Abstract
Innate immunity is generally initiated with recognition of conserved pathogen-associated molecular patterns (PAMPs). PAMPs are perceived by pattern recognition receptors (PRRs), leading to activation of a series of immune responses, including the expression of defense genes, ROS production and activation of MAP kinase. Recent progress has indicated that receptor-like cytoplasmic kinases (RLCKs) are directly activated by ligand- activated PRRs and initiate pattern -triggered immunity (PTI) in both Arabidopsis and rice. To suppress PTI, pathogens inhibit the RLCKs by many types of effectors, including AvrAC, AvrPphB and Xoo1488. In this review, we summarize recent advances in RLCK-mediated PTI in plants.
Keywords: RLCK, PRR, MAPK, immunity, PAMP
The first layer of defense against pathogen attack is triggered by recognition of PAMPs by PRRs, called PTI.1 PRRs consist of a variety of receptor like kinases (RLK) and receptor like proteins, including FLS2, CERK1, CEBiP, LYM1, LYM3, LYP4, and LYP6.2-9 These PRRs directly recognize PAMPs, such as flagellin, chitin, or peptidoglycan (PGN). The activation of PTI leads to a series of immune responses, which include callose deposition, production of reactive oxygen species (ROS), transcriptional induction of defense genes, and activation of mitogen-activated protein kinase (MAPK) cascades.1,10 However, it is not clear how ligand-activated PRRs transmit signals downstream. Thus, this mini review will focus on recent work about the dynamics of ligand-activated PRRs and roles of RLCKs, the downstream components of PRRs.
Dynamics of Pattern Recognition Receptors by Perception of PAMPs
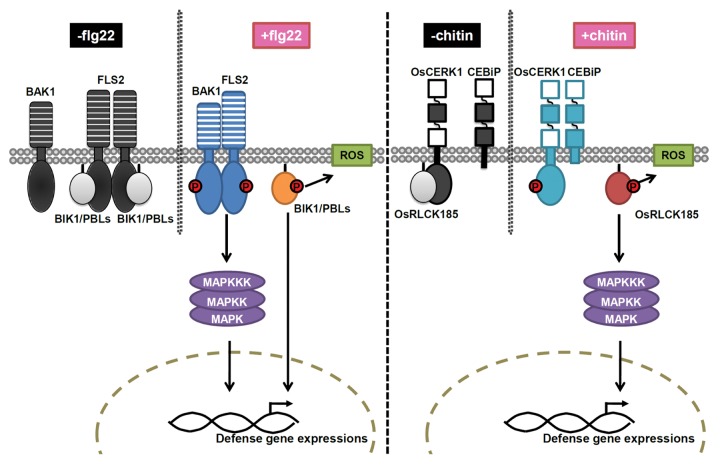
Flagellin is a highly conserved PAMP among bacterial pathogens. A conserved 22–amino acid peptide (flg22) in the N terminus of flagellin is specifically recognized by the leucine-rich repeat (LRR)-RLK, FLS2.2 FLS2 forms a homodimer in the absence of flg22 (Fig. 1).11 Upon flg22 perception, FLS2 rapidly forms a complex with another LRR-RLK, BAK1 (Fig. 1).12-14 This association occurs within seconds and leads to rapid phosphorylation of FLS2 and BAK1.13,14 Activated complexes transmit the signals to downstream components to initiate flg22-dependent PTI.15
Figure 1. Model for the signal transduction of PTI mediated by RLCKVII family. Flg22 perception and signaling in Arabidopsis is shown on the left, and chitin perception and signaling in rice is shown on the right. FLS2 and OsCERK1 interact with BIK1/PBLs and OsRLCK185 in the absence of flg22 and chitin, respectively. Treatment with flg22 or chitin induces an interaction between BAK1 and FLS2 or OsCERK1 and CEBiP, respectively. This interaction leads to trans-phosphorylation of BIK1/PBLs and OsRLCK185 by the FLS2-BAK1 complex and OsCERK1, respectively. The phosphorylated BIK1/PBLs and OsRLCK185 dissociate from the receptor complexes and initiate downstream immune responses such as ROS production and gene expressions. OsRLCK185 but not BIK1/PBLs functions upstream of MAPK cascades.
