Abstract
The egg apparatus-secreted polymorphic EA1 peptide is required for micropylar pollen tube (PT) guidance in maize, the last step of the PT journey during the double fertilization process in flowering plants. In a recent study we have shown that maize PTs are attracted in vitro by EA1 and that their growth is arrested at high peptide concentrations. Moreover, we have also shown that maize PTs are guided in vitro in a species-preferential manner to the micropylar opening of transgenic Arabidopsis ovules secreting the EA1-GFP fusion protein. In support of these findings, we have improved the ligand-receptor labeling assay and report here that the EA1 peptide interacts in vitro with the maize PT apex in a species-specific manner. Bound peptide gets internalized in large vesicles and is degraded. This finding indicates that the pollen tube remains sensitive to the attractant by its rapid internalization.
Keywords: fertilization, pollen tube guidance, signaling peptide, ligand-receptor interaction, EA1, maize
After successful adhesion, hydration and germination on a receptive stigma, flowering plants (angiosperms) transport and deliver the two non-motile sperm cells to the female gametophyte via a highly specialized cell type, the pollen tube, for the achievement of double fertilization. Genetic studies have shown that the pollen tube path is controlled by various cross-incompatible (interspecific) and self-incompatible (intraspecific) hybridization mechanisms likely involving a larger number of species-specific molecular players.1-3 Among the molecules involved, maize EA1 (Egg Apparatus 1) was the first female gametophyte expressed signaling molecule identified to be required for pollen tube guidance during the last step of the journey.4 The egg apparatus specifically expressed EA1 is encoding a 94 amino acids hydrophobic precursor protein with a predicted N-terminal transmembrane domain. We have previously shown that a GFP fusion protein of EA1 is secreted from the egg apparatus and accumulates in the cell walls of micropylar nucellar cells, and that its knockdown triggers loss of micropylar pollen tube guidance.4 EA1 belongs to a novel class of hydrophobic and polymorphic peptides, of which until now only one additional member, EAL1 (EA1-like1) has been functionally characterized in maize and was shown to be involved in regulating cell fate of female gametophyte cells.5
EA1-like peptides do not exist in eudicotyledonous plant species. In the eudicot model plant Torenia fournieri cysteine-rich defensin-like proteins were identified as LUREs to attract own pollen tubes in a species-preferential manner.6 LUREs have been identified recently also in Torenia concolor (TcCRP1) and in Arabidopsis species (AtLURE1 and AlLURE1).7,8 In a recent publication we could show that EA1 acts as a species-preferential direct attractant of maize pollen tubes in vitro. Arabidopsis ovules secreting the peptide are capable to guide maize pollen tubes in a species-preferential manner toward the micropylar opening of the ovule.9
In addition to the above work we have now visualized the interaction of EA1 peptide derived from the maize inbred line A188 with pollen tubes of various maize inbred lines, a maize relative and two non-grass species in vitro. Therefore we performed several modified in vitro pollen tube binding assays based on Márton et al.9 For each experiment we used freshly germinated pollen of the maize inbred lines B73, A188 and HiIIA, of Tripsacum dactyloides, Lilium “Stargazer” and Nicotiana benthamiana, respectively. Pollen tubes were incubated either with synthetic predicted mature EA1 or its homologous peptide EAL2 (EA1-like2)2 that were both labeled with the green fluorophore DyLight 488 NHS Ester. A protocol is attached as Suppl. Materials. Binding of labeled EA1 could be observed at the pollen tube apex for all maize lines (Fig. 1A–H), but was weaker with HiIIA pollen compared with the other inbred lines (see also Table 1). The peptide could also be detected inside vesicles within the pollen tube apex region. These data indicate that the peptide is recognized specifically at the pollen tube membrane and gets immediately internalized. Binding events could not be observed with the related EAL2 control peptide (Fig. S1) demonstrating a highly specific interaction of EA1 with its receptor.
