Abstract
Signal transduction via phospholipids is mediated by phospholipases such as phospholipase C (PLC) and D (PLD), which catalyze hydrolysis of plasma membrane structural phospholipids. Phospholipid signaling is also involved in plant responses to phytohormones such as salicylic acid (SA). The relationships between phospholipid signaling, SA, and secondary metabolism are not fully understood. Using a Capsicum chinense cell suspension as a model, we evaluated whether phospholipid signaling modulates SA-induced vanillin production through the activation of phenylalanine ammonia lyase (PAL), a key enzyme in the biosynthetic pathway. Salicylic acid was found to elicit PAL activity and consequently vanillin production, which was diminished or reversed upon exposure to the phosphoinositide-phospholipase C (PI-PLC) signaling inhibitors neomycin and U73122. Exposure to the phosphatidic acid inhibitor 1-butanol altered PLD activity and prevented SA-induced vanillin production. Our results suggest that PLC and PLD-generated secondary messengers may be modulating SA-induced vanillin production through the activation of key biosynthetic pathway enzymes.
Keywords: Phospholipids, Salicylic acid, Phospholipase C, Phospholipase D, Vanillin
Introduction
Phospholipids are structural components of the cell plasma membrane and are important messengers that regulate plant growth and development and cellular response to environmental change or stress.1 Phospholipid-generated signal transduction involves a family of phospholipases that catalyze the hydrolysis of plasma membrane phospholipids to generate secondary messengers. Phospholipase C (PLC) hydrolyzes phosphatidylinositol 4,5 bisphosphate (PtdIns(4,5)P2) to generate inositol 1,4,5 trisphosphate (Ins(1,4,5)P3) and diacylglycerol (DAG). In plants, Ins(1,4,5)P3 may be converted instead to inositol hexaphosphate (IP6), which has been shown to stimulate the release of Ca2+ from intracellular stores in guard cells.2 Phospholipase D (PLD) hydrolyzes membrane phospholipids, generating structural phosphatidic acid (PA) and releasing the polar head of the phospholipid.3 Phospholipid signaling is involved in plant response to phytohormones such as salicylic acid (SA), an important endogenous signaling molecule in plant defense.4-7 SA also regulates several plant physiological processes and is essential for the expression of some defense genes.8,9
Two spatially separated SA biosynthetic pathways of have been proposed in higher plants: the cytoplasmic and chloroplastic routes. The cytosolic pathway initiates from phenylalanine, whereas the chloroplastic one does it from chorismate, via isochorismate (IC).10 SA accumulated in response to pathogens, is produced in the chloroplast in different species, such as Arabidopsis, Nicotiana benthamiana, and tomato (Solanum lycopersicum).11-13 If this is the case in C. chinense has yet to be established however, since other members of the Solanaceae family, including tomato12 use the chloroplastic pathway, a similar scenario can be inferred.
As for the possible receptors of SA (see ref. 8 for a review), Fu et al.14 reported a small family of pathogenesis-related genes (NPR1, NPR3, and NPR4) that might function as SA receptors in the immune response of Arabidopsis thaliana. However, besides these proteins, a plasma membrane receptor for SA has not been yet identified.
Salicylic acid has been applied in different plants to elicit the production of some secondary metabolites. In the genus Capsicum, SA has been used to induce secondary metabolites and increase capsaicinoid content in in vitro cultures.15-17 Capsaicinoid formation can originate from the phenylpropanoid pathway and via branched-chain amino acids such as valine or leucine.18 Phenylalanine ammonia-lyase (PAL), a key enzyme in the phenylpropanoid pathway, forms cinnamic acid by the deamination of phenylalanine. PAL activity can be induced in response to various stress-inducing factors including freezing,19,20 wounding,21 UV light,22 and phytohormones such as SA. Increased PAL activity is associated with the accumulation of secondary metabolites (e.g., anthocyanins, flavonoids, and other phenolic compounds) in the tissues of plants such as pear,23 grape,24,25 tomato,26 apple,27 strawberry28 and tangerine.29 This supports evidence suggesting that PAL is an environmental stress indicator in different plant tissues.19,30 However, the relationship between phospholipid signaling, SA, and PAL has not yet been established.
Several inhibitory substances have been used to elucidate the role of phospholipid enzymes in cellular responses.31 In pharmacological studies using the inhibitors of phosphoinositide-PLC (PI-PLC) neomycin and U73122, it was shown to play a role in cellular response in plant models such as rice, soybean, peas cell suspensions.32-34
An evaluation of PLD involvement in different plant cellular processes has been conducted using a primary alcohol such as 1-butanol (1-But), which can inhibit PLD-mediated PA production through competing with water by generating the phosphatidyl-butanol group.35 This lipid is not normally present in cells but can be easily synthesized in vivo when cells are pre-incubated with low concentrations (0.1–0.5%) of 1-butanol.36 In this method, PLD-induced PA production is required to regulate increased production of secondary metabolites such as silymarin in Silybum marianum cell suspensions37 or scopoletin in tobaccum suspensions.38 These studies suggest that the products of the phospholipid signaling cascade may function as secondary messengers during the stimulation of secondary metabolism in plants.3,39
Our research group has observed that the treatment of C. chinense cell suspensions with 100 and 200 μM SA modulates the in vitro enzymatic activities of PLC and PLD, resulting in increased vanillin content.6 However, when vanillin content was evaluated in the presence of neomycin, the SA-induced vanillin production was inhibited. Therefore, we focused on elucidating the relationship between phospholipid signaling, PAL activity, and vanillin accumulation, which are all events closely related to the SA induction response. Our goal was to analyze biochemical evidence supporting PLC and PLD involvement in SA-induced signal transduction in the presence of neomycin, U73122, and 1-But and evaluate vanillin accumulation and PAL activity in C. chinense suspension cells.
Results
Effect of SA on PAL enzymatic activity and vanillin levels
SA-induced response and activity of PAL was studied in C. chinense suspension cells after culturing them for 14 d. Cells were harvested by transferring 1 g of the samples into flasks containing 25 ml fresh Murashige and Skoog (MS) medium. After a 15 min adjustment period, one sample was exposed to 200 μM SA for 30 min, and a control sample was left unexposed for the same period of time. Cells were then harvested by vacuum filtration and immediately frozen in liquid nitrogen.