Chitin, a major component of fungal cell walls, is a linear polymer of β-1,4-linked N-acetyl-glucosamine.16 In Arabidopsis, chitin is recognized by a PRR, AtCERK1, with extracellular lysine motif (LysM)-domains.5,17,18 Chitin induces homodimerization of AtCERK1, which is essential for activation of downstream signaling.19 In contrast, OsCERK1 does not bind to chitn in rice.20 In response to chitin, OsCERK1 forms a heterodimer with CEBiP, encoding a LysM receptor protein that directly binds chitin (Fig. 1),7,21 and subsequently activates PTI. Thus, chitin perception occurs in a different manner between Arabidopsis and rice.
PGN is an essential cell wall component in bacteria and has a similar structure to chitin. In Arabidopsis, AtCERK1 is involved in recognition of PGN together with AtLYM1 and AtLYM3, LysM-containing proteins. In rice, OsLYP4 and OsLYP6, rice homologs of AtLYM1 and AtLYM3, also directly bind PGN.9 It suggests that OsCERK1 may recognize PGN with OsLYP4 and OsLYP6, although direct evidence remains to be addressed.
RLCK is a Key Regulator in PRR-Mediated Signaling
One of the RLK superfamily lacks an extracellular domain, which was designated as RLCK. A large number of RLCK genes has been found in plants; rice and Arabidopsis contain 379 and 200 RLCK genes, respectively.22 RLCKs are divided into 13 subfamilies (RLCKs I–XIII), based on phylogenetic clades.22 BIK1, a member of the RLCKVII subfamily, interacts with FLS2 and EFR in Arabidopsis. By stimulation with flg22, BIK1 is phosphorylated in an FLS2- and BAK1-dependent manner and dissociated from FLS2.23,24 Subsequently, BIK1 induces callose deposition and ROS production. PBS1, PBL1 and PBL2, members of the RLCKVII subfamily and BSK1, a member of the RLCKXII subfamily, also mediate flg22-dependent PTI signaling similarly to BIK1,23,25 indicating that these RLCK proteins redundantly function in the downstream signaling of FLS2. Recently, we have identified OsRLCK185, a member of the RLCKVII subfamily, as an important player in rice immune signaling.26 OsRLCK185 is directly phosphorylated by OsCERK1 in response to chitin and subsequently dissociates from OsCERK1 complex. Silencing of OsRLCK185 showed reduction of chitin and PGN-induced PTI responses, including the expression of defense genes and ROS production. Activation of MAP kinases is also suppressed in OsRLCK185 silencing cells, suggesting that OsRLCK185 transmits signals from OsCERK1 to the MAPK cascade. Thus, increasing evidence indicates that the RLCK family plays an important role in PTI signal transduction from ligand-activated PRRs in plants.
RLCKs as Targets of Pathogen Effector Proteins
Recent progress in understanding effector-mediated PTI inhibition revealed that many pathogen effectors target RLCKs to suppress PTI. Pseudomonas syringae effector AvrPphB is a cysteine protease. AvrPphB is capable of proteolytically cleaving the activation domain conserved in the RLCKs, including BIK1 and PBL1 (Fig. 2).23,27 Xanthomonas campestris effector AvrAC possesses uridylyltransferase activity and targets BIK1 and RIPK. RIPK, a member of the RLCKVII subfamily, is involved in RPM1-mediated effector triggered immunity.28 AvrAC adds uridine 5-monophosphate (UMP) to important serine and threonine residues in the activation domain of BIK1 and RIPK, preventing their activation by autophosphorylation (Fig. 2).
