Abstract
Tumor cells that survive radiation are more sensitive to T-cell-mediated lysis due to a spectrum of biological adaptations to cellular stress (defined as immunogenic modulation), including enhanced antigen processing and cell-surface presence of calreticulin. This mechanism can be exploited to maximize clinical benefit in patients receiving radiotherapy plus immunotherapy.
Keywords: radiation therapy, immunogenic modulation, antigen-processing machinery, calreticulin, ER-stress, CTL-mediated lysis, therapeutic cancer vaccine, immunotherapy
Introduction
Radiation therapy (RT) is standard-of-care for multiple malignancies, aimed at direct tumor destruction. However, systemic disease, treatment resistance, or the need for sublethal dosing to minimize normal tissue toxicity, often translates into surviving tumor cell populations and disease progression.1,2
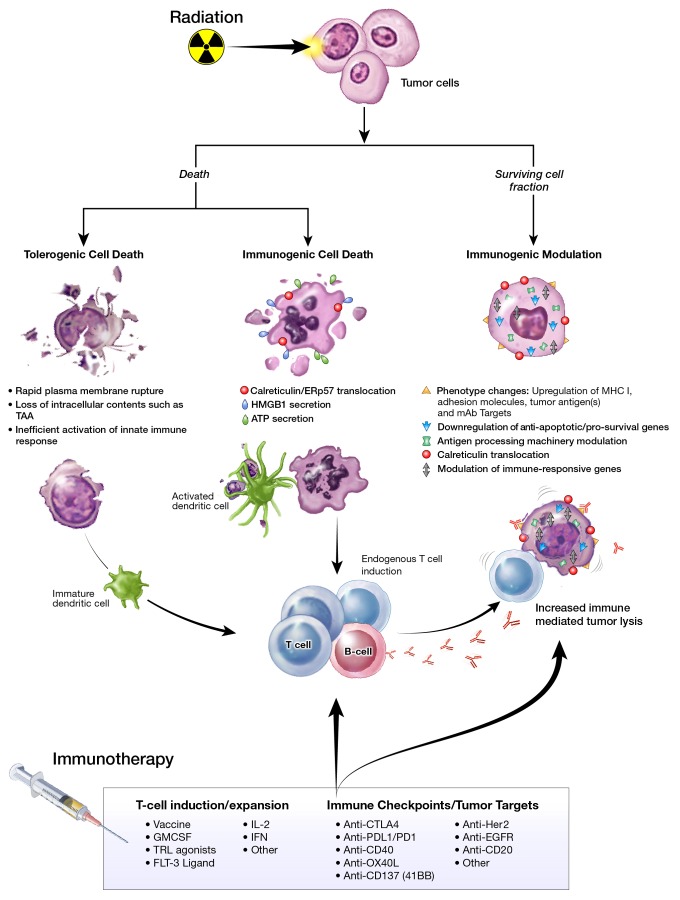
Mounting evidence suggests that radiation can modulate the tumor to become an immunostimulatory milieu, through direct effects on the immune system and (or) the tumor.1 Radiation can induce a continuum of immunogenic alterations on dying and surviving tumor cells (Fig. 1). Lethal irradiation induces tumor cell death while promoting antitumor immunity, defined as immunogenic cell death (ICD).3 Although immune responses in cancer patients receiving RT alone are often weak and rarely translate into protective immunity, the immunogenic effects of RT can be exploited to promote synergistic clinical benefit for patients receiving combination regimens with immunotherapy.1,2,4,5
Figure 1. Radiation-induced immunogenic modulation may enhance immunotherapy. Multiple immunogenic consequences of radiation therapy (RT) that can be harnessed to promote synergy with immunotherapeutic regimens.
Molecular Mechanisms of Radiation-induced Immunogenic Modulation
We have previously described a novel anticancer immune mechanism complementary to ICD and distinct from autophagy–termed immunogenic modulation–whereby radiation, and other anticancer therapies, induces a spectrum of molecular alterations in the biology of surviving tumor cells rendering them more sensitive to cytotoxic T lymphocyte (CTL)-mediated lysis.6 These include changes in the cancer cell-surface phenotype, the expression of antiapoptotic and/or immune-responsive genes, and the levels of antigen-processing machinery (APM) components, as well as the translocation of calreticulin to the cell surface.1,6,7 Radiation has been shown to also modulate the expression of tumor ligands required for monoclonal antibody therapies, including those targeting immune checkpoints, such as the programmed cell death 1 axis (PD1/PDL1) and cytotoxic T lymphocyte associated protein 4 (CTLA4).2,5,8 However, the molecular mechanism(s) underlying immunogenic modulation in tumor cells that survive radiation have not been fully investigated.