PAL activity was found to be doubled in the 200 μM SA treatment compared with the control (Fig. 1A). Because increased PAL activity is associated with secondary metabolite accumulation, the present model was used to evaluate the effect of SA on vanillin accumulation. Addition of 200 μM SA to the cell suspensions 3 times stimulated vanillin production (Fig. 1B), suggesting that SA-induced vanillin biosynthesis yield is correlated with increased PAL enzyme activity.
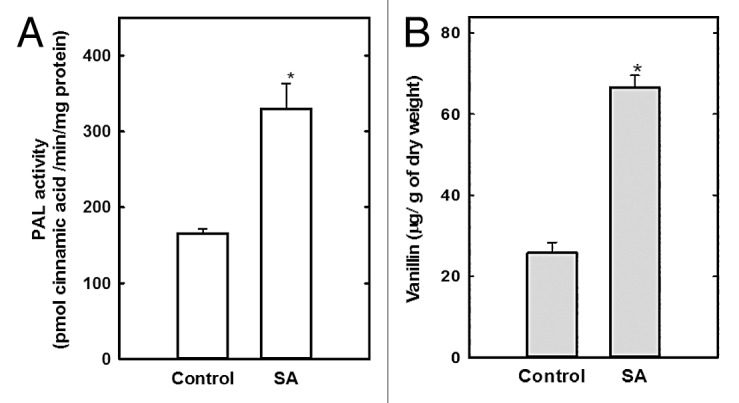
Figure 1. Salicylic acid increases PAL activity and vanillin content in C. chinense cells. After a 14-d culture cycle, cells were treated with 200 μM SA or untreated (control) and then PAL activity (A) and vanillin production (B) assessed. Results represent the mean of 3 independent experiments ± SE, *P < 0.001.
U73122 and neomycin reduce PAL activity and SA-stimulated vanillin levels
Initially, the effect of inhibitors of PI-PLC (neomycin and U73122) and PLD inhibitor (1-But) on cell suspensions was evaluated, followed by analysis of the cellular structure using scanning electron microscopy. The treatments with SA and inhibitors did not cause any morphological damage to the cell structure that might compromise metabolic activity (data not shown).
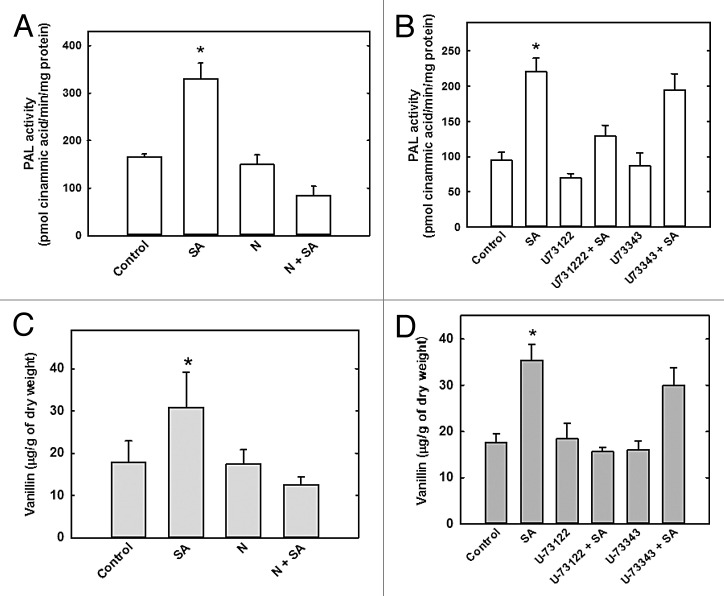
To determine the role of PLC in the regulation of vanillin accumulation in C. chinense cells, the cells were first treated with neomycin and U73122 and then treated with SA. Cell suspensions were placed in 25 ml fresh MS medium containing neomycin and/or U73122 for 15 min, and a sample without inhibitor treatment was used as the control. Salicylic acid (200 μM) was then added to some of the cell/inhibitor mixtures for 30 min, while the others were not treated with SA. Our results showed that upon neomycin-only treatment, PAL activity was similar to that of the control sample (Fig. 2A) but higher than the neomycin + SA treated sample, suggesting SA-induced stimulation. Treatment with U73122 lowered PAL activity only by 26% compared with the control. The U73122 + SA treatment further decreased PAL activity, especially compared with the SA only treatment (Fig. 2B). Treatment of cells with U73343, the inactive analog of U73122, did not alter PAL activity. These results suggest that SA-induced increases in PAL activity can be regulated by PLC-mediated signaling.
Figure 2. PLC-inhibitors suppress vanillin accumulation and PAL activity in C. chinense suspension cells. Cells were treated with 100 μM neomycin (N), 10 μM U73122 or 10 μM U73343 (inactive analog) for 15 min before SA addition and the PAL activity (A and B) and vanillin content (C and D) were evaluated. Data represent the mean of 3 independent experiments ± SE, *P < 0.001.
The above results also imply that PLC signaling and metabolite (i.e., vanillin) accumulation are both related to the SA induction response. To further evaluate the effect of neomycin and U73122 treatment on vanillin levels, cell suspensions were placed in 25 ml fresh MS medium containing neomycin or U73122 for 15 min before the addition of SA for 30 min (Fig. 2C and D). Neomycin-only treatment produced vanillin levels similar to control levels (Fig. 2C), but neomycin-SA treatment reversed any SA-stimulated increases in vanillin accumulation. When treated with U73122 only, vanillin levels remained at basal levels similar to those observed in the neomycin-only treated cells (Fig. 2D). Combined U73122-SA treatment produced lower levels of vanillin than in the SA-only treatment. The inhibitory analog U73343 had no effect on vanillin levels (Fig. 2D).