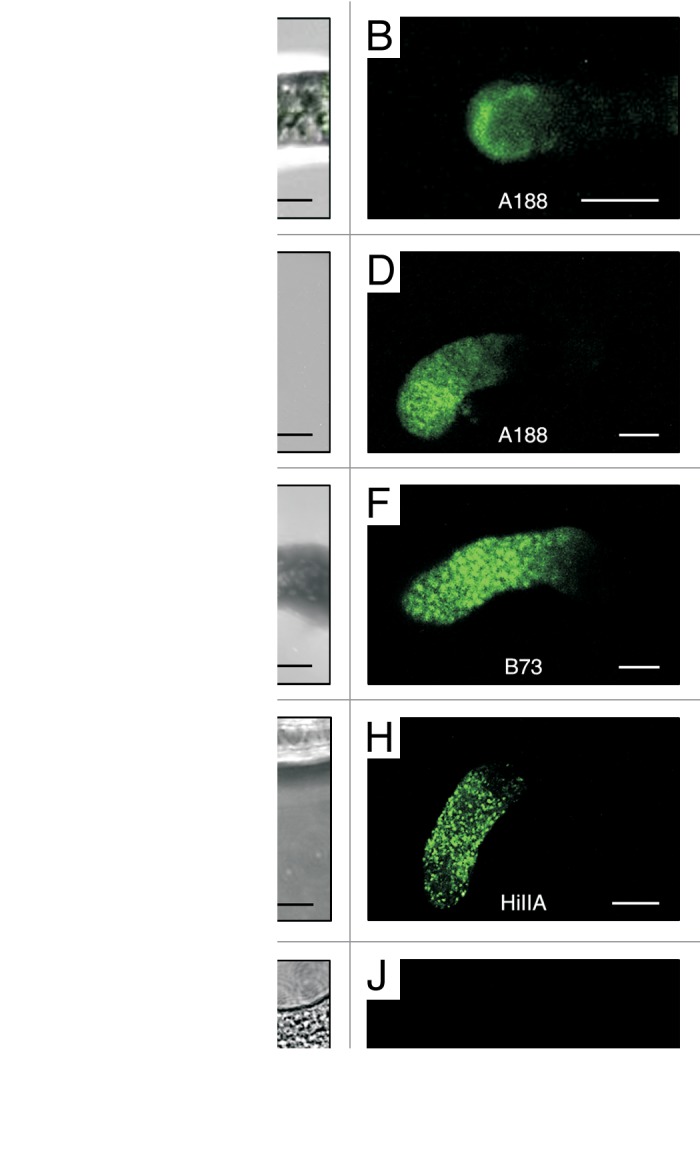
Figure 1. EA1 peptide interacts with the maize pollen tube apex in a species-specific manner. In vitro pollen tube binding assays of three maize inbred lines and the maize relative Tripsacum dactyloides (T.d.) were performed with synthetic predicted mature EA1 peptide labeled with the green fluorophore DyLight 488 NHS Ester. Z-projections of confocal image stacks of 6 to 20 μm thick sections are shown in each panel. (A, C, E, G and I) Merged bright field and fluorescence micrographs. (B, D, F, H and J) UV-fluorescence images. (A–B) Labeled EA1 fluorescence is visible at the apical membrane region of a A188 pollen tube tip. (C–H) Pollen tubes of A188, B73, and HiIIA, respectively, displaying fluorescence at their apical region in vesicle-like structures, whereas (I–J) fluorescence is not detectable from T. d. pollen tubes. Scale bars represent 10 μm.
Table 1. Summary of in vitro pollen tube binding assays of three maize inbred lines, Tripsacum dactyloides, Lilium “Stargazer” and Nicotiana benthamiana, respectively. Experiments were either performed with synthetic predicted mature EA1 or EAL2 peptides both labeled with the green fluorophore DyLight 488 NHS Ester.
