Abstract
Lateral roots (LRs) play important roles in increasing the absorptive capacity of roots as well as to anchor the plant in the soil. In rice, exposure to auxin, methyl jasmonate (MJ), apocynin, and CoCl2 has been shown to increase LR formation. This review provides evidence showing a close link between rice heme oxygenase (HO) and LR formation. The effect of auxin and MJ is nitric oxide (NO) dependent, whereas that of apocynin requires H2O2. The effect of CoCl2 on the LR formation could be by some other pathway unrelated to NO and H2O2. This review also highlights future lines of research that should increase our knowledge of HO-involved LR formation in rice.
Keywords: heme oxygenase, hydrogen peroxide, nitric oxide, lateral root formation
In recent years, root development has emerged as a central focus of research in many laboratories across the world. Lateral roots (LRs) play important roles in water and nutrient acquisition and anchorage. Root architecture, including LR, has been suggested to be one of the promising feature of crops in a new green revolution.(1) Thus, understanding the regulation LR development is of agronomic importance.
Heme oxygenase (HO; EC1.14.99.3) catabolizes heme into carbon monoxide (CO), biliverdin (BV), and free iron. Three isoforms of HO (HO-1, HO-2, and HO-3) have been identified, which are products of distinct genes.2 HO-1 is highly inducible, whereas HO-2 and HO-3 are constitutively expressed. In plants, HO-1 has been shown to be associated with LR formation.3,4 Plant HO-1 also plays roles in phytochrome chromophore synthesis and protection against oxidative cell damage.5,6 The expression of HO-1 has been shown to be induced by nitric oxide (NO)7 and H2O2.8-10 In this mini-review, which is not intended to be comprehensive, we shall be concerned strictly with the role of rice HO in LR formation, and mostly with the work from our laboratory.
HO and LR Formation Caused by Auxin and Methyl Jasmonate
A large body of work has indicated that auxin, a plant hormone that influences many aspects of plant development, is a primary regulator of LR formation. Plants that are treated with auxin or that overproduce it form more LRs, whereas plants that are impaired in auxin signaling form fewer.11 Nitric oxide (NO), a free radical active gas, is now recognized as an ubiquitous signal involved in diverse physiological process in plants.12 Correa-Aragunde et al.13 provided the first evidence about the interplays between auxin and NO during LR formation in tomato. In rice, NO-donor sodium nitroprusside (SNP) and indole-3-butyric acid (IBA, a naturally occurring auxin) were able to induce LR formation.14 This effect is specific for NO because the NO scavenger 2-(4-carboxyphenyl)-4,4,5,5- tetramethylimidazoline-1-oxyl-3-oxide (cPTIO) blocked the action of SNP and IBA.14 Moreover, SNP and IBA-induced NO production were localized in root area corresponding to emergence of LRs.14 In another recent report, we demonstrated that SNP and IBA could induce OsHO1 mRNA expression.15 LR formation and HO activity induced by SNP and IBA was reduced by cPTIO and Zn protoporphyrin (ZnPPIX, the specific inhibitor of HO).15 It seems that HO is required for auxin- and NO-induced LR formation in rice (Fig. 1).
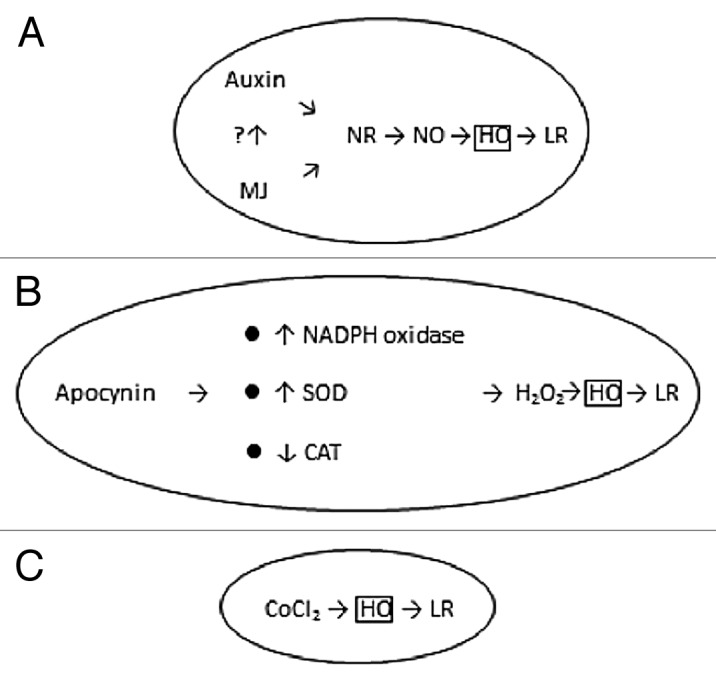
Figure 1. Diagram showing the pathways by which auxin and MJ (A), apocynin (B), and CoCl2 might induce LR formation in rice.