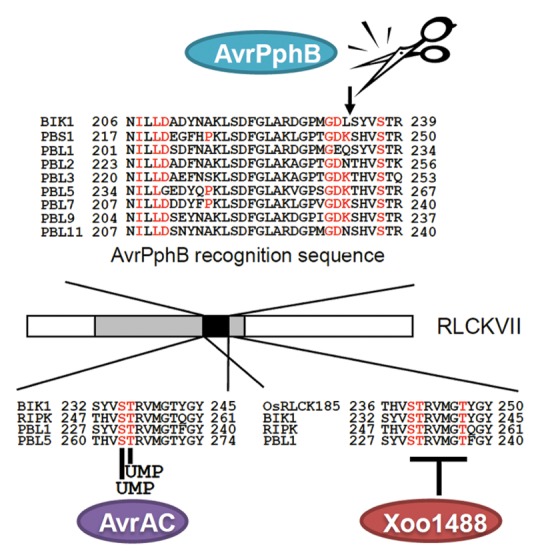
Figure 2. Target sites of effector proteins in RLCKVII family proteins. Gray region denotes the kinase domain and black region indicates the activation domain (AD) in RLCKVII family proteins. AvrPphB cleave their recognition site in AD of RLCKVII family proteins (upper position). Putative AvrPphB recognition sequences are highlighted in black. AvrAC uridylylates conserved serine and threonine residues in AD (left position). The UMP modification sites are highlighted in black. Xoo1488 inhibits the phosphorylation of 3 amino acid residues in AD of OsRLCK185 by unknown mechanism (right position). The predicted phosphorylation sites of OsRLCK185 by OsCERK1 are highlighted in black. Gene IDs are: BIK1, At2g39660; PBS1, At5g13160); PBL1, At3g55450; PBL2, At1g14370; PBL3, At2g02800; PBL5, At1g07870; PBL6, At2g28590; PBL7, At5g02800; PBL9, At1g07570; PBL11, At5g02290; RIPK, At2g05940; and OsRLCK185, Os05 g0372100.
Recently, we have identified that Xanthomonas oryzae effector Xoo1488, with unknown function, targets OsRLCK185.26 Expression of Xoo1488 in rice cells compromises OsRLCK185-mediated immune responses,26 which is consistent with the fact that Xoo1488 inhibits trans-phosphorylation of the activation domain of OsRLCK185 by OsCERK1 (Fig. 2).26 Interestingly, Xoo1488 is phosphorylated by OsRLCK185, suggesting that modification of Xoo1488 in host cell may affect their virulent activity.26
Link Between PRR and MAPK Cascade
Activation of MAP kinases often occurs in response to PAMPs. However, it remains unknown how the MAPK cascades are engaged downstream of PRRs.10 Although BIK1, PBS1, PBL1, PBL2, and BSK1 play roles in FLS2-mediated signaling, no mutants of these RLCKs compromise flg22-induced MAPK activation.27,29 Interestingly, AvrAC inhibit flg22-induced activation of MAP kinases,27 suggesting that unidentified RLCKVII family proteins targeted by AvrAC may transmit the signal from FLS2 to the downstream MAP kinase cascade. As mentioned above, OsRLCK185 was identified as the first factor to transmit a signal from a PRR to MAPK cascade.26 In rice OsMPK3, OsMPK4 and OsMPK6 are rapidly phosphorylated by chitin,30 and OsMPK3/OsMPK6 and OsMPK4 are regulated by different pathways.30 Chitin-induced activation of OsMPK3 and OsMPK6 but not OsMPK4 is reduced in OsRLCK185 silencing cells, indicating that OsRLCK185 acts upstream of OsMPK3 and OsMPK6, but not OsMPK4.26 Although OsMKK4, a MAPK kinase, was reported to function as MAPKK for OsMPK3 and OsMPK6, the MAPKK kinase regulating the OsMKK4-OsMPK3/OsMPK6 cascade remains to be identified. Further investigation of OsRLCK185-mediated MAPK activation will reveal the molecular link between PRR and the MAPK cascade.
