Abstract
The Arabidopsis thaliana 4/1 (At-4/1) protein has a highly α-helical structure with potential to interact both with itself and other protein ligands, including the movement proteins of some plant viruses; the Nicotiana tabacum ortholog (Nt-4/1) has similar structure. Here we describe localization of GUS expression in transgenic N. tabacum seedlings under control of the Nt-4/1 promoter, which indicates that transcription is associated with the veins at certain developmental stages, and especially in the hypocotyl. Viroid accumulation and movement was altered in plants in which 4/1 expression was reduced by virus-induced gene silencing. These localization studies support a role of 4/1 in signaling in the vasculature, including mobility of pathogen-related and cellular RNAs.
The Arabidopsis thaliana 4/1 protein (At-4/1) has previously been shown to interact with the tospovirus and nepovirus tubule-forming movement proteins (MPs) in a yeast 2-hybrid system (ref. 1, and S. von Bargen, personal communication). Later, the ability of At-4/1 protein to localize near plasmodesmata (PD) with a membrane-bound MP of another plant virus, and to move between cells via PD suggested its involvement in cell-to-cell communication2. The mostly α-helical structure of 4/1 protein with pronounced coiled-coil elements covering more than two-thirds of its length implies a potential for self-interaction and binding to multiple protein ligands.2,3 These features make the 4/1 protein an attractive candidate with which to study novel protein-protein interaction pathways involved in the cell-to-cell macromolecular trafficking in plants.
Significant information is also available concerning the structural characteristics and subcellular localization of the Nicotiana tabacum 4/1 ortholog (Nt-4/1).3,4,5 In particular, the Nt-4/1 protein was found to undergo nuclear-cytoplasmic transport specified by nuclear localization signal and nuclear export signal (NES), and a specific NES mutant of Nt-4/1 was localized to the nucleoplasm where it formed large spherical bodies.4,5 Structural studies confirmed the α-helical nature of the Nt-4/1 protein, revealing that it consists of 3 possible structural domains and is capable of self-interaction.5 Data available in the NCBI gene expression database (GEO) show that levels of Arabidopsis and rice 4/1 mRNAs increase in response to several biotic and abiotic stress factors (NCBI GEO accessions GDS3243, GDS2136, GDS1785, GDS1448, GDS2631, GDS2480), suggesting that 4/1 expression may be controlled to maintain plant homeostasis. The precise function(s) of 4/1 are still unknown, however.
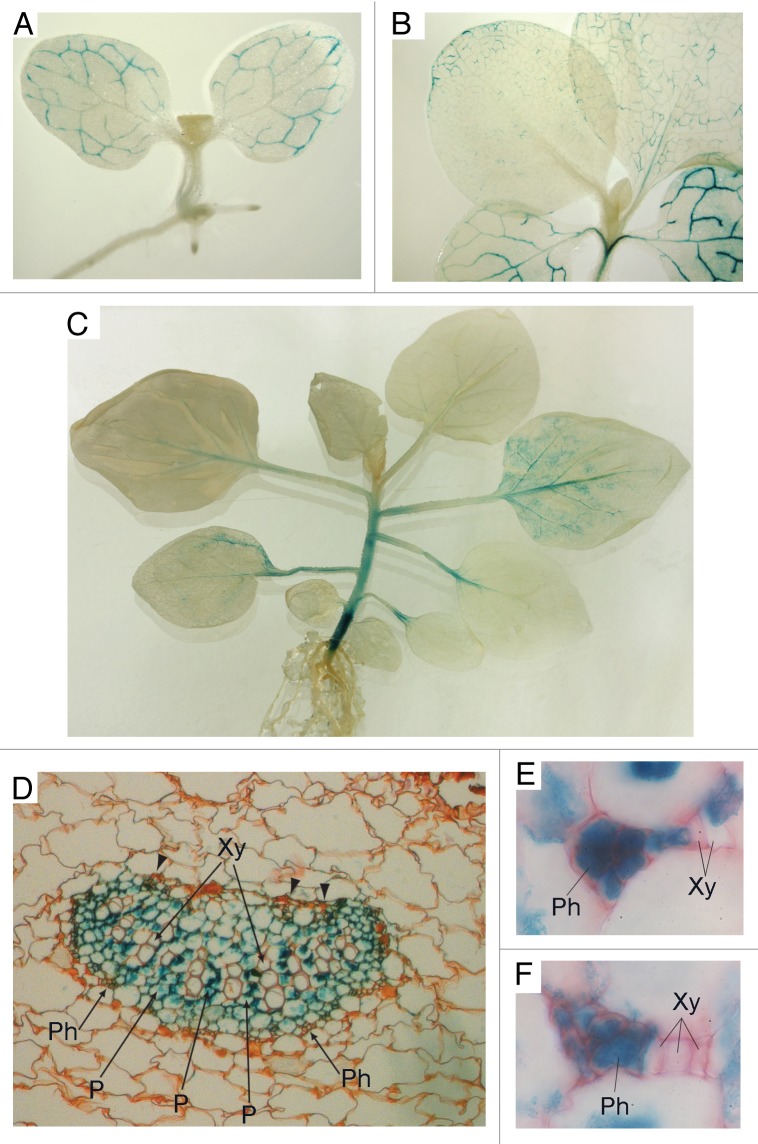
To analyze the tissue specificity and developmental regulation of Nt-4/1 gene expression, we constructed transgenic Nicotiana tabacum plants carrying the GUS gene under the control of the Nt-4/1 promoter. Analyses of germinating seeds and growing plants at different developmental stages revealed that GUS staining was first observed in cotyledons in association with veins but not in other parts of the plant (Fig. 1A). Later, at the 2–4-leaf stage, GUS staining was found in cotyledon veins and in veins of blades of first 2 foliage leaves, as well as in petioles and the hypocotyl (Fig. 1B). As the plants continued to grow, GUS staining disappeared from the cotyledons and older leaves and was found in association with veins of newer leaves. The youngest leaves never exhibited GUS staining (Fig. 1C). Staining was also observed in the stem of older plants, with the hypocotyl being the most intensively stained (Fig. 1C). This pattern of GUS staining was markedly different from that in control plants carrying the GUS gene under the control of the constitutive Cauliflower mosaic virus 35S promoter (data not shown). Taken together, these observations indicate that the Nt-4/1 promoter is transcriptionally active in veins of cotyledons and leaves at a certain developmental stage, and additionally in petioles and the stem.