Jasmonic acid (JA) and methyl jasmonate (MJ), originally identified as the major component of jasmine scent, play a universal role in the plant kingdom and are involved in the regulation of diverse aspects of plant biology, including growth, development, metabolism, and interaction with the environment.16 Application of MJ to rice seedlings induced LR formation, OsHO1 mRNA expression, and HO activity.17,18 Using NO scavenger cPTIO and HO inhibitor ZnPPIX, we were able to show that LR formation caused by MJ is NO dependent and that HO participates in MJ-promoted LR formation in rice roots.17,18 Sun et al.19 demonstrated that JA-mediated auxin biosynthesis in the Arabidopsis roots is critical for LR formation. Whether auxin biosynthesis is required for MJ-induced LR formation in rice remains to be examined (Fig. 1).
To study the contribution of the NO sources in LR formation in rice, pharmacological approaches using both nitrate reductase (NR) and nitric oxide synthase inhibitors have been employed. Application of NR inhibitor sodium tungstate completely inhibited IBA- and MJ-induced NO production and LR formation.14,17 Our results support the participation of NR-catalyzed NO synthesis in IBA- and MJ-induced LR formation in rice.
HO and LR Formation Caused by Apocynin
Apocynin (acetovanillone) is a compound originally isolated from the medicinal plant Picrorhiza kurroa, a small perennial herb that grows in Himalyas. In animal system, apocynain induces NO synthesis.20 Tossi et al.21 also demonstrated that apocynin increases NO production in maize leaves. This is the only work showing that apocynin increases NO production in plants. The effect of apocynin on LR formation in rice has been examined.22 Our data suggest that both H2O2 and HO are required for apocynin-induced LR formation in rice (Fig. 1). Our conclusion is supported by the observations that 1) treatment with apocynin induced LR formation and increased H2O2 production, but had no effect on NO production; 2) H2O2 production caused by H2O2 and apocynin was localized in the root area corresponding to the LR emergence; 3) treatment with H2O2 and apocynin also increased OsHO1 mRNA expression and HO activity; and 4) LR formation and HO activity induced by H2O2 and apocynin were reduced by ZnPPIX (the specific inhibitor of HO).
Plasma-membrane NADPH oxidase is a protein that transfers electrons from cytoplasmic NADPH to an O2 to form O2-, followed by dimutation of O2- to H2O2.23 Diphenyleneiodonium chloride, an inhibitor of NADPH oxidase, was effective in reducing apocynin-induced H2O2 production and LR formation.22 This indicates that apocynin-dependent H2O2 production in rice originated at least in part, from plasma-membrane NADPH oxidase. The increase in superoxide dismutase (SOD) activity and the decrease in catalase (CAT) activity could result in H2O2 accumulation. Apocynin was also observed to increase SOD activity and decreased CAT activity in rice roots.22 These results suggest that apocynin may exert other effects besides its ability to induce NADPH oxidase, for instance, it increase SOD activity and decrease CAT activity.
HO and LR Formation Caused by CoCl2
Cobalt is usually considered as a non-essential element. Gad and Atta-Aly24 provided the first evidence showing that cobalt induces adventitious root formation in cucumber and tomato. Later work by Xu et al.25 demonstrated that CoCl2 induces LR formation in tomato. Recently, the effect of CoCl2 on LR formation in rice has been examined.26 CoCl2 has been shown to increase the number of LRs in rice, induce the expression of OsHO1 gene and the activity of HO. Moreover, ZnPPIX reduced LR formation, OsHO1 mRNA expression, and HO activity caused by CoCl2. Our data support the importance of HO in regulating CoCl2-increased LR formation (Fig. 1). Moreover, CoCl2 had no effect on H2O2 content and NO production. Therefore, the effect of CoCl2 could be mediated by some other pathway unrelated to H2O2 and NO. Exposure of plants to mild chronic stress can cause induction of specific, stress-induced morphogenic responses (SIMRs) characterized by an inhibition of root elongation, and enhanced formation of LRs.27 CoCl2 treatment causes a typical SIMR in rice.(26) It is most likely that rice HO is involved in SIMR caused by CoCl2.
