Abstract
Auxin is one of the most prominent phytohormones regulating many aspects of fleshy fruit development including fruit set, fruit size through the control of cell division and cell expansion, and fruit ripening. To shed light on the role of auxin fruit ripening, we have previously shown that Sl-ARF4 is a major player in mediating the auxin control of sugar metabolism in tomato fruit (cv MicroTom). Further extending this study, we show here that down-regulation of Sl-ARF4 in tomato alters some ripening-related fruit quality traits including enhanced fruit density at mature stage, increased firmness, prolonged shelf-life and reduced water (weight) loss at red ripe stage. These findings suggest that Sl-ARF4 plays a role in determining fruit cell wall architecture and thus providing a potential genetic marker for improving post-harvest handling and shelf life of tomato fruits.
Keywords: auxin, auxin response factor, ARF4, fruit, firmness, shelf life, tomato
Fruit ontogeny and ripening are genetically regulated processes involving a complex multi-hormonal control. While the role of ethylene in triggering and regulating the ripening of climacteric fruit have been clearly demonstrated, little is known about the contribution of other hormones.1 The plant hormone Auxin plays a vital role in all stages of reproductive growth through its important implication in cell division and cell expansion during fruit development.2-5 This findings were consolidated by the discovery of the involvement of many gene members belonging to two important auxin response gene families; ARFs (auxin response factors) and Aux/IAA in tomato fruit set and growth.3,6-8 Other studies also showed the involvement of auxin in regulating the fruit ripening process and fruit quality traits in many crop species.9-11 During fruit ripening, different regulatory factors involved in auxin signaling and response3,4,12,13 were shown to be also involved in the modification of cell wall structure and composition.14,15 Qualification and orchestration of these proteins remain an important area of research aimed at uncovering mechanisms of degradation of the cell wall which is responsible of fruit softening.16 Previous study showed that down-regulation of the DR12/Sl-ARF4 resulted in late-occurring cell divisions in the pericarp tissue and enhanced firmness at the red-ripe stage.4 Further investigation indicated that the altered firmness does not result from a major impairment of ripening-related pectin metabolism, but rather involves differences in pectin fine structure associated with changes in tissue architecture.3 We have recently shown that down-regulation of Sl-ARF4 gene in tomato fruits enhanced photosynthetic activity, chlorophyll and starch accumulation in immature tomato fruits, suggesting that Sl-ARFs may play a key role in controlling sugar content, an essential feature of fruit quality.10 In this work, we show new findings on Sl-ARF4 down-regulated tomato fruits supporting the role of this gene in the control of fruit softening and shelf life.
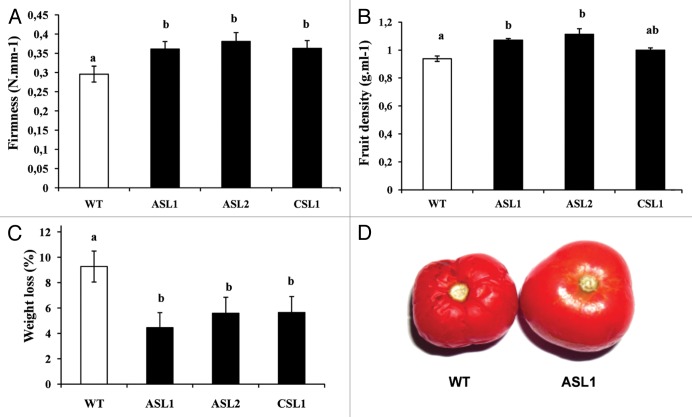
