Abstract
Coordinated regulation of the many genes controlling leaf, flower, and root development determines the phenotypes of plants; this regulation requires exquisite control of many transcription factors, including the WUSCHEL-related homeobox (WOX) family. We recently reported that rice (Oryza sativa) WUSCHEL-related homeobox 3A (OsWOX3A) plays important roles in organ development, including lateral-axis outgrowth and vasculature patterning in leaves, lemma and palea morphogenesis in spikelets, and the numbers of tillers and lateral roots. OsWOX3A is encoded by NARROW LEAF2 (NAL2) and NAL3, a pair of duplicated genes. In this study, further analysis of nal2 nal3 (hereafter nal2/3) double mutants revealed that, in addition to its role in lateral root development, OsWOX3A also acts in the control of root hair formation. Based on this new finding, we describe a possible mechanism by which OsWOX3A regulation of auxin transport genes acts in root development.
Keywords: auxin, lateral root, nal2 nal3, OsWOX3A, PIN, rice, root hair
Studying root organ defective mutants in the dicot model plant Arabidopsis has greatly advanced our understanding of taproot differentiation and organization.1 However, the mechanisms underlying the development of the fibrous root system in monocot plants remain unclear. Rice, a monocot model crop plant, has a fibrous root system including primary, adventitious (or crown) and lateral roots, and root hairs.2 Examination of the mechanisms of root development in rice will advance our understanding of the differentiation and organization of the monocot root system.
WOX genes are well-known developmental regulators; there are 14 Arabidopsis and 12 rice WOX family members,3 and some of these WOX genes play important roles in regulating cell division and differentiation during root system development in both monocot and dicot plants. For example, in Arabidopsis, WOX2 and WOX8 regulate cell fates during basal embryonic root formation.4WOX5 functions in stem cell maintenance5 and WOX9/STIMPY acts in early embryonic growth in the root apices.6 In rice, WOX11 plays an important role in the emergence and growth of adventitious roots, and directly represses the cytokinin type-A responsive regulator RR2, which is expressed in adventitious root primordia.7
Our recent study demonstrated that OsWOX3A regulates the development of lateral roots, but not adventitious roots.3 The OsWOX3A transcriptional activator contains a WUS homeodomain and a WUS-box domain, homologous to NARROW SHEATH 1 (NS1) and NS2 in maize and PRESSED FLOWER (PRS) in Arabidopsis.3 OsWOX3A is encoded by duplicated genes, NAL2 and NAL3 (hereafter NAL2/3). nal2/3 mutants produce narrow-curly leaves, more tillers, fewer lateral roots, opened spikelets and narrow-thin grains, indicating that OsWOX3A regulates development of several organs. Although we demonstrated the role of OsWOX3A in lateral root development,3 its possible involvement in root hair development remained unclear. Thus, we examined the nal2/3 root hair phenotype to elucidate the specific role of OsWOX3A in root organ development. As previously reported,3 lateral root number was significantly reduced in nal2/3 (Fig. 1A and B). By contrast, root hair growth was markedly increased in nal2/3; both the number and elongation rate of root hairs increased considerably in nal2/3 (Fig. 1C and D), suggesting that OsWOX3A is involved in root hair development as well as lateral root development.
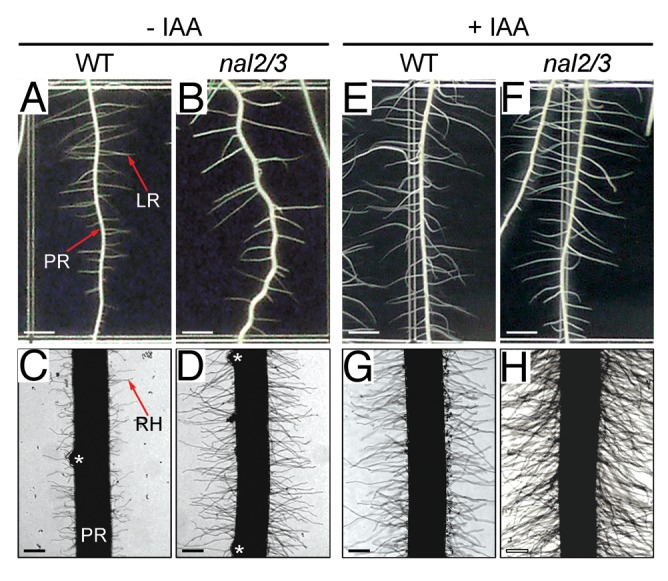
Figure 1. Lateral root and root hair phenotype of wild-type (WT) and nal2/3 plants with exogenous auxin treatment. Surface-sterilized rice seeds were germinated and grown on Murashige and Skoog (MS) agar medium for 1 or 2 d. Then, germinated seeds were transferred to MS agar plates containing 0.1 μM IAA (+IAA), or to control plates (-IAA) and grown vertically in the growth chamber. Two-week-old plants were observed. (A-H) Lateral roots and root hair phenotypes of WT and nal2/3 seedlings grown on +IAA (A-D) or -IAA (E-H) agar plates. White asterisks indicate emerging lateral roots. LR, lateral root; PR, primary root; RH, root hair. Bars = 2 mm in (A-D) and 0.3 mm in (E-H).