PLD regulates PAL activity and accumulation of vanillin in C. chinense
To assess the role of PLD signaling in PAL regulation, 1-But was used as an inhibitor of PLD-induced PA formation. Because 1-But may have a toxic effect on metabolite production, different working concentrations (% v/v) were evaluated to identify the concentration that did not affect basal vanillin levels in cell suspensions. After 15 min treatment with 1-But, cells were filtered, lyophilized, and vanillin levels quantified. At the 1% concentration, 1-But reduced vanillin levels by 53% compared with the control, but concentrations < 0.75% had no effect on vanillin levels (Fig. 3A). Specifically, 1-But at 0.5% did not affect basal vanillin levels compared with the control and therefore, this concentration was used to evaluate the effect of 1-But on PAL activity and SA-stimulated vanillin accumulation. After 14 d in culture, cell suspension samples were placed in 25 ml fresh MS medium containing 0.5% 1-But for 15 min before the addition of SA. Tert-butanol, an inactive 1-But isomer, was used as a positive control (Fig. 3B). In the 1-But treatment, vanillin levels were similar to that of the control (Fig. 3B). In contrast, 1-But-SA treatment decreased the vanillin content by 84%, effectively reversing SA-stimulated vanillin accumulation. Tert-butanol had no effect on basal levels of vanillin content in the cell suspension.
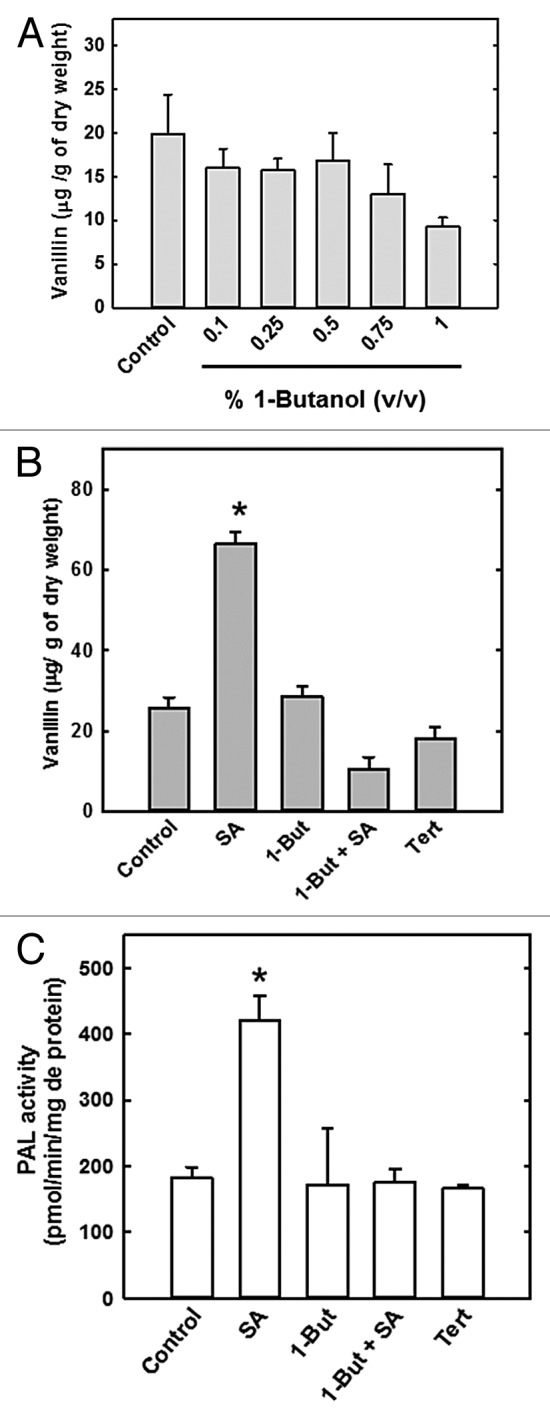
Figure 3. Reduction of vanillin accumulation and PAL activity in C. chinense cells in the presence of 1-butanol. Vanillin content was evaluated in cells treated with increasing concentrations of 1-butanol for 15 min (A). Cells were incubated in 1-butanol (1-But, 0.5%), 200 μM SA, 1-But + SA, or 0.5% Tert-butanol (Tert). Vanillin content (B) and PAL activity (C) were then evaluated. Data represent the mean of 3 independent experiments ± SE, *P < 0.001.
In the 1-But-only treatment, PAL activity was similar to control (Fig. 3C). However, PAL activity was 58% lower in the 1-But + SA treatment than in the SA-only treatment. Tert-butanol did not modify PAL activity. These results suggest that SA-stimulated increases in PAL activity are mediated by PLD signaling.
Total endogenous SA production
Total endogenous SA level in the cells was quantified in the presence of neomycin, U73122 or 1-But to determine whether these inhibitors modify intracellular SA levels. Exogenous SA application produced a 3-fold increase in total endogenous SA content compared with the basal content in cells (Fig. 4A). However, upon U73122 + SA and neomycin + SA treatments SA levels decreased by 43% and 53%, respectively (Fig. 4A), and by 43% after 1-But treatment (Fig. 4B). Cells treated only with the inhibitors did not affect basal levels of total SA.
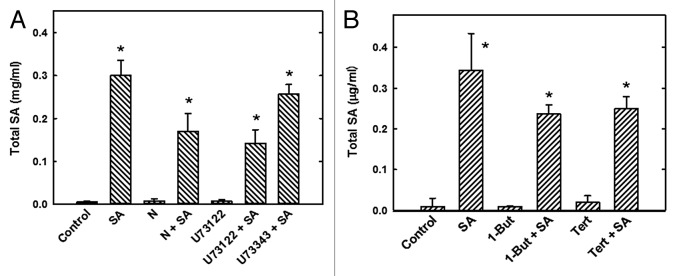
Figure 4. Effect of phospholipase inhibitors on total endogenous SA levels in C. chinense suspension cells. Cells were treated with the PLC inhibitors 100 μM neomycin (N), 10 μM U73122, or 10 μM U73343 with 200 μM SA (A), or the PLD inhibitor 0.5% 1-butanol or 0.5% Tert-butanol (Tert) with 200 μM SA (B). In all treatment groups, cells were pre-incubated with the inhibitors for 15 min before the 30 min SA treatment. The SA level in the cells after the different treatments was evaluated by HPLC (see Materials and Methods). The data represent the mean of 3 independent experiments ± SE, *P < 0.001
Discussion
Elicitors increase secondary metabolite accumulation in cultured cells, thereby facilitating the study of metabolite biosynthesis regulation mechanisms.40 Phytohormones such as SA increase the accumulation of metabolites such as capsaicinoids in cell suspensions of C. annum C. frutescens, and C. chinense.6,15-17 This study evaluated the role of the phospholipase signaling pathway in SA-stimulated vanillin production in C. chinense cell suspensions. Pharmacological substances that inhibit PLC and PLD signaling were used and their effect on PAL activity and vanillin content quantified.