Three tomato lines under-expressing Sl-ARF4 and corresponding to either antisense (ASL1, ASL2) or sense co-suppressed (CSL1) were used in this study.10 Several parameters such as fruit density, firmness and shelf life were assessed and compared with those of wild type tomatoes. A significant increase in fruit firmness was observed at ten days after breaker in Sl-ARF4-AS fruit compared with WT (Fig. 1A). On the other hand, transgenic fruit displayed a lower water loss at post-breaker stage (breaker + 10 d) when compared with WT fruit at the same stage. The water loss was assessed by assessing following weight evolution of Sl-ARF4-AS and WT harvested fruit and stored at 25 °C for 40 d. The results (Fig. 1C) showed a lower water loss between breaker and breaker+10 d stages for the transgenic fruits (0.3 to 0.37 g fresh weight) in comparison to WT fruit at the same stages (0.54 g fresh weight). Sl-ARF4 down-regulated fruits showed also significantly higher density than that of WT (Fig. 1B). The difference in fruit density was also revealed by the fact that when immersed into water, WT fruits float while transgenic ones sink (data not shown). These observations are consistent with the elevated total soluble solids concentration in the Sl-ARF4-AS fruits shown previously.10
Figure 1. Density, firmness and weight loss measured on WT and Sl-ARF4 down-regulated fruits. (A) Firmness of WT and Sl-ARF4 down-regulated fruits measured at br + 10 d (B) Density of WT and Sl-ARF4 down-regulated fruits measured at br + 10 d (C) Weight loss measured on WT and Sl-ARF4 down-regulated fruits between the two stages breaker and breaker + 10 d. Small letters show significant differences between the WT and every downregulated line using ANOVA at P < 0.05. Values represent means ± SE (n = 30). (D) Prolonged conservation of Sl-ARF4 down-regulated fruit. Fruits were harvested at br stage and conserved at 25 °C for 45 d. (br = breaker stage, br + 10= ten days after breaker stage)
The enhanced fruit firmness and lower water loss of Sl-ARF4-AS fruits prompted us to assess the post-harvest behavior of Sl-ARF4 downregulated fruit to determine shelf-life potential, WT and Sl-ARF4-AS fruits were harvested at the breaker stage (45 d after anthesis) and stored at 25 °C until they reached complete deterioration. As shown in Figure 1D, when stored at 25 °C, WT fruits first displayed severe shrinking and then undergo effusion of juice contents associated with loss of texture and integrity at 20 d after storage. By contrast, in the same conditions, the Sl-ARF4 downregulated fruits did not display such signs of deterioration even after 45 d of storage (Fig. 1D).
Altogether, our study show that fruits under-expressing Sl-ARF4 significantly exhibit enhanced density, firmness, prolonged shelf life and lower water (weight) loss at red ripe fruit stage.
Material and methods
Tomato plants (Lycopersicon esculentum cv MicroTom) were grown and transformed as described previously.10 Density was determined by measuring fruit weight in air and in water (volume), this parameter was calculated following the equation (density = mass/volume). Firmness was determined by measuring the diameter and deformability of red ripe fruits (breaker + 10 d) using a compression clamp, which determines the difference between fruit diameter without pressure and the diameter after clamp pressure. Firmness value was calculated using the following equation: (((0 0108 × deformability) + 5 897) × 0 1005) / deformability). For shelf life, fruits at the breaker stage were detached and kept at room temperature (25 °C and 55~60% relative humidity) for approximately 45 d. Six replicates were taken for each individual plant. Average fresh weight loss was determined between the breaker and breaker + 10 d stages.