Figure 1. Tissue-specific activity of the Nt-4/1 promoter at different developmental stages. Promoter activity was analyzed in transgenic Nicotiana tabacum cv Samsun nn plants carrying the GUS gene under the transcriptional control of a DNA fragment comprising 2000 base pairs upstream of the Nt-4/1 gene transcription start site experimentally mapped earlier.5 This region, presumably containing the Nt-4/1 promoter, was cloned from the “long” Nt-4/1 gene, which is expressed 3 times more efficiently than the “short” Nt-4/1 gene.3 The resulting construct was transformed into N. tabacum as described earlier.6 Detection of GUS was performed according to Jefferson et al.7 (A) Seedling with 2 expanded cotyledons and emerging foliage leaves. (B) 2-leaf stage. (C) 8-leaf stage. At this stage, staining was found in the petioles of leaves 1–3 but not in the cotyledons, and was also associated with the veins of leaf 4. In leaf 5 weak staining was found in the petiole and larger veins; in leaf 6 only faint staining of the midrib was observed; and no staining was found in the youngest leaves. At this developmental stage the hypocotyl stained very intensively, whereas the rest of the stem exhibited much weaker staining. (D–F) Anatomy of stained leaves in transverse section. A leaf which exhibited pronounced vein staining was taken from a 6-leaf plant for sectioning. For light microscope observations, material was fixed in 70% ethanol, dehydrated, embedded and then sectioned with a rotary microtome using standard methods of Paraplast embedding and serial sectioning at 10–16 µm thickness. Sections were stained with safranin and mounted in DPX mounting medium. (D) Thick vein with well-developed secondary xylem whose cells are arranged in regular radial rows. Arrowheads point to adaxial phloem. (E and F) minor veins composed exclusively of primary xylem and phloem. Xy, xylem; Ph, phloem; P, parenchyma cells.
Anatomical examination under the light microscope revealed that, in larger veins showing secondary growth, staining was associated mostly with the xylem and phloem parenchyma (Fig. 1D) but not with conducting elements (especially in xylem), whereas in young or smaller veins without secondary growth it was found in the primary phloem cells (Fig. 1E and F). In both cases, staining was associated with cells that retained living cytoplasm. In stem tissue, similar to mature leaves, GUS staining was observed in vascular bundles, mostly in xylem parenchyma and additionally in other live cells of the vascular tissue (data not shown).
In accordance with the observed transcriptional activity of Nt-4/1 promoter in leaf veins, a transiently expressed Nt-4/1-GFP fusion protein appeared to be associated with minor veins of N. benthamiana leaves. In our previous experiments, when Nt-4/1-GFP was co-expressed with tombusvirus silencing suppressor p19 to increase the accumulation of fusion protein, Nt-4/1-GFP was distributed ubiquitously among leaf cells (Fig. 2A).5 However, when Nt-4/1-GFP was expressed by agroinoculation in the absence of p19, the fusion protein accumulated primarily in cells of minor leaf veins (Fig. 2B and C). This observation suggests that silencing-related inactivation of 4/1 mRNA could be less pronounced in veins as compared with cells of other leaf tissues. We propose that the cells where 4/1 protein performs its function(s) may be better adapted to maintain the optimal half-life of the Nt-4/1 mRNA and/or protein.