PAL is the first enzyme in the activated phenylpropanoid biosynthesis pathway and is responsive to elicitor stimulation. Enzymatic activity was initially evaluated on day 14 of C. chinense cell suspension culture, and SA application was found to increase it. The use of neomycin and U73122 show that PLC signaling could be involved in the SA-stimulated vanillin production. This may occur as a result of the regulation of phenylpropanoid pathway (Fig. 2A and B). Induction of PAL may occur via a PLC-modulated signal, since neomycin is a phosphoinositide turnover and PtdIns(4,5)P2 inhibitor.41
Kamada and Muto42 evaluated the effect of the protein kinase inhibitors K252 and staurosporine on PAL activity and phosphoinositide turnover in N. tobacum cell suspensions after stimulation with an elicitor prepared from Phytophthora nicotianae. The inducing agent was found to stimulate phosphoinositide turnover and increase PAL activity, while the addition of K252 and staurosporine inhibited both these responses. The results suggested that phosphoinositide turnover plays an important role in stimulating PAL activity via kinases. Neomycin has been shown to a have a similar inhibitory effect on PAL elicitation when used in combination with an elicitor prepared from the cell wall of the pathogenic fungus Fusarium oxysporum that infiltrated Pisum sativum leaves43 or Larix decidua cell suspensions.44 Neomycin treatment reduced PAL activity when used in combination with the elicitor.
Effects of neomycin and U73122 on vanillin accumulation suggest that blocking one of the processes coupled to Ins(1,4,5)P3 or IP6, such is the cytosolic Ca2+ increment, could be affecting important signaling components responsible for PAL activation. This would affect vanillin levels in the C. chinense cell suspensions. This process was no identified in the present study, but there are reports of PAL activity regulation via phosphorylation,45,46 although it is not clear if these effects occur either directly on PAL or on some regulatory element mediating the response to a stimulus.
For experimental purposes, PLD activity can be manipulated by the addition of 1-But. This strategy was previously used to show that PLD-induced PA production is necessary for increased production of the metabolite silymarin in Silybum marianum cell suspensions.37
In this way, the effect of inhibited PA derivative production on PLD-stimulated vanillin accumulation in C. chinense cell suspensions treated with SA was analyzed by adding 1-But. Both SA-stimulated PAL activity (Fig. 3B) and vanillin accumulation (Fig. 3C) were reduced by addition of 1-But. The same effect has been reported in N. tobacum cell suspensions treated with 1-But,38 where it was shown to reduce riboflavin-stimulated scopoletin accumulation (activating defense response), whereas exogenous application of PA reversed the effect. This indicates that PLD and the PA products are important components in riboflavin-activated phytoalexin biosynthesis regulation. When 1-But was applied to mechanically injured plants, both PAL activity and phenolic compounds were reduced.47
That the inhibition of PLD in SA-exposed C. chinense cells had abolished PAL and vanillin suggests that this signaling pathway participate in this response. PA is a vital molecule that has been characterized as a multifunctional phospholipid with direct and indirect impacts on many cellular processes.48,49 For example, in a study of how PA may activate MAPK-type protein kinases in soybean (Glycine max L.) under stress, inhibition of PA production by 1-But resulted in wounding, and MAPK activation was also affected.50 No reports exist in the literature on the direct role of PA in PAL activity, although some studies have suggested that Ca2+, calmodulin (CaM) and ion channels are important components in the signal transduction pathway that stimulates PAL activity and that PA may be acting as a modulator of these signaling components.51
Capsicum chinense cells are sensitive to exogenous SA treatment and exhibit a significant increase in total SA in response to exogenous SA. These results are consistent with those from a study of SA stimulation in Hypericum perforatum L. shoots, callus, and cell suspensions in which growth of callus or shoots in cell suspensions was facilitated by close contact between the cells and the elicitor.52 In another study, treatment with SA was reported to induce de novo synthesis via activation of gene expression of protein involved in the SA biosynthesis pathway.8,53
Neomycin is widely used due to its affinity to form electroneutral complexes with PIP and PtdIns(4,5)P2, thereby blocking the binding of PIP and PtdIns(4,5)P2 with PLC.54 This antibiotic has also been reported as an inhibitor of protein synthesis in bacteria and chloroplasts.55 Chloroplast/plastids are important for lipid metabolism and the generation of lipid derived signal9 and because neomycin effects in these organelles, we cannot rule out the possibility that it could have affected protein synthesis, affecting the chloroplast-localized SA pathway. However, we found that 100 μM neomycin did not affect the endogenous total SA. This result added to the effect of U73122 on total SA level support our hypothesis that neomycin is affecting the PLC signaling and not protein synthesis in chloroplast.
The results presented here suggest that PLC and PLD signaling inhibitors may be interfering at some level with SA biosynthesis, and consequently, with vanillin production.
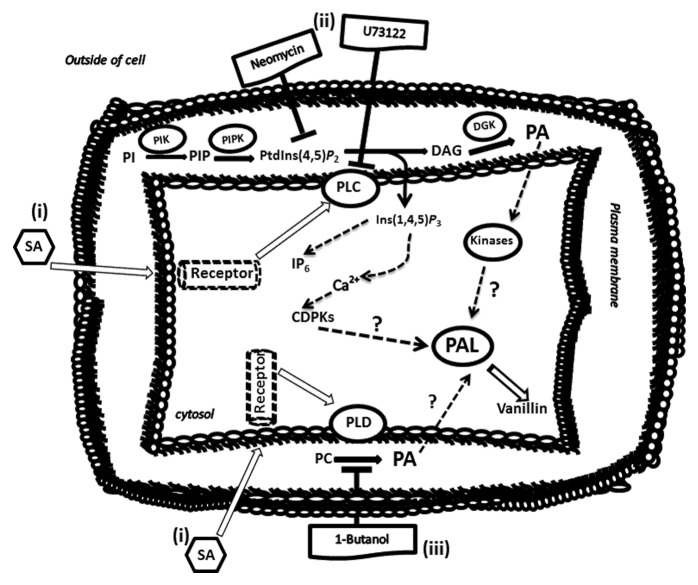
Our results are integrated into a model (Fig. 5) that suggests that SA regulates PAL activity, and consequently, increasing vanillin content in the cell suspensions. However, treatment of cells with PI-PLC and PLD inhibitors prior to SA addition leads to a simultaneous decrease in PAL activity and vanillin.