Acknowledgments
This research was supported by Grants-in-Aid for Scientific Research (B)(23380028), for Scientific Research on Innovative Areas (24113519 and 25114517), and by Strategic Project to Support the Formation of Research Bases at Private Universities: Matching Fund Subsidy from the Ministry of Education, Culture, Sports, Science and Technology, 2011–2015 (S1101035) to TK.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Jones JD, Dangl JL. The plant immune system. Nature. 2006;444:323–9. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 2.Chinchilla D, Bauer Z, Regenass M, Boller T, Felix G. The Arabidopsis receptor kinase FLS2 binds flg22 and determines the specificity of flagellin perception. Plant Cell. 2006;18:465–76. doi: 10.1105/tpc.105.036574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zipfel C, Kunze G, Chinchilla D, Caniard A, Jones JD, Boller T, et al. Perception of the bacterial PAMP EF-Tu by the receptor EFR restricts Agrobacterium-mediated transformation. Cell. 2006;125:749–60. doi: 10.1016/j.cell.2006.03.037. [DOI] [PubMed] [Google Scholar]
- 4.Lee SW, Han SW, Sririyanum M, Park CJ, Seo YS, Ronald PC. A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science. 2009;326:850–3. doi: 10.1126/science.1173438. [DOI] [PubMed] [Google Scholar]
- 5.Miya A, Albert P, Shinya T, Desaki Y, Ichimura K, Shirasu K, et al. CERK1, a LysM receptor kinase, is essential for chitin elicitor signaling in Arabidopsis. Proc Natl Acad Sci USA. 2007;104:19613–8. doi: 10.1073/pnas.0705147104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wan J, Zhang XC, Neece D, Ramonell KM, Clough S, Kim SY, et al. A LysM receptor-like kinase plays a critical role in chitin signaling and fungal resistance in Arabidopsis. Plant Cell. 2008;20:471–81. doi: 10.1105/tpc.107.056754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaku H, Nishizawa Y, Ishii-Minami N, Akimoto-Tomiyama C, Dohmae N, Takio K, et al. Plant cells recognize chitin fragments for defense signaling through a plasma membrane receptor. Proc Natl Acad Sci USA. 2006;103:11086–91. doi: 10.1073/pnas.0508882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willmann R, Lajunen HM, Erbs G, Newman MA, Kolb D, Tsuda K, et al. Arabidopsis lysin-motif proteins LYM1 LYM3 CERK1 mediate bacterial peptidoglycan sensing and immunity to bacterial infection. Proc Natl Acad Sci USA. 2011;108:19824–9. doi: 10.1073/pnas.1112862108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu B, Li JF, Ao Y, Qu J, Li Z, Su J, et al. Lysin motif-containing proteins LYP4 and LYP6 play dual roles in peptidoglycan and chitin perception in rice innate immunity. Plant Cell. 2012;24:3406–19. doi: 10.1105/tpc.112.102475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tena G, Boudsocq M, Sheen J. Protein kinase signaling networks in plant innate immunity. Curr Opin Plant Biol. 2011;14:519–29. doi: 10.1016/j.pbi.2011.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun W, Cao Y, Jansen Labby K, Bittel P, Boller T, Bent AF. Probing the Arabidopsis flagellin receptor: FLS2-FLS2 association and the contributions of specific domains to signaling function. Plant Cell. 2012;24:1096–113. doi: 10.1105/tpc.112.095919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heese A, Hann DR, Gimenez-Ibanez S, Jones AME, He K, Li J, et al. The receptor-like kinase SERK3/BAK1 is a central regulator of innate immunity in plants. Proc Natl Acad Sci USA. 2007;104:12217–22. doi: 10.1073/pnas.0705306104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinchilla D, Zipfel C, Robatzek S, Kemmerling B, Nürnberger T, Jones JD, et al. A flagellin-induced complex of the receptor FLS2 and BAK1 initiates plant defence. Nature. 2007;448:497–500. doi: 10.1038/nature05999. [DOI] [PubMed] [Google Scholar]