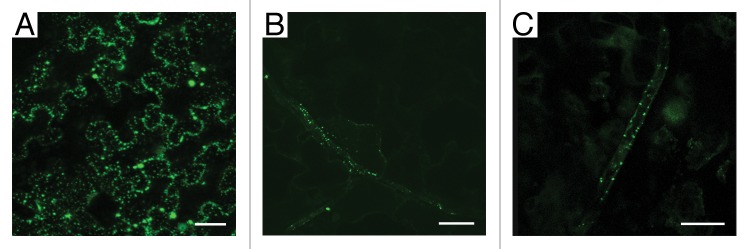
Figure 2. Tissue-specific inactivation of the Nt-4/1 mRNA. The GFP-fusion of Nt-4/1 protein described previously5 was transiently expressed in young N. benthamiana leaves by agroinfiltration. Fluorescence was observed by confocal laser scanning microscopy in detached leaves 3 d after infiltration. (A) Nt-4/1-GFP expression in the presence of transiently co-expressed tombusvirus silencing suppressor p19; (B and C) Nt-4/1-GFP expression in the absence of p19. Scale bars, 50 µm in (A and B) 25 µm in (C).
Three factors—the accumulation of 4/1 protein in vascular tissue, activation of gene transcription in response to stress factors, and the ability of 4/1 protein to move both intra- and intercellularly2—suggest that 4/1 protein may influence signaling in the vasculature, in particular the mobility of pathogen-related and cellular RNAs. In this work we report evidence for a possible role of 4/1 protein in the long-distance vascular movement of a molecular pathogen, potato spindle tuber viroid (PSTVd).6 PSTVd was inoculated onto N. benthamiana plants where the level of endogenous 4/1 mRNA was downregulated by virus-induced gene silencing (VIGS) employing a previously described Alternanthera mosaic potexvirus (AltMV) silencing construct7 carrying a fragment of Nt-4/1 cDNA. Despite the fact that accumulation of PSTVd in inoculated leaves of 4/1-silenced plants was significantly lower than in control AltMV-infected plants, the level of viroid RNA accumulation in the 4th, 6th and 7th systemic leaves above the PSTVd-inoculated leaf was significantly higher in 4/1-silenced plants compared with control plants (Fig. 3). It appears that long-distance movement of viroid into developing young leaves above the inoculated leaf was much more efficient in silenced plants.
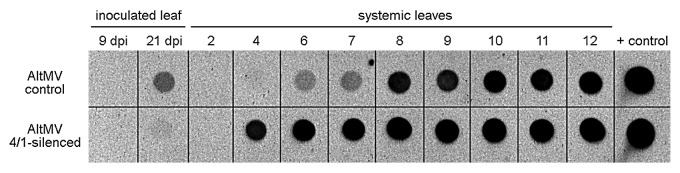
Figure 3. Dot-blot detection of PSTVd in leaves of N. benthamiana plants. Two leaves from the young N.benthamiana plants were inoculated with AltMV.4/1 or control AltMV vector constructs. Quantitative reverse transcription (qRT-PCR) was performed to assess the relative 4/1 mRNA levels using an endogenous gene (actin) as an internal standard. At 13 dpi 4/1 expression was found to be strongly (approximately 8-fold) downregulated in systemic leaves of plants inoculated with the AltMV.4/1 construct (data not shown). At this time, the uppermost 1.5–2 cm leaf of both AltMV.4/1-infected and AltMV-infected control plants was challenge inoculated with infectious PSTVd RNA transcripts. Samples (1 disk per leaf) were collected from plants infected with each construct 9 and 21 d after inoculation with PSTVd (inoculated leaf) and 21 d (2nd–12th leaves above inoculated) for dot blot analysis using chemiluminescent detection with digoxygenin-labeled cPSTVd RNA probe and CDP-Star substrate.8 Note that each spot represents a pooled sample contained discs from 5 individual plants in that treatment. For systemic leaves numbers indicate the position of leaves above the challenge-inoculated leaf. “+ control”, PSTVd RNA transcripts used as a control.