We have interrogated the immunogenic consequences and molecular mechanisms underlying radiation-induced immunogenic modulation in human carcinomas of the breast, lung, and prostate.9 CTL killing relies on the recognition of specific CD8+-restricted epitopes/MHC class I complexes on the surface of tumor cells, which is dictated by the cooperative action of several APM proteins. We hypothesized that radiation could elicit immunogenic modulation by altering the expression of proteins implicated in CTL-mediated tumor lysis, including calreticulin and APM components, thereby augmenting productive interactions between CTLs and tumor. We observed secretion of ATP and high mobility group box 1 (HMGB1) protein, two cardinal signs of ICD, from both dying and surviving carcinoma cells. Tumor irradiation in vitro and in vivo significantly augmented the expression of multiple APM components, and promoted cell-surface expression of calreticulin. Increased CTL lysis was primarily dictated by the presence of calreticulin on the surface of tumor cells, as increased CTL-mediated lysis was abrogated in the presence of a calreticulin blocking peptide, or when calreticulin translocation was inhibited. Moreover, CTL lysis of tumor targets increased significantly in the presence of exogenous calreticulin.10 These findings suggest a direct interaction between exposed calreticulin and CTLs, highlighting a novel function for calreticulin in this context, contrasting with its role in ICD in which calreticulin exposure is insufficient to elicit efficacious antitumor responses.3,9
Further interrogation of the mechanisms underlying radiation-induced immunogenic modulation revealed that radiation induces endoplasmic reticulum (ER) stress in carcinoma cells, triggering a survival response through the PERK branch of the unfolded protein response (UPR). This translates into increased expression of multiple APM components and other immune-relevant proteins, as we observed in tumor cells exposed to the ER-stress inducer thapsigargin.10 These findings strongly suggest that immunogenic modulation, and the resulting increase in tumor sensitivity to CTL lysis, is a result of this survival response stimulated in reaction to radiation-induced ER stress. However, other mechanisms such as autophagy and (or) branches of the UPR may also be complementary, contributing further to the enhanced susceptibility of radiation-treated cancer cells to cytolysis.10
Overall, our studies provide evidence that radiation induces a continuum of immunogenic alterations in tumor biology, ranging from immunogenic modulation to ICD. One can envision harnessing these manifestations in order to achieve optimal synergy with therapeutic cancer vaccines, monoclonal antibodies, and other immunotherapy regimens, thereby maximizing clinical benefit, even in the case of patients who have failed RT or who have limited treatment options.
Clinical Implications
These findings have translated into promising clinical benefits for patients receiving RT plus immunotherapy.1,2,4 In a multi-center Phase II trial, metastatic CRPC patients (n = 44) were randomized to receive 153Sm-EDTMP (Quadramet®, an FDA-approved radiopharmaceutical targeting bone metastasis) alone or in combination with PSA-TRICOM vaccine. Time to progression significantly improved (P = 0.03) with combination therapy (3.7 mo) compared with 153Sm-EDTMP alone (1.7 mo).4
Challenges and Promise for Radiation Plus Immunotherapy
Improving clinical outcomes for patients receiving RT plus immunotherapy relies on deepening our understanding of the specific molecular entities involved as well as finding answers to pertinent questions regarding immunogenic modulation:
1. What role do dosing and fractionation regimens play in RT’s ability to alter tumor biology, thus fostering tumor cell susceptibility to immune attack?
2. Are there differences in how ICD and immunogenic modulation synergize with immunotherapy? Can those differences be exploited to enhance RT’s role as an effective adjuvant to immunotherapy?
3. What is the optimal dose and sequence for combined RT/immunotherapy?
4. Can other forms of radiation, i.e., α particles or proton therapy also elicit immunogenic modulation and synergize with immunotherapy?
5. Can the combination of RT and therapeutic vaccines be an effective regimen for patients resistant to standard anticancer treatments?
Disclosure of Potential Conflicts of Interest
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed. No writing assistance was utilized in the production of this manuscript.
Acknowledgments
The authors thank Jeffrey Schlom for his support and helpful suggestions and Bonnie L Casey and Debra Weingarten for editorial assistance in the preparation of this manuscript. This research was supported by the Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health.
Glossary
Abbreviations:
- APM
antigen-processing machinery
- CTL
cytotoxic T lymphocyte
- ER
endoplasmic reticulum
- ICD
immunogenic cell death
- RT
radiation therapy
- UPR
unfolded protein response
Citation: Gameiro SR, Ardiani A, Kwilas A, Hodge JW. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. OncoImmunology 2014; 3:e28643; 10.4161/onci.28643
References
- 1.Kwilas AR, Donahue RN, Bernstein MB, Hodge JW. In the field: exploiting the untapped potential of immunogenic modulation by radiation in combination with immunotherapy for the treatment of cancer. Front Oncol. 2012;2:104. doi: 10.3389/fonc.2012.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Formenti SC, Demaria S. Combining radiotherapy and cancer immunotherapy: a paradigm shift. J Natl Cancer Inst. 2013;105:256–65. doi: 10.1093/jnci/djs629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 4.Heery CR, Madan RA, Bilusic M, Kim JW, Singh NK, Rauckhorst M, Steinberg SM, Dahut WL, Chen C, DiPaula RS, et al. A phase II randomized clinical trial of samarium-153 EDTMP (Sm-153) with or without PSA-tricom vaccine in metastatic castration-resistant prostate cancer (mCRPC) after docetaxel. ASCO Genitourinary Cancer Symposium. Orlando, Florida: J Clin Oncol 31 (suppl 6; abstr 102), 2013. [Google Scholar]
- 5.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hodge JW, Kwilas A, Ardiani A, Gameiro SR. Attacking malignant cells that survive therapy: Exploiting immunogenic modulation. Oncoimmunology. 2013;2:e26937. doi: 10.4161/onci.26937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Makinde AY, John-Aryankalayil M, Palayoor ST, Cerna D, Coleman CN. Radiation survivors: understanding and exploiting the phenotype following fractionated radiation therapy. Mol Cancer Res. 2013;11:5–12. doi: 10.1158/1541-7786.MCR-12-0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wattenberg MM, Kwilas AR, Gameiro SR, Dicker AP, Hodge JW. Expanding the use of monoclonal antibody therapy of cancer by using ionising radiation to upregulate antibody targets. Br J Cancer. 2014;110:1472–80. doi: 10.1038/bjc.2014.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weiner LM, Lotze MT. Tumor-cell death, autophagy, and immunity. N Engl J Med. 2012;366:1156–8. doi: 10.1056/NEJMcibr1114526. [DOI] [PMC free article] [PubMed] [Google Scholar]