- 14.Schulze B, Mentzel T, Jehle AK, Mueller K, Beeler S, Boller T, et al. Rapid heteromerization and phosphorylation of ligand-activated plant transmembrane receptors and their associated kinase BAK1. J Biol Chem. 2010;285:9444–51. doi: 10.1074/jbc.M109.096842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roux M, Schwessinger B, Albrecht C, Chinchilla D, Jones A, Holton N, et al. The Arabidopsis leucine-rich repeat receptor-like kinases BAK1/SERK3 and BKK1/SERK4 are required for innate immunity to hemibiotrophic and biotrophic pathogens. Plant Cell. 2011;23:2440–55. doi: 10.1105/tpc.111.084301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tanaka K, Nguyen CT, Liang Y, Cao Y, Stacey G. Role of LysM receptors in chitin-triggered plant innate immunity. Plant Signal Behav. 2012;8:e22598. doi: 10.4161/psb.22598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iizasa E, Mitsutomi M, Nagano Y. Direct binding of a plant LysM receptor-like kinase, LysM RLK1/CERK1, to chitin in vitro. J Biol Chem. 2010;285:2996–3004. doi: 10.1074/jbc.M109.027540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Petutschnig EK, Jones AM, Serazetdinova L, Lipka U, Lipka V. The lysin motif receptor-like kinase (LysM-RLK) CERK1 is a major chitin-binding protein in Arabidopsis thaliana and subject to chitin-induced phosphorylation. J Biol Chem. 2010;285:28902–11. doi: 10.1074/jbc.M110.116657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu T, Liu Z, Song C, Hu Y, Han Z, She J, et al. Chitin-induced dimerization activates a plant immune receptor. Science. 2012;336:1160–4. doi: 10.1126/science.1218867. [DOI] [PubMed] [Google Scholar]
- 20.Shinya T, Motoyama N, Ikeda A, Wada M, Kamiya K, Hayafune M, et al. Functional characterization of CEBiP and CERK1 homologs in arabidopsis and rice reveals the presence of different chitin receptor systems in plants. Plant Cell Physiol. 2012;53:1696–706. doi: 10.1093/pcp/pcs113. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu T, Nakano T, Takamizawa D, Desaki Y, Ishii-Minami N, Nishizawa Y, et al. Two LysM receptor molecules, CEBiP and OsCERK1, cooperatively regulate chitin elicitor signaling in rice. Plant J. 2010;64:204–14. doi: 10.1111/j.1365-313X.2010.04324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shiu SH, Karlowski WM, Pan R, Tzeng YH, Mayer KFX, Li WH. Comparative analysis of the receptor-like kinase family in Arabidopsis and rice. Plant Cell. 2004;16:1220–34. doi: 10.1105/tpc.020834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J, Li W, Xiang T, Liu Z, Laluk K, Ding X, et al. Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe. 2010;7:290–301. doi: 10.1016/j.chom.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Lu D, Wu S, Gao X, Zhang Y, Shan L, He P. A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA. 2010;107:496–501. doi: 10.1073/pnas.0909705107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi H, Shen Q, Qi Y, Yan H, Nie H, Chen Y, et al. BR-Signaling KinaSE1 physically associates with Flagellin Sensing2 and regulates plant innate immunity in Arabidopsis. Plant Cell. 2013;25:1143–57. doi: 10.1105/tpc.112.107904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi K, Yamada K, Ishikawa K, Yoshimura S, Hayashi N, Uchihashi K, et al. A receptor-like cytoplasmic kinase targeted by a plant pathogen effector is directly phosphorylated by the chitin receptor and mediates rice immunity. Cell Host Microbe. 2013;13:347–57. doi: 10.1016/j.chom.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 27.Feng F, Yang F, Rong W, Wu X, Zhang J, Chen S, et al. A Xanthomonas uridine 5′-monophosphate transferase inhibits plant immune kinases. Nature. 2012;485:114–8. doi: 10.1038/nature10962. [DOI] [PubMed] [Google Scholar]
- 28.Liu J, Elmore JM, Lin ZJ, Coaker G. A receptor-like cytoplasmic kinase phosphorylates the host target RIN4, leading to the activation of a plant innate immune receptor. Cell Host Microbe. 2011;9:137–46. doi: 10.1016/j.chom.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Zhou JM. Plant immunity triggered by microbial molecular signatures. Mol Plant. 2010;3:783–93. doi: 10.1093/mp/ssq035. [DOI] [PubMed] [Google Scholar]
- 30.Kishi-Kaboshi M, Okada K, Kurimoto L, Murakami S, Umezawa T, Shibuya N, et al. A rice fungal MAMP-responsive MAPK cascade regulates metabolic flow to antimicrobial metabolite synthesis. Plant J. 2010;63:599–612. doi: 10.1111/j.1365-313X.2010.04264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]