These data point to an involvement of 4/1 protein in viroid transport in the vasculature. Moreover, preliminary data revealed that the Nt-4/1 protein is also capable of preferential binding to PSTVd RNA in vitro (to be published elsewhere). Assuming that the 4/1 protein normally functions in the absence of viroid infection, our data generally suggest a role for the 4/1 protein in RNA translocation in vascular tissue. Phloem-mobile RNAs may act as long-distance signaling molecules, and systemic movement of cellular mRNAs has been found to regulate leaf architecture, tuberization and flowering.9–11 Furthermore, small interfering RNAs are components of mobile systemic signals for RNA silencing defense, and miRNAs are mobile as well.12 One can speculate that 4/1 affects the direction of flow–or at least the balance between downward and upward flow of phloem RNAs–and that the expression in the hypocotyls regulates exchange between the inner and outer vascular bundles. If this is the case, 4/1 silencing might change the balance, allowing PSTVd to transition from “rootward” to “shootward” flow without first needing to replicate in the roots. To follow up on this experimentally, it would be necessary to assay roots and upper leaves (simultaneously at various time points) after PSTVd inoculation for both 4/1 and PSTVd levels, and preferably the hypocotyls as well. Thus, further work is required to elucidate the role of 4/1 protein in signaling through plant conductive tissue.
Acknowledgments
This work was supported in part by grant 12-04-00139-а from the Russian Foundation for Basic Research. We are grateful to Dr Susanne von Bargen (Humboldt-Universität zu Berlin) for communicating experimental data prior to publication.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.von Bargen S, Salchert K, Paape M, Piechulla B, Kellmann J-W. Interactions between the Tomato spotted wilt virus movement protein and plant proteins showing homologies to myosin, kinesin and DnaJ-like chaperones. Plant Physiol Biochem. 2001;39:1083–93. doi: 10.1016/S0981-9428(01)01331-6. [DOI] [Google Scholar]
- 2.Paape M, Solovyev AG, Erokhina TN, Minina EA, Schepetilnikov MV, Lesemann DE, et al. At-4/1, an interactor of the Tomato spotted wilt virus movement protein, belongs to a new family of plant proteins capable of directed intra- and intercellular trafficking. Mol Plant Microbe Interact. 2006;19:874–83. doi: 10.1094/MPMI-19-0874. [DOI] [PubMed] [Google Scholar]
- 3.Makarova SS, Minina EA, Makarov VV, Semenyuk PI, Kopertekh L, Schiemann J, et al. Orthologues of a plant-specific At-4/1 gene in the genus Nicotiana and the structural properties of bacterially expressed 4/1 protein. Biochimie. 2011;93:1770–8. doi: 10.1016/j.biochi.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 4.Minina EA, Erokhina TN, Garushyants SK, Solovyev AG, Morozov SY. Subcellular localization of the new plant protein 4/1 and analysis of heterologous protein-protein interactions indicate its ability for nuclear-cytoplasmic transport. Dokl Biochem Biophys. 2009;429:296–300. doi: 10.1134/S1607672909060039. [DOI] [PubMed] [Google Scholar]
- 5.Solovyev AG, Minina EA, Makarova SS, Erokhina TN, Makarov VV, Kaplan IB, et al. Subcellular localization and self-interaction of plant-specific Nt-4/1 protein. Biochimie. 2013;95:1360–70. doi: 10.1016/j.biochi.2013.02.015. [DOI] [PubMed] [Google Scholar]
- 6.Owens RA, Sano T, Duran-Vila N. Plant viroids: isolation, characterization/detection, and analysis. Methods Mol Biol. 2012;894:253–71. doi: 10.1007/978-1-61779-882-5_17. [DOI] [PubMed] [Google Scholar]
- 7.Lim HS, Vaira AM, Domier LL, Lee SC, Kim HG, Hammond J. Efficiency of VIGS and gene expression in a novel bipartite potexvirus vector delivery system as a function of strength of TGB1 silencing suppression. Virology. 2010;402:149–63. doi: 10.1016/j.virol.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 8.Podleckis EV, Hammond RW, Hurtt SS, Hadidi A. Chemiluminescent detection of potato and pome fruit viroids by digoxigenin-labeled dot blot and tissue blot hybridization. J Virol Methods. 1993;43:147–58. doi: 10.1016/0166-0934(93)90072-Y. [DOI] [PubMed] [Google Scholar]
- 9.Cho SK, Kang IH, Carr T, Hannapel DJ. Using the yeast three-hybrid system to identify proteins that interact with a phloem-mobile mRNA. Front Plant Sci. 2012;3:189. doi: 10.3389/fpls.2012.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin T, Sharma P, Gonzalez DH, Viola IL, Hannapel DJ. The impact of the long-distance transport of a BEL1-like messenger RNA on development. Plant Physiol. 2013;161:760–72. doi: 10.1104/pp.112.209429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jackson SD, Hong Y. Systemic movement of FT mRNA and a possible role in floral induction. Front Plant Sci. 2012;3:127. doi: 10.3389/fpls.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parent JS, Martínez de Alba AE, Vaucheret H. The origin and effect of small RNA signaling in plants. Front Plant Sci. 2012;3:179. doi: 10.3389/fpls.2012.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]