Conclusions and Perspectives
Overall, the data summarized here indicate a close link between rice HO and LR formation. OsHO1 mRNA expression and HO activity could be mediated by NO, H2O2 or pathway unrelated to NO and H2O2. A critical issue to be further explored is the detailed function of OsHO1, a rice HO-1 gene in LR formation. Evaluation of overexpression of OsHO1 in transgenic rice plants will uncover the detailed function in LR formation. There are still important aspects related to LR formation that require characterization. It is well established that cell cycle regulatory genes are the regulatory genes of LR formation. It is of great interest to know whether cell cycle regulatory genes might be target for HO-involved LR formation in rice induced by auxin, MJ, NO, H2O2 or CoCl2.
Acknowledgments
The author is grateful to Dr Frantisek Baluska for kindly inviting this review. The author would like to acknowledge with gratitude the contribution of colleagues and students to the personal research reported. Research in the author’s laboratory has been supported by grants from the National Science Council of the Republic of China.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Den Herder G, Van Isterdael G, Beeckman T, De Smet I. The roots of a new green revolution. Trends Plant Sci. 2010;15:600–7. doi: 10.1016/j.tplants.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 2.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–54. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 3.Guo K, Xia K, Yang Z-M. Regulation of tomato lateral root development by carbon monoxide and involvement in auxin and nitric oxide. J Exp Bot. 2008;59:3443–52. doi: 10.1093/jxb/ern194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han B, Xu S, Xie Y-J, Huang J-J, Wang L-J, Yang Z, et al. ZmHO-1, a maize haem oxygenase-1 gene, plays a role in determining lateral root development. Plant Sci. 2012;184:63–74. doi: 10.1016/j.plantsci.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Terry MJ, Linley PJ, Kohchi T. Making light of it: the role of plant haem oxygenases in phytochrome chromophore synthesis. Biochem Soc Trans. 2002;30:604–9. doi: 10.1042/BST0300604.. [DOI] [PubMed] [Google Scholar]
- 6.Shekhawat GS, Verma K. Haem oxygenase (HO): an overlooked enzyme of plant metabolism and defence. J Exp Bot. 2010;61:2255–70. doi: 10.1093/jxb/erq074. [DOI] [PubMed] [Google Scholar]
- 7.Noriega GO, Yannarelli GG, Balestrasse KB, Batlle A, Tomaro ML. The effect of nitric oxide on heme oxygenase gene expression in soybean leaves. Planta. 2007;226:1155–63. doi: 10.1007/s00425-007-0561-8. [DOI] [PubMed] [Google Scholar]
- 8.Yannarelli G, Noriga GO, Battle A, Tomaro ML. Heme oxygengase upregulation in ultraviolet B irradiated soybean plants involves reactive oxygen species. 2006;224:1154–62. doi: 10.1007/s00425-006-0297-x. [DOI] [PubMed] [Google Scholar]
- 9.Chen XY, Ding X, Xu S, Wang R, Xuan W, Cao ZY, et al. Endogenous hydrogen peroxide plays a positive role in the upregulation of heme oxygenase and acclimation to oxidative stress in wheat seedling leaves. J Integr Plant Biol. 2009;51:951–60. doi: 10.1111/j.1744-7909.2009.00869.x. [DOI] [PubMed] [Google Scholar]
- 10.Wei M-Y, Chao Y-Y, Kao CH. NaCl induced heme oxygenase in roots of rice seedlings is mediated through hydrogen peroxide. Plant Growth Regul. 2013;69:299–14. doi: 10.1007/s10725-012-9762-7. [DOI] [Google Scholar]
- 11.Fukaki H, Okushima Y, Tasaka M. Auxin-mediated lateral root formation in higher plants. Int Rev Cytol. 2007;256:111–37. doi: 10.1016/S0074-7696(07)56004-3. [DOI] [PubMed] [Google Scholar]