Figure 5. The conceptual model for salicylic acid role on vanillin synthesis involving phospholipid signaling pathway. (i) Salicylic acid can be sensed on or near the plasma membrane by a receptor and activate a signaling cascade through phospholipases (PLC and/or PLD) function. This is followed by the regulation of PAL enzymatic activity and increased vanillin content. (ii) In the presence of U73122 or neomycin (inhibitors of PLC signaling) the levels of DAG and Ins(1,4,5)P3 (second messengers), reduced which lead to modifications of intracellular Ca2+ levels that may affect the activity of PAL as well as the promoter of reduced vanillin production. (iii) The inhibitory function of 1-butanol on formation of PA could be affecting phosphorylation processes through the regulation of protein kinases activities that may be responding to PA levels. This event also could be affecting PAL activity following vanillin production. Therefore, response to SA resulting in the production of second messengers such as DAG, Ins(1,4,5)P3, and PA produced in phospholipid signaling pathway may be involved in regulating of PAL activity, and consequently vanillin production.
Therefore, PAL may be an important enzyme in vanillin biosynthesis, which in turn suggests that signal molecules such as phosphoinositides (PI, PIP, and PtdIns(4,5)P2) and PA are involved in regulating PAL activity. Overall, PLC and PLD inhibitors were found to reduce PAL activity and metabolite (vanillin) accumulation, suggesting that the PLC and PLD signaling pathways are involved in this SA-induced process.
Materials and Methods
Materials
Neomycin sulfate, U73122, U73343, 1-But, tert-butanol, and sodium salicylate were purchased from Sigma-Aldrich. U73122 and U73343 were dissolved in dimethyl sulfoxide to make stock solutions. Bicinchoninic acid (BCA) protein assay reagent (Pierce Chemical Co). All other chemicals were supplied by Sigma-Aldrich.
Cell culture and SA treatment
Capsicum chinense suspension cells were obtained by callus desegregation followed by culture in MS medium at pH 5.6.56 The MS medium was supplemented with 0.5 mM myo-inositol, 0.02 mM thiamine, 0.2 mM cysteine, 4 μM 2,4-dyclorophenoxiacetic acid, and 3% sucrose. Cells were subcultured every 14 d. For the induction treatments, 1 g (fresh weight) cell suspension per flask was inoculated into 25 ml culture medium and maintained as described above for 14 d prior to SA exposure. After the culture period, 200 μM SA was added to the cell suspension, and water was added in the control sample. Both samples were kept at 25 °C on a rotary shaker at 100 rpm for 30 min. The cells were then harvested, frozen in liquid nitrogen, and stored at –80 °C until protein extraction.
Phospholipase inhibitor treatment
Before being added to cell suspensions, the inhibitors neomycin (100 μM), U73122 (10 μM), and 1-But (0.5%) were sterilized by filtration. Each inhibitor was added to a cell suspension at the end of the 14-d culture period and 15 min before SA addition.
Protein extract preparation
For protein extraction, 1 g of cells was homogenized in a mortar and pestle in 2 ml extraction buffer (50 mM Tris-HCl, pH 8.8, 15 mM β-mercaptoethanol) at 4 °C. The resulting mixture was centrifuged at 1747 × g for 30 min and the supernatant used as the PAL enzyme source in the activity assay. Sample protein concentration was measured by the BCA assay57 using bovine serum albumin (BSA) as a standard.
PAL activity
Enzymatic activity assay was performed in 2 ml reaction volume containing 0.5 ml enzyme extract (5–20 μg protein), 1 ml 50 mM Tris-HCl (pH 8.8), and 0.5 ml 10 mM l-phenylalanine. After incubating this mixture at 37 °C for 1 h, 500 μl 6 M HCl was added to stop the reaction and then centrifuged at 10000 × g for 10 min. The supernatant was removed to quantify PAL activity in a spectrophotometer at 290 nm. The boiled extract was white in appearance and contained other reaction mixture components. A calibration curve was generated using cinnamic acid, and one unit of enzyme activity was designated as being equivalent to the amount required to produce 1 pmol cinnamic acid / min.16
Vanillin determination
Vanillin was acetone extracted from freeze-dried cultures and quantified by in situ TLC densitometry using a Shimadzu CS-930 dual wavelength chromatoscanner equipped with a DR 2 data collector (Shimadzu Corporation, Kyoto, Japan).58
Total SA Measurement
Endogenous total SA levels were measured in total extract by adding 800 μl buffer (50 mM NaCl, 1 mM EGTA, 250 mM sucrose, 10% glycerol, 50 mM Tris-HCl pH 7.4, 10 mM sodium pyrophosphate, 0.2 mM sodium orthovanadate), 1.24 mM phenylmethylsulfonyl fluoride (PMSF), and 1 mM β-mercaptoethanol per gram of plant tissue, homogenizing the mixture and centrifuging it at 1747 × g for 30 min at 4 °C. Organic phase extraction was performed by adding 1 ml 1M HCl to 0.5 ml protein extract, mixing for 20 s, adding 2 ml dichloromethane/isopropanol (9:1 v/v), stirring for 5 min and centrifuging at 3,351.04 × g for 5 min. The organic phase (bottom layer) was evaporated in a CentriVap (DyNA Vap) at 400 mbar for 1 h. The concentrate was resuspended in 50 μl mobile phase [pH 3.6 acetate buffer / methanol (72:28 v/v)]. SA separation was done by high performance liquid chromatography (HPLC) (Agilent 1100) using a 4.6 × 150 mm ion exchange column (Eclipse XDB-C18). SA was eluted with the mobile phase at a rate of 1 ml/min at room temperature and quantified SA by UV spectrometry at 280 nm.
