary endothelial barrier integrity. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1067–77. doi: 10.1152/ajplung.00190.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Meiniel A. The secretory ependymal cells of the subcommissural organ: which role in hydrocephalus? Int J Biochem Cell Biol. 2007;39:463–8. doi: 10.1016/j.biocel.2006.10.021. [DOI] [PubMed] [Google Scholar]
- 132.Castañeyra-Perdomo A, Meyer G, Carmona-Calero E, Bañuelos-Pineda J, Méndez-Medina R, Ormazabal-Ramos C, Ferres-Torres R. Alterations of the subcommissural organ in the hydrocephalic human fetal brain. Brain Res Dev Brain Res. 1994;79:316–20. doi: 10.1016/0165-3806(94)90138-4. [DOI] [PubMed] [Google Scholar]
- 133.Overholser MD, Whitley JR, O’Dell BL, Hogan AG. The ventricular system in hydrocephalic rat brains produced by a deficiency of vitamin B12 or of folic acid in the maternal diet. Anat Rec. 1954;120:917–33. doi: 10.1002/ar.1091200407. [DOI] [PubMed] [Google Scholar]
- 134.Zhang D, Zeldin DC, Blackshear PJ. Regulatory factor X4 variant 3: a transcription factor involved in brain development and disease. J Neurosci Res. 2007;85:3515–22. doi: 10.1002/jnr.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lang B, Song B, Davidson W, MacKenzie A, Smith N, McCaig CD, Harmar AJ, Shen S. Expression of the human PAC1 receptor leads to dose-dependent hydrocephalus-related abnormalities in mice. J Clin Invest. 2006;116:1924–34. doi: 10.1172/JCI27597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Dietrich P, Shanmugasundaram R, Shuyu E, Dragatsis I. Congenital hydrocephalus associated with abnormal subcommissural organ in mice lacking huntingtin in Wnt1 cell lineages. Hum Mol Genet. 2009;18:142–50. doi: 10.1093/hmg/ddn324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fernández-Llebrez P, Grondona JM, Pérez J, López-Aranda MF, Estivill-Torrús G, Llebrez-Zayas PF, Soriano E, Ramos C, Lallemand Y, Bach A, et al. Msx1-deficient mice fail to form prosomere 1 derivatives, subcommissural organ, and posterior commissure and develop hydrocephalus. J Neuropathol Exp Neurol. 2004;63:574–86. doi: 10.1093/jnen/63.6.574. [DOI] [PubMed] [Google Scholar]
- 138.Vio K, Rodríguez S, Navarrete EH, Pérez-Fígares JM, Jiménez AJ, Rodríguez EM. Hydrocephalus induced by immunological blockage of the subcommissural organ-Reissner’s fiber (RF) complex by maternal transfer of anti-RF antibodies. Exp Brain Res. 2000;135:41–52. doi: 10.1007/s002210000474. [DOI] [PubMed] [Google Scholar]
- 139.Ortloff AR, Vío K, Guerra M, Jaramillo K, Kaehne T, Jones H, McAllister JP, 2nd, Rodríguez E. Role of the subcommissural organ in the pathogenesis of congenital hydrocephalus in the HTx rat. Cell Tissue Res. 2013;352:707–25. doi: 10.1007/s00441-013-1615-9. [DOI] [PubMed] [Google Scholar]
- 140.Johansson PA, Dziegielewska KM, Ek CJ, Habgood MD, Møllgård K, Potter A, Schuliga M, Saunders NR. Aquaporin-1 in the choroid plexuses of developing mammalian brain. Cell Tissue Res. 2005;322:353–64. doi: 10.1007/s00441-005-1120-x. [DOI] [PubMed] [Google Scholar]
- 141.Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Oshio K, Watanabe H, Song Y, Verkman AS, Manley GT. Reduced cerebrospinal fluid production and intracranial pressure in mice lacking choroid plexus water channel Aquaporin-1. FASEB J. 2005;19:76–8. doi: 10.1096/fj.04-1711fje. [DOI] [PubMed] [Google Scholar]
- 143.Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res. 2010;7:15. doi: 10.1186/1743-8454-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Venero JL, Vizuete ML, Machado A, Cano J. Aquaporins in the central nervous system. Prog Neurobiol. 2001;63:321–36. doi: 10.1016/S0301-0082(00)00035-6. [DOI] [PubMed] [Google Scholar]
- 145.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–80. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Gunnarson E, Zelenina M, Aperia A. Regulation of brain aquaporins. Neuroscience. 2004;129:947–55. doi: 10.1016/j.neuroscience.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 147.Yukutake Y, Yasui M. Regulation of water permeability through aquaporin-4. Neuroscience. 2010;168:885–91. doi: 10.1016/j.neuroscience.2009.10.029. [DOI] [PubMed] [Google Scholar]