- 12.Simontacchi M, García-Mata C, Bartoli CG, Santa-María GE, Lamattina L. Nitric oxide as a key component in hormone-regulated processes. Plant Cell Rep. 2013;32:853–66. doi: 10.1007/s00299-013-1434-1. [DOI] [PubMed] [Google Scholar]
- 13.Correa-Aragunde N, Graziano M, Lamattina L. Nitric oxide plays a central role in determining lateral root development in tomato. Planta. 2004;218:900–5. doi: 10.1007/s00425-003-1172-7. [DOI] [PubMed] [Google Scholar]
- 14.Chen YH, Kao CH. Calcium is involved in nitric oxide- and auxin-induced lateral root formation in rice. Protoplasma. 2012;249:187–95. doi: 10.1007/s00709-011-0277-2. [DOI] [PubMed] [Google Scholar]
- 15.Chen YH, Chao Y-Y, Hsu YY, Hong C-Y, Kao CH. Heme oxygenase is involved in nitric oxide- and auxin-induced lateral root formation in rice. Plant Cell Rep. 2012;31:1085–91. doi: 10.1007/s00299-012-1228-x. [DOI] [PubMed] [Google Scholar]
- 16.Wasternack C. Jasmonates: an update on biosynthesis, signal transduction and action in plant stress response, growth and development. Ann Bot. 2007;100:681–97. doi: 10.1093/aob/mcm079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsu Y-Y, Kao CH. Nitric oxide is involved in methyl jasmonate-induced lateral root formation in rice. Crop Environ Bioinform. 2012;8:160–7. [Google Scholar]
- 18.Hsu Y-Y, Chao Y-Y, Kao CH. Methyl jasmonate-induced lateral root formation in rice: the role of heme oxygenase and calcium. J Plant Physiol. 2013;170:63–9. doi: 10.1016/j.jplph.2012.08.015. [DOI] [PubMed] [Google Scholar]
- 19.Sun JQ, Xu YX, Ye SQ, Jiang HL, Chen Q, Liu F, et al. Arabidopsis ASA1 is important for jasmonate-mediated regulation of auxin biosynthesis and transport during lateral root formation. Plant Cell. 2009;21:1495–511. doi: 10.1105/tpc.108.064303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riganti C, Costamagna C, Doublier S, Miraglia E, Polimeni M, Bosia A, et al. The NADPH oxidase inhibitor apocynin induces nitric oxide synthesis via oxidative stress. Toxicol Appl Pharmacol. 2008;228:277–85. doi: 10.1016/j.taap.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 21.Tossi V, Cassia R, Lamattina L. Apocynin-induced nitric oxide production confers antioxidant protection in maize leaves. J Plant Physiol. 2009;166:1336–41. doi: 10.1016/j.jplph.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 22.Chen Y-H, Chao Y-Y, Hsu Y-Y, Kao CH. Heme oxygenase is involved in H(2)O (2)-induced lateral root formation in apocynin-treated rice. Plant Cell Rep. 2013;32:219–26. doi: 10.1007/s00299-012-1356-3. [DOI] [PubMed] [Google Scholar]
- 23.Auh C-K, Murphy TM. Plasma membrane redox enzyme is nvolved in the synthesis of O2- and H2O2 by Phytophthora elicitor-stimulated rose cells. Plant Physiol. 1995;107:1241–7. doi: 10.1104/pp.107.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gad BN, Atta-Aly MA. Effect of cobalt on the formation, growth and development of adventitious roots in tomato and cucumber cuttings. J Appl Sci Res. 2006;2:423–9. [Google Scholar]
- 25.Xu S, Zhang B, Cao Z-Y, Ling T-F, Shen W-B. Heme oxygenase is involved in cobalt chloride-induced lateral root development in tomato. Biometals. 2011;24:181–91. doi: 10.1007/s10534-010-9386-1. [DOI] [PubMed] [Google Scholar]
- 26.Hsu Y-Y, Chao Y-Y, Kao CH. Cobalt chloride-induced lateral root formation in rice: The role of heme oxygenase. J Plant Physiol. 2013;170:1075–81. doi: 10.1016/j.jplph.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Potters G, Pasternak TP, Guisez Y, Palme KJ, Jansen MAK. Stress-induced morphogenic responses: growing out of trouble? Trends Plant Sci. 2007;12:98–105. doi: 10.1016/j.tplants.2007.01.004. [DOI] [PubMed] [Google Scholar]