Data presentation
All experiments were repeated at least 3 times using extracts prepared on separate occasions, and all produced similar results. Data were analyzed using a Student t-test. Analyses were run using the Sigma Stat ver. 3.1 program (2004).
Acknowledgments
This research was supported by a grant from the Consejo Nacional de Ciencia y Tecnología (No. 98352), a fellowship grant BARJ (No. 204969), and grant YACG (No. 4422) by the Sistema Nacional de Investigadores.
Glossary
Abbreviations:
- 1-But
1-butanol
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- CaM
calmodulin
- CDPK
calcium-dependent protein kinases
- DAG
diacylglycerol
- DGK
diacylglycerol kinase
- IC
isochorismate
- Ins(1,4,5)P3
inositol 1,4,5-trisphosphate
- IP6
inositol hexaphosphate
- MAPK
mitogen-activated protein kinase
- CaM
calmodulin
- N
neomycin
- NPR1
pathogenesis-related genes 1
- PC
phosphatidylcholine
- PIK
phosphoinositide 3-kinase
- PIP
phosphatidylinositol 4-phosphate
- PtdIns(4,5)P2
phosphatidylinositol 4,5-bisphosphate
- PLC
phospholipase C
- PI-PLC
phosphoinositide-phospholipase C
- PLD
phospholipase D
- PA
phosphatidic acid
- PAL
phenylalanine ammonia-lyase
- SA
salicylic acid, Tert, ter-butanol
- TLC
thin layer chromatography
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Xue HW, Chen X, Mei Y. Function and regulation of phospholipid signalling in plants. Biochem J. 2009;421:145–56. doi: 10.1042/BJ20090300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munnik T, Vermeer JEM. Osmotic stress-induced phosphoinositide and inositol phosphate signalling in plants. Plant Cell Environ. 2010;33:655–69. doi: 10.1111/j.1365-3040.2009.02097.x. [DOI] [PubMed] [Google Scholar]
- 3.Munnik T, Nielsen E. Green light for polyphosphoinositide signals in plants. Curr Opin Plant Biol. 2011;14:489–97. doi: 10.1016/j.pbi.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Kalachova T, Iakovenko O, Kretinin S, Kravets V. Involvement of phospholipase D and NADPH-oxidase in salicylic acid signaling cascade. Plant Physiol Biochem. 2013;66:127–33. doi: 10.1016/j.plaphy.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Liu HT, Liu YY, Pan QH, Yang HR, Zhan JC, Huang WD. Novel interrelationship between salicylic acid, abscisic acid, and PIP2-specific phospholipase C in heat acclimation-induced thermotolerance in pea leaves. J Exp Bot. 2006;57:3337–47. doi: 10.1093/jxb/erl098. [DOI] [PubMed] [Google Scholar]
- 6.Altúzar-Molina AR, Muñoz-Sánchez JA, Vázquez-Flota F, Monforte-González M, Racagni-Di Palma G, Hernández-Sotomayor SMT. Phospholipidic signaling and vanillin production in response to salicylic acid and methyl jasmonate in Capsicum chinense. Plant Physiol Biochem. 2011;49:151–8. doi: 10.1016/j.plaphy.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Muñoz-Sánchez A, Altúzar-Molina AR, Hernández-Sotomayor S. M.T. Phospholipase signaling is modified differentially by phytoregulators in Capsicum chinense cells. Plant Signal Behav. 2013;79:1103–5. doi: 10.4161/psb.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawano T, Bouteau F. Crosstalk between intracellular and extracellular salicylic acid signaling events leading to long-distance spread of signals. Plant Cell Rep. 2013;32:1125–38. doi: 10.1007/s00299-013-1451-0. [DOI] [PubMed] [Google Scholar]
- 9.Shah J. The salicylic acid loop in plant defense. Curr Opin Plant Biol. 2003;6:365–71. doi: 10.1016/S1369-5266(03)00058-X. [DOI] [PubMed] [Google Scholar]
- 10.Dempsey DA, Vlot AC, Wildermuth MC, Klessig DF. Salicylic acid biosynthesis and metabolism. The Arabidopsis book 2011; 9: e0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wildermuth MC, Dewdney J, Wu G, Ausubel FM. Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature. 2001;414:562–5. doi: 10.1038/35107108. [DOI] [PubMed] [Google Scholar]
- 12.Catinot J, Buchala A, Abou-Mansour E, Métraux JP. Salicylic acid production in response to biotic and abiotic stress depends on isochorismate in Nicotiana benthamiana. FEBS Lett. 2008;582:473–8. doi: 10.1016/j.febslet.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 13.Uppalapati SR, Ishiga Y, Wangdi T, Kunkel BN, Anand A, Mysore KS, Bender CL. The phytotoxin coronatine contributes to pathogen fitness and is required for suppression of salicylic acid accumulation in tomato inoculated with Pseudomonas syringae pv. tomato DC3000. Mol Plant Microbe Interact. 2007;20:955–65. doi: 10.1094/MPMI-20-8-0955. [DOI] [PubMed] [Google Scholar]
- 14.Fu ZQ, Yan S, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, et al. NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature. 2012;486:228–32. doi: 10.1038/nature11162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sudha G, Ravishankar GA. Influence of methyl jasmonate and salicylic acid in the enhancement of capsaicin production in cell suspension cultures of Capsicum frutescens. Mill. Curr Sci. 2003;85:1212–7. doi: 10.1078/0176-1617-00928. [DOI] [PubMed] [Google Scholar]
- 16.Ochoa-Alejo N, Gómez-Peralta JE. Activity of enzymes involved in capsaicin biosynthesis in callus tissue and fruits of chili pepper (Capsicum annuum L.) J Plant Physiol. 1993;141:147–52. doi: 10.1016/S0176-1617(11)80751-0. [DOI] [Google Scholar]