Polar auxin transport is directional; asymmetric localization of auxin-efflux carriers such as PIN family proteins drives active auxin flow through the neighboring cells.10 Auxin concentration affects the development of various root types in rice.8,9 For example, OsPIN1-RNAi plants, in which polar auxin distribution was compromised, showed a severe defect in adventitious root development, and exogenous auxin treatment complemented this phenotype.11 OsWOX3A directly or indirectly regulates the expression of auxin transport-related genes such as AUXIN RESPONSE FACTOR (ARF), YABBY (YAB), YUCCA (YUC) and PIN-FORMED (PIN).3 Moreover, exogenous IAA treatment rescued the reduced number of lateral roots in nal2/3, suggesting that altered expression of OsPIN1 and OsPIN2 may cause the nal2/3 tiller and lateral root phenotypes. Therefore, OsWOX3A may act by affecting OsPIN expression and thus altering the distribution of auxin. Indeed, exogenous auxin treatment also rescued the reduced number of lateral roots in nal2/3 (Fig. 1E and F), suggesting that the altered expression of OsPIN genes in nal2/3 perturbs the precise distribution of endogenous auxin, as previously observed in the rice crown rootless (crl) and pin mutants.8,11-13 We further tested the effect of exogenous auxin treatment on root hair development in nal2/3. Interestingly, treatment with exogenous auxin further increased root hair growth in nal2/3 mutants (Fig. 1G and H).
Auxin flows from the shoot meristem and young leaves (auxin source) to the root tip (auxin sink), and moves up toward the root differentiation zone through the epidermal cells.14 Auxin transporters play important roles in specifying lateral root initiation and growth by establishing auxin maxima in the pericycle cells. Auxin transporters also affect root hair formation and elongation by regulating intracellular auxin concentration in the epidermal cells.15-17 In Arabidopsis, the polar localization of PIN1, PIN3, and PIN7 at the basal side of stele cells and PIN4 at the basal side of root stem cells mediate rootward auxin flow; similarly, PIN2 at the upper side of root epidermis and at the basal side of root cortex mediates shootward auxin flow.14,18,19 Arabidopsis and rice PIN1-overexpressing plants showed increased numbers of lateral roots, and pin1 mutants displayed a defect in lateral root initiation.11,17 These results suggest that PIN1 activity for lateral root initiation is conserved in angiosperms. Thus, it can be speculated that the reduced PIN1 expression in nal2/3 mutants disrupts formation of auxin maxima in the stele cells, resulting in a reduction of lateral root initiation.
In addition, root hair development was also altered in nal2/3 mutants (Fig. 1C and D) and OsPIN1 expression was significantly downregulated.3 In Arabidopsis, root hair-specific overexpression of AtPIN1 inhibits root hair growth.20 As the intracellular auxin concentration in the epidermal cells regulates root hair initiation and growth, it appears that, in nal2/3, reduced OsPIN1 expression leads to increased intracellular auxin levels in the root hair cells, promoting root hair growth (Fig. 1D). This is further supported by results of exogenous auxin treatment, which should increase intracellular auxin concentration in the epidermal cells. Indeed, exogenous auxin stimulated more root hair formation in both wild-type and nal2/3 seedlings (Fig. 1G and H).
Based on these results, we propose a model for the growth of lateral roots and root hairs regulated by auxin concentration in the pericycle and epidermal cells (Fig. 2). Lateral root formation initiates from regularly spaced primordia with the establishment of auxin maxima at the primordial tips in the pericycle cells. In nal2/3, altered expression of OsPIN genes inhibits lateral root development by attenuating local auxin gradient formation in the pericycle cells. In addition, disruption of OsPIN activities enhances intracellular auxin concentrations in the epidermal cells above the threshold required for root hair initiation, increasing the number of root hairs in nal2/3. In summary, here we demonstrate that OsWOX3A regulates root hair development in addition to lateral root development, probably by modulating the expression of OsPIN genes, correlated with local auxin concentration in the pericycle and epidermal cells of rice roots. Further analysis to examine local auxin accumulation in these root cells in