| Peptide vs. PTs |
+ DyLight 488-labeled sEA1 |
+ DyLight 488-labeled EAL2 |
|---|---|---|
| Zea mays A188 | ++ | − |
| Zea mays B73 | ++ | − |
| Zea mays HiIIA | + | − |
| Tripsacum dactyloides | − | − |
| Lilium “Stargazer“ | − | − |
| Nicotiana benthamiana | − | − |
PT, pollen tubes; vs.,vs.; +, interaction; −, no interaction
Until now only few ligand-receptor pairs for cell-to-cell communication are described in plants and they mainly involve receptor-like kinases (RLKs). One of the best characterized RLKs is CLAVATA1 (CLV1) which is bound by the signaling peptide CLAVATA3 (CLV3) to control stem cell fate in the shoot apical meristem.10 Other signaling peptides include EPIDERMAL PATTERNING FACTOR 1/2 (EPF1/2) which is regulating stomatal patterning via interaction with the ERECTA-family RLKs11 and INFLORESCENCE DEFICIENT IN ABSCISSION (IDA) which is signaling through the RLKs HAESA (HAE) and HAESA-LIKE2 (HSL2) during floral abscission and lateral root emergence.12,13 In the Brassicacea binding of the pollen-coat protein S-Locus Cysteine-Rich/S-Protein-11 (SCR/SP11) to the stigma-specific S-locus Receptor Kinase (SRK) regulates sporophytic self-incompatibility, which is a reproductive strategy for preventing self-fertilization and allowing genetic diversity to be maintained.14 Although it still has to be shown whether the EA1 peptide also acts through a proteinaceous membrane receptor, it is conceivable that it may be recognized either by a tip-localized transmembrane RLK protein or an ion channel. The RLK superfamily forms a large receptor group in plants with more than 100 RLKs expressed in pollen of Arabidopsis.15,16 A subsequent signal transduction pathway could lead to reorientation of the polar tip growth toward the source of the attractant, since the direction of pollen tube growth is controlled at its tip involving Rho GTPase signaling.16 The receptor might also represent an ion channel, which have been shown to be involved in pollen tube growth and guidance.17 To avoid circular tube growth, one would expect such a reorientation signal to be transient and short for fine-tuning of the growth direction. A ligand-receptor complex thus must be inactivated, dissociated, degraded or internalized very shortly after signal transduction pathway(s) are initiated. Usually disassociation and inactivation of peptide ligands occurs in endosomes.18-20 As the fluorophore Dylight 488 is stable from pH 4 to pH 9, its insensitivity against the acid environment inside endosomal vesicles is consistent with the remaining strong signal inside the pollen tube apex after removing excess of labeled peptide from the pollen tubes (Fig. 2).
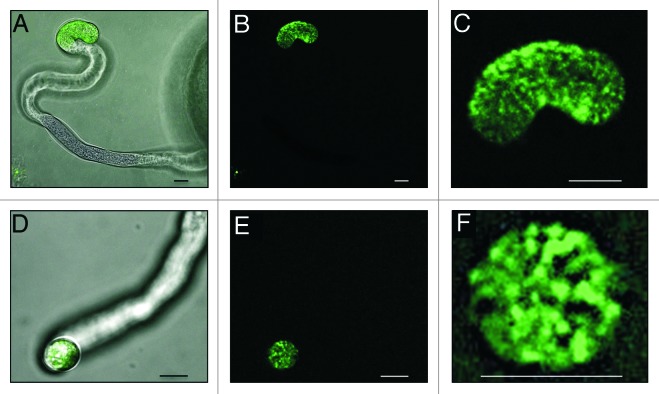
Figure 2. DyLight-labeled synthetic EA1 peptide gets internalized in vesicles at the apical region of the maize pollen tube tip. (A and D) Merged bright field and fluorescence micrographs. (B, C, E and F) UV-fluorescence micrographs. (A–B) Side-view of a pollen tube interacting with labeled EA1 peptide at its apical and sub-apical region. (C) Close-up of (B) displaying labeled EA1 in vesicle-like structures inside the pollen tube tip region. (D‒E) A pollen tube tip facing toward the observer displays labeled EA1 peptide in the tube tip. (F) Close-up of (E) displaying labeled EA1 in vesicles inside the pollen tube tip. Single optical sections are shown in (B, C, E and F). Scale bars represent 10 μm.