- 17.Gutiérrez-Carbajal MG, Monforte-González M, Miranda-Ham ML, Godoy-Hernández G, Vázquez-Flota F. Induction of capsaicinoid synthesis in Capsicum chinense cell cultures by salicylic acid or methyl jasmonate. Biol Plant. 2011;54:430–4. doi: 10.1007/s10535-010-0078-z. [DOI] [Google Scholar]
- 18.Vázquez-Flota F, Miranda-Ham ML, Monforte-González M, Gutiérrez-Carbajal G, Velázquez-García C, Nieto-Pelayo Y. La Biosíntesis de capsaicinoides, el principio picante del chile. Rev Fitot Mex. 2007;30:353–60. [Google Scholar]
- 19.Sánchez-Ballesta MT, Zacarías L, Granell A, Lafuente MT. Accumulation of PAL transcript and PAL activity as affected by heat-conditioning and low-temperature storage and its relation to chilling sensitivity in mandarin fruits. J Agric Food Chem. 2000;48:2726–31. doi: 10.1021/jf991141r. [DOI] [PubMed] [Google Scholar]
- 20.Lafuente MT, Sala JM, Zacarías L. Active oxygen detoxifying enzymes and phenylalanine ammonia-lyase in the ethylene-induced chilling tolerance in citrus fruit. J Agric Food Chem. 2004;52:3606–11. doi: 10.1021/jf035185i. [DOI] [PubMed] [Google Scholar]
- 21.Campos-Vargas R, Nonogaki H, Suslow T, Saltveit ME. Heat shock treatments delay the increase in woud-induced phenylalanine ammonia-lyase activity by altering its expression, not induction in romaine lettuce (Lactuca sattive) tissue. Physiol Plant. 2005;132:82–91. doi: 10.1111/j.1399-3054.2005.00446.x. [DOI] [Google Scholar]
- 22.Teklemariam TA, Blake TJ. Phenylalanine ammonia lyase-induced freezing tolerance in jack pine (Pinus banksiana) seedlings treated with low ambient levels of ultraviolet-B- radiation. Physiol Plant. 2004;122:244–53. doi: 10.1111/j.0031-9317.2004.00396.x. [DOI] [Google Scholar]
- 23.Billot J, Hartmann C, Macheix J, Rateau J. Les composes phenoliques au cours de la croissance de la poire Passe-Crassane. Physiol Veg. 1978;16:693–714. [Google Scholar]
- 24.Kataoka I, Kubo Y, Sugiura A, Tomana T. Changes in L-phenylalanine ammonia-lyase activity and anthocyanin synthesis during berry ripening of three grape cultivars. J Jpn Soc Hortic Sci. 1983;52:273–9. doi: 10.2503/jjshs.52.273. [DOI] [Google Scholar]
- 25.Chen JY, Wen PF, Kong WF, Pan QH, Zhan JC, Li JM, Wan SB, Huang WD. Effect of salicylic acid on phenylpropanoids and phenylalanine ammonia-lyase in harvested grape berries. Posharvest Biol Technol. 2006;40:64–72. doi: 10.1016/j.postharvbio.2005.12.017. [DOI] [Google Scholar]
- 26.Fleuriet A, Macheix JJ. Orientation nouvelle du métabolisme des acides hydroxycinnamiques dans les fruits de tomates blessés (Lycopersicon esculentum) Physiol Plant. 1984;61:64–8. doi: 10.1111/j.1399-3054.1984.tb06101.x. [DOI] [Google Scholar]
- 27.Arakawa O, Hori Y, Ogata R. Characteristics of color development and relationship between anthocyanin synthesis and phenylalanine ammonia lyase activity in ‘Starking Delicious’ ‘Fuji’ and ‘Mutsu’ apple fruits. J Jpn Soc Hortic Sci. 1986;54:424–30. [Google Scholar]
- 28.Given NK. Phenylalanine ammonia-lyase activity and anthocyanin synthesis in ripening strawberry fruit. J Plant Physiol. 1988;133:25–30. doi: 10.1016/S0176-1617(88)80079-8. [DOI] [Google Scholar]
- 29.Oufedjikh H, Mahrouz M, Amiot MJ, Lacroix M. Effect of γ-irradiation on phenolic compounds and phenylalanine ammonia-lyase activity during storage in relation to peel injury from peel of Citrus clementina hort. Ex. tanaka. J Agric Food Chem. 2000;48:559–65. doi: 10.1021/jf9902402. [DOI] [PubMed] [Google Scholar]
- 30.Leyva A, Jarillo JA, Salinas J, Martínez-Zapater JM. Low temperature induces the accumulation of phenylalanine ammonia-lyase and chalcone synthase mRNA of Arabidopsis thaliana in a light-dependent manner. Plant Physiol. 1995;108:39–46. doi: 10.1104/pp.108.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Toyoda K, Kawahara T, Ichinose Y, Yamada T, Shiraishi T. Potentiation of phytoalexin accumulation in elicitor-treated epicotyls of pea (Pisum sativum) by a diacylglicerol kinase inhibitor. J Phytopathol. 2000;148:633–6. doi: 10.1046/j.1439-0434.2000.00568.x. [DOI] [Google Scholar]
- 32.Yamaguchi T, Minami E, Ueki J, Shibuya N. Elicitor-induced activation of phospholipases plays an important role for the induction of defense responses in suspension-cultured rice cells. Plant Cell Physiol. 2005;46:579–87. doi: 10.1093/pcp/pci065. [DOI] [PubMed] [Google Scholar]
- 33.Legendre L, Yueh YG, Crain R, Haddock N, Heinstein PF, Low PS. Phospholipase C activation during elicitation of the oxidative burst in cultured plant cells. J Biol Chem. 1993;268:24559–63. [PubMed] [Google Scholar]
- 34.Staxen I, Pical C, Montgomery LT, Gray JE, Hetherington AM, McAinsh MR. Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C. Proc Natl Acad Sci U S A. 1999;96:1779–84. doi: 10.1073/pnas.96.4.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Testerink C, Munnik T. Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci. 2005;10:368–75. doi: 10.1016/j.tplants.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 36.Chen G, Snyder C, Greer MS, Randall J. Biology and biochemistry of plant phospholipases. Crit Rev Plant Sci. 2011;30:239–58. doi: 10.1080/07352689.2011.572033. [DOI] [Google Scholar]