Acknowledgments
This work was supported by funds from the Laboratoire d'Excellence (LABEX) entitled TULIP (ANR-10-LABX-41) and supported by the European Integrated Project EU-SOL (FOOD-CT-2006-016214). The work benefited from the networking activities within the European COST Action FA1106.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Bouzayen M, Latché A, Nath P, Pech JC. Mechanism of Fruit Ripening. In: Pua EC, Davey MR, eds. Plant Developmental Biology - Biotechnological Perspectives: Springer Berlin Heidelberg, 2010:319-39. [Google Scholar]
- 2.Abel S, Theologis A. Early genes and auxin action. Plant Physiol. 1996;111:9–17. doi: 10.1104/pp.111.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guillon F, Philippe S, Bouchet B, Devaux M-F, Frasse P, Jones B, et al. Down-regulation of an Auxin Response Factor in the tomato induces modification of fine pectin structure and tissue architecture. J Exp Bot. 2008;59:273–88. doi: 10.1093/jxb/erm323. [DOI] [PubMed] [Google Scholar]
- 4.Jones B, Frasse P, Olmos E, Zegzouti H, Li ZG, Latché A, et al. Down-regulation of DR12, an auxin-response-factor homolog, in the tomato results in a pleiotropic phenotype including dark green and blotchy ripening fruit. Plant J. 2002;32:603–13. doi: 10.1046/j.1365-313X.2002.01450.x. [DOI] [PubMed] [Google Scholar]
- 5.Ruan YL, Patrick JW, Bouzayen M, Osorio S, Fernie AR. Molecular regulation of seed and fruit set. Trends Plant Sci. 2012;17:656–65. doi: 10.1016/j.tplants.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 6.de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development. Plant J. 2009;57:160–70. doi: 10.1111/j.1365-313X.2008.03671.x. [DOI] [PubMed] [Google Scholar]
- 7.Vriezen WH, Feron R, Maretto F, Keijman J, Mariani C. Changes in tomato ovary transcriptome demonstrate complex hormonal regulation of fruit set. New Phytol. 2008;177:60–76. doi: 10.1111/j.1469-8137.2007.02254.x. [DOI] [PubMed] [Google Scholar]
- 8.Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, et al. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling. Plant Cell. 2009;21:1428–52. doi: 10.1105/tpc.108.060830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cohen JD. In vitro tomato fruit cultures demonstrate a role for indole-3-acetic acid in regulating fruit ripening. J Am Soc Hortic Sci. 1996;121:520–4. [Google Scholar]
- 10.Sagar M, Chervin C, Mila I, Hao Y, Roustan JP, Benichou M, et al. SlARF4, an auxin response factor involved in the control of sugar metabolism during tomato fruit development. Plant Physiol. 2013;161:1362–74. doi: 10.1104/pp.113.213843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vendrell M. Dual effect of 2, 4-D on ethylene production and ripening of tomato fruit tissue. Physiol Plant. 1985;64:559–63. doi: 10.1111/j.1399-3054.1985.tb08539.x. [DOI] [Google Scholar]
- 12.Wang H, Jones B, Li Z, Frasse P, Delalande C, Regad F, et al. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis. Plant Cell. 2005;17:2676–92. doi: 10.1105/tpc.105.033415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zegzouti H, Jones B, Frasse P, Marty C, Maitre B, Latch A, et al. Ethylene-regulated gene expression in tomato fruit: characterization of novel ethylene-responsive and ripening-related genes isolated by differential display. Plant J. 1999;18:589–600. doi: 10.1046/j.1365-313x.1999.00483.x. [DOI] [PubMed] [Google Scholar]
- 14.Brownleader MD, Jackson P, Mobasheri A, Pantelides AT, Sumar S, Trevan M, et al. Molecular aspects of cell wall modifications during fruit ripening. Crit Rev Food Sci Nutr. 1999;39:149–64. doi: 10.1080/10408399908500494. [DOI] [PubMed] [Google Scholar]
- 15.Brummell DA, Harpster MH. Cell wall metabolism in fruit softening and quality and its manipulation in transgenic plants. Plant Mol Biol. 2001;47:311–40. doi: 10.1023/A:1010656104304. [DOI] [PubMed] [Google Scholar]
- 16.Seymour GB, Manning K, Eriksson EM, Popovich AH, King GJ. Genetic identification and genomic organization of factors affecting fruit texture. J Exp Bot. 2002;53:2065–71. doi: 10.1093/jxb/erf087. [DOI] [PubMed] [Google Scholar]
- 17.Ecarnot M, Baczyk P, Tessarotto L, Chervin C. Rapid phenotyping of the tomato fruit model, Micro-Tom, with a portable VIS-NIR spectrometer. Plant Physiol Biochem. 2013;70:159–63. doi: 10.1016/j.plaphy.2013.05.019. [DOI] [PubMed] [Google Scholar]