Within many plant species, several reproductive crossing barriers have evolved to avoid fertilization by undesirable or alien pollen tubes and sperm cells, respectively.2,21 EA1 as a short-range pollen tube attractor is a candidate for being involved in such a control mechanism. To examine whether EA1 acts species-specific we investigated its ability to bind to pollen tubes of other plant species than maize. Neither germinated pollen of the eudicot model plant Nicotiana benthamiana nor of the monocot plant lily “Stargazer” was able to recognize EA1 in the assay (Fig. S1; Table 1). Tripsacum dactyloides was chosen as a grass model to analyze species-specificity of EA1 interaction. Based on ribosomal ITS sequences, Zea and Tripsacum are closely related and form a clade that is clearly differentiated from other Poacea.22 Despite this close relationship, EA1 as well as the control peptide EAL2 are not capable to bind to pollen tube tips of T. dactyloides (Fig. 1I–J, Fig. S1). This result is substantiated by the finding that pollen tubes of T. dactyloides were not attracted by EA1 secreted from Arabidopsis ovules.9 Regarding the fact that in vivo pollination of shortened Z. mays silks with T. dactyloides pollen results in fertilization and seed set,1,23 these pollen tubes might need to grow through the sporophytic tissue of the transmitting tract in order to become competent for attraction, as it was reported for Arabidopsis and Torenia.24,25
Taken together we presented a method for visualizing the specific interaction of a peptide ligand with its unknown receptor at the apex of a growing pollen tube. Recently, Okuda et al. reported an alternative method for displaying the binding activity of LURE2, a pollen tube attractor of Torenia fournieri.6,26 After incubation with pollen tubes LURE2 could be detected via crosslinking and immunostaining at the pollen tube tip region. Both methods demonstrate the binding of a pollen tube attractor to the pollen tube tip. The usage of a fluorophore coupled peptide for in vitro binding assays with non-fixed pollen tubes additionally allows the detection of the internalized peptide after being bound to the tip. It will now be the major future challenge to identify and characterize the exact binding mechanism, to identify the receptor and the corresponding signal pathway that leads to a reorientation of the pollen tube growth.
Supplementary Material
Acknowledgment
We thank Annemarie Taffner for excellent technical assistance, Günther Peissig and Ursula Wittmann for plant care. Financial support by the German Research Foundation DFG (grants MA 3976/1–2 to M.L.M. and SFB924 TP A03 to TD) is gratefully acknowledged.
Glossary
Abbreviations:
- EA1
Egg Apparatus 1
- PT
pollen tube
- GFP
green-fluorescent protein
- EAL2
EA1-like 2
- NHS
N-hydroxysuccinimid
- RLK
receptor-like kinase
- CLV1/3
CLAVATA1/3
- EPF1/2
EPIDERMAL PATTERNING FACTOR 1/2
- IDA
INFLORESCENCE DEFICIENT IN ABSCISSION
- HAE
HAESA
- HSL2
HAESA-LIKE2
- SCR/SP11
S-Locus Cystein-Rich/S-Protein-11
- SRK
S-Locus Receptor Kinase
- ITS
internal transcribed spacer
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Lausser A, Kliwer I, Srilunchang KO, Dresselhaus T. Sporophytic control of pollen tube growth and guidance in maize. J Exp Bot. 2010;61:673–82. doi: 10.1093/jxb/erp330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dresselhaus T, Lausser A, Márton ML. Using maize as a model to study pollen tube growth and guidance, cross-incompatibility and sperm delivery in grasses. Ann Bot. 2011;108:727–37. doi: 10.1093/aob/mcr017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palanivelu R, Tsukamoto T. Pathfinding in angiosperm reproduction: pollen tube guidance by pistils ensures successful double fertilization. Wiley Interdiscip Rev Dev Biol. 2012;1:96–113. doi: 10.1002/wdev.6. [DOI] [PubMed] [Google Scholar]
- 4.Márton ML, Cordts S, Broadhvest J, Dresselhaus T. Micropylar pollen tube guidance by egg apparatus 1 of maize. Science. 2005;307:573–6. doi: 10.1126/science.1104954. [DOI] [PubMed] [Google Scholar]
- 5.Krohn NG, Lausser A, Juranić M, Dresselhaus T. Egg cell signaling by the secreted peptide ZmEAL1 controls antipodal cell fate. Dev Cell. 2012;23:219–25. doi: 10.1016/j.devcel.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 6.Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, Yui R, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–61. doi: 10.1038/nature07882. [DOI] [PubMed] [Google Scholar]