- 37.Madrid E, Corchete P. Silymarin secretion and its elicitation by methyl jasmonate in cell cultures of Silybum marianum is mediated by phospholipase D-phosphatidic acid. J Exp Bot. 2010;61:747–54. doi: 10.1093/jxb/erp339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang L, Zhu X, Liu J, Chu X, Jiao J, Liang Y. Involvement of phospholipases C and D in the defence responses of riboflavin-treated tobacco cells. Protoplasma. 2013;250:441–9. doi: 10.1007/s00709-012-0426-2. [DOI] [PubMed] [Google Scholar]
- 39.Yamaguchi T, Tanabe S, Minami E, Shibuya N. Activation of phospholipase D induced by hydrogen peroxide in suspension-cultured rice cells. Plant Cell Physiol. 2004;45:1261–70. doi: 10.1093/pcp/pch150. [DOI] [PubMed] [Google Scholar]
- 40.Moreno-Valenzuela OA, Monforte-González M, Muñoz-Sánchez JA, Méndez-Zeel M, Loyola-Vargas M, Hernández-Sotomayor SMT. Effect of macerozyme on secondary metabolism plant product production and phospholipase C activity in Catharanthus roseus hairy roots. J Plant Physiol. 1999;155:447–52. doi: 10.1016/S0176-1617(99)80038-8. [DOI] [Google Scholar]
- 41.Kashem MA, Itoh K, Iwabuchi S, Hori H, Mitsui T. Possible involvement of phosphoinositide-Ca2+ signaling in the regulation of alpha-amylase expression and germination of rice seed (Oryza sativa L.) Plant Cell Physiol. 2000;41:399–407. doi: 10.1093/pcp/41.4.399. [DOI] [PubMed] [Google Scholar]
- 42.Kamada Y, Muto S. Protein kinase inhibitors inhibit stimulation of inositol phospholipid turnover and induction of phenylalanine ammonia-lyase in fungal elicitor-treated tobacco suspension culture cells. Plant Cell Physiol. 1994;35:405–9. [Google Scholar]
- 43.Toyoda K, Shiraishi T, Yamada T, Ichinose Y, Oku H. Rapid changes in polyphosphoinositide metabolism in pea in response to fungal signals. Plant Cell Physiol. 1993;34:729–35. [Google Scholar]
- 44.Bach M, Ulrich-Seitz H. Elicitor-induced defense responses of a suspension-cultured woody plant (Larix deciduas) and possible mechanisms of signal transduction. Can J Bot. 1997;75:1243–51. doi: 10.1139/b97-838. [DOI] [Google Scholar]
- 45.Bolwell GP. A role for phosphorylation in the down-regulation of phenylalanine ammonia-lyase in suspension-cultured cells of French bean. Phytochemistry. 1992;31:4081–6. doi: 10.1016/0031-9422(92)80418-E. [DOI] [Google Scholar]
- 46.Allwood EG, Davies DR, Gerrish C, Bolwell GP. Regulation of CDPKs, including identification of PAL kinase, in biotically stressed cells of French bean. Plant Mol Biol. 2002;49:533–44. doi: 10.1023/A:1015502117870. [DOI] [PubMed] [Google Scholar]
- 47.Choi YJ, Tomás-Barberán FA, Saltveit ME. Wound-induced phenolic accumulation and browning in lettuce (Lactuca sativa L.) leaf tissue is reduced by exposure to n-alcohols. Postharvest Biol Technol. 2005;37:47–55. doi: 10.1016/j.postharvbio.2005.03.002. [DOI] [Google Scholar]
- 48.Wang XM, Devaiah SP, Zhang WH, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–78. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 49.Testerink C, Munnik T. Molecular, cellular, and physiological responses to phosphatidic acid formation in plants. J Exp Bot. 2011;62:2349–61. doi: 10.1093/jxb/err079. [DOI] [PubMed] [Google Scholar]
- 50.Lee S, Hirt H, Lee Y. Phosphatidic acid activates a wound-activated MAPK in Glycine max. Plant J. 2001;26:479–86. doi: 10.1046/j.1365-313x.2001.01037.x. [DOI] [PubMed] [Google Scholar]
- 51.Blanco FA, Zanetti ME, Daleo GR. Calcium-dependent protein kinases are involved in potato signal transduction in response to elicitors from the oomycete Phytophtora infestans. J Phytopathol. 2008;156:53–61. doi: 10.1111/j.1439-0434.2007.01344.x. [DOI] [Google Scholar]
- 52.Gadzovska S, Maury S, Delaunay A, Spasenoski M, Hagege D, Courtois D, Joseph C. The influence of salicylic acid elicitation of shoots, callus and cell suspension cultures on production of naphtodianthrones and phenylpropanoids in Hypericum perforatum L. Plant Cell Tissue Organ Cult. 2013;113:25–39. doi: 10.1007/s11240-012-0248-0. [DOI] [Google Scholar]
- 53.Szalai G, Pál M, Janda T. Abscisic acid may alter the salicylic acid-related abiotic stress response in maize. Acta Biologica Szegediensis. 2011;55:155–7. [Google Scholar]
- 54.Chen Q, Boss WF. Neomycin inhibits the phosphatidylinositol monophosphate and phosphatidylinositol bisphosphate stimulation of plasma membrane ATPase activity. Plant Physiol. 1991;96:340–3. doi: 10.1104/pp.96.1.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Conte S, Stevenson D, Furner I, Lloyd A. Multiple antibiotic resistance in Arabidopsis is conferred by mutations in a chloroplast-localized transport protein. Plant Physiol. 2009;151:559–73. doi: 10.1104/pp.109.143487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murashige T, Skoog F. A revised medium for rapid growth on bioassays with tobacco tissue culture. Physiol Plant. 1962;15:608–12. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 57.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 58.Monforte-González M, Medina-Lara F, Gutiérrez-Carbajal G, Vázquez-Flota F. Capsaicinoid quantification by in situ densitometry of thin layer chromatography plates. J Liquid Chromatogr Relat Technol. 2007;30:1697–704. doi: 10.1080/10826070701225041. [DOI] [Google Scholar]