- 148.Yool AJ. Aquaporins: multiple roles in the central nervous system. Neuroscientist. 2007;13:470–85. doi: 10.1177/1073858407303081. [DOI] [PubMed] [Google Scholar]
- 149.Amiry-Moghaddam M, Ottersen OP. The molecular basis of water transport in the brain. Nat Rev Neurosci. 2003;4:991–1001. doi: 10.1038/nrn1252. [DOI] [PubMed] [Google Scholar]
- 150.Shen XQ, Miyajima M, Ogino I, Arai H. Expression of the water-channel protein aquaporin 4 in the H-Tx rat: possible compensatory role in spontaneously arrested hydrocephalus. J Neurosurg. 2006;105(Suppl):459–64. doi: 10.3171/ped.2006.105.6.459. [DOI] [PubMed] [Google Scholar]
- 151.Feng X, Papadopoulos MC, Liu J, Li L, Zhang D, Zhang H, Verkman AS, Ma T. Sporadic obstructive hydrocephalus in Aqp4 null mice. J Neurosci Res. 2009;87:1150–5. doi: 10.1002/jnr.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Li X, Kong H, Wu W, Xiao M, Sun X, Hu G. Aquaporin-4 maintains ependymal integrity in adult mice. Neuroscience. 2009;162:67–77. doi: 10.1016/j.neuroscience.2009.04.044. [DOI] [PubMed] [Google Scholar]
- 153.Papadopoulos MC, Verkman AS. Aquaporin water channels in the nervous system. Nat Rev Neurosci. 2013;14:265–77. doi: 10.1038/nrn3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23:2929–36. doi: 10.1111/j.1460-9568.2006.04829.x. [DOI] [PubMed] [Google Scholar]
- 155.Tourdias T, Dragonu I, Fushimi Y, Deloire MSA, Boiziau C, Brochet B, Moonen C, Petry KG, Dousset V. Aquaporin 4 correlates with apparent diffusion coefficient and hydrocephalus severity in the rat brain: a combined MRI-histological study. Neuroimage. 2009;47:659–66. doi: 10.1016/j.neuroimage.2009.04.070. [DOI] [PubMed] [Google Scholar]
- 156.Bloch O, Auguste KI, Manley GT, Verkman AS. Accelerated progression of kaolin-induced hydrocephalus in aquaporin-4-deficient mice. J Cereb Blood Flow Metab. 2006;26:1527–37. doi: 10.1038/sj.jcbfm.9600306. [DOI] [PubMed] [Google Scholar]
- 157.Skjolding AD, Holst AV, Broholm H, Laursen H, Juhler M. Differences in distribution and regulation of astrocytic aquaporin-4 in human and rat hydrocephalic brain. Neuropathol Appl Neurobiol. 2012 doi: 10.1111/j.1365-2990.2012.01275.x. [DOI] [PubMed] [Google Scholar]
- 158.Mishra PK, Teale JM. Transcriptome analysis of the ependymal barrier during murine neurocysticercosis. J Neuroinflammation. 2012;9:141. doi: 10.1186/1742-2094-9-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Deckert-Schlüter M, Schlüter D, Hof H, Wiestler OD, Lassmann H. Differential expression of ICAM-1, VCAM-1 and their ligands LFA-1, Mac-1, CD43, VLA-4, and MHC class II antigens in murine Toxoplasma encephalitis: a light microscopic and ultrastructural immunohistochemical study. J Neuropathol Exp Neurol. 1994;53:457–68. doi: 10.1097/00005072-199409000-00005. [DOI] [PubMed] [Google Scholar]
- 160.Nieminen M, Henttinen T, Merinen M, Marttila-Ichihara F, Eriksson JE, Jalkanen S. Vimentin function in lymphocyte adhesion and transcellular migration. Nat Cell Biol. 2006;8:156–62. doi: 10.1038/ncb1355. [DOI] [PubMed] [Google Scholar]
- 161.Deren KE, Packer M, Forsyth J, Milash B, Abdullah OM, Hsu EW, McAllister JP., 2nd Reactive astrocytosis, microgliosis and inflammation in rats with neonatal hydrocephalus. Exp Neurol. 2010;226:110–9. doi: 10.1016/j.expneurol.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 162.Olopade FE, Shokunbi MT, Sirén A-L. The relationship between ventricular dilatation, neuropathological and neurobehavioural changes in hydrocephalic rats. Fluids Barriers CNS. 2012;9:19. doi: 10.1186/2045-8118-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miller JM, McAllister JP., 2nd Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5. doi: 10.1186/1743-8454-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eskandari R, Harris CA, McAllister JP., 2nd Reactive astrocytosis in feline neonatal hydrocephalus: acute, chronic, and shunt-induced changes. Childs Nerv Syst. 2011;27:2067–76. doi: 10.1007/s00381-011-1552-4. [DOI] [PubMed] [Google Scholar]