- 7.Kanaoka MM, Kawano N, Matsubara Y, Susaki D, Okuda S, Sasaki N, et al. Identification and characterization of TcCRP1, a pollen tube attractant from Torenia concolor. Ann Bot. 2011;108:739–47. doi: 10.1093/aob/mcr111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takeuchi H, Higashiyama T. A species-specific cluster of defensin-like genes encodes diffusible pollen tube attractants in Arabidopsis. PLoS Biol. 2012;10:e1001449. doi: 10.1371/journal.pbio.1001449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Márton ML, Fastner A, Uebler S, Dresselhaus T. Overcoming hybridization barriers by the secretion of the maize pollen tube attractant ZmEA1 from Arabidopsis ovules. Curr Biol. 2012;22:1194–8. doi: 10.1016/j.cub.2012.04.061. [DOI] [PubMed] [Google Scholar]
- 10.Ogawa M, Shinohara H, Sakagami Y, Matsubayashi Y. Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science. 2008;319:294. doi: 10.1126/science.1150083. [DOI] [PubMed] [Google Scholar]
- 11.Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, et al. Direct interaction of ligand-receptor pairs specifying stomatal patterning. Genes Dev. 2012;26:126–36. doi: 10.1101/gad.179895.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Butenko MA, Patterson SE, Grini PE, Stenvik GE, Amundsen SS, Mandal A, et al. Inflorescence deficient in abscission controls floral organ abscission in Arabidopsis and identifies a novel family of putative ligands in plants. Plant Cell. 2003;15:2296–307. doi: 10.1105/tpc.014365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumpf RP, Shi CL, Larrieu A, Stø IM, Butenko MA, Péret B, et al. Floral organ abscission peptide IDA and its HAE/HSL2 receptors control cell separation during lateral root emergence. Proc Natl Acad Sci USA. 2013;110:5235–40. doi: 10.1073/pnas.1210835110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iwano M, Takayama S. Self/non-self discrimination in angiosperm self-incompatibility. Curr Opin Plant Biol. 2012;15:78–83. doi: 10.1016/j.pbi.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc Natl Acad Sci USA. 2001;98:10763–8. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin Y, Yang Z. Rapid tip growth: insights from pollen tubes. Semin Cell Dev Biol. 2011;22:816–24. doi: 10.1016/j.semcdb.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dresselhaus T, Franklin-Tong N. Male-Female Cross-Talk during Pollen Germination, Tube Growth and Guidance, and Double Fertilization. Mol Plant. 2013 doi: 10.1093/mp/sst061. [DOI] [PubMed] [Google Scholar]
- 18.Reyes FC, Buono R, Otegui MS. Plant endosomal trafficking pathways. Curr Opin Plant Biol. 2011;14:666–73. doi: 10.1016/j.pbi.2011.07.009. [DOI] [PubMed] [Google Scholar]
- 19.Geldner N, Robatzek S. Plant receptors go endosomal: a moving view on signal transduction. Plant Physiol. 2008;147:1565–74. doi: 10.1104/pp.108.120287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Irani NG, Russinova E. Receptor endocytosis and signaling in plants. Curr Opin Plant Biol. 2009;12:653–9. doi: 10.1016/j.pbi.2009.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Wheeler MJ, de Graaf BH, Hadjiosif N, Perry RM, Poulter NS, Osman K, et al. Identification of the pollen self-incompatibility determinant in Papaver rhoeas. Nature. 2009;459:992–5. doi: 10.1038/nature08027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckler ES, 4th, Holtsford TP. Zea systematics: ribosomal ITS evidence. Mol Biol Evol. 1996;13:612–22. doi: 10.1093/oxfordjournals.molbev.a025621. [DOI] [PubMed] [Google Scholar]
- 23.Mangelsdorf PC, Reeves RG. Hybridization of maize, Tripsacum, and Euchlaena. J Hered. 1931;22:329–43. [Google Scholar]
- 24.Higashiyama T, Kuroiwa H, Kawano S, Kuroiwa T. Guidance in vitro of the pollen tube to the naked embryo sac of torenia fournieri. Plant Cell. 1998;10:2019–32. doi: 10.1105/tpc.10.12.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin Y, Leydon AR, Manziello A, Pandey R, Mount D, Denic S, et al. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet. 2009;5:e1000621. doi: 10.1371/journal.pgen.1000621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Okuda S, Suzuki T, Kanaoka MM, Mori H, Sasaki N, Higashiyama T. Acquisition of LURE-Binding Activity at the Pollen Tube Tip of Torenia fournieri. Mol Plant. 2013 doi: 10.1093/mp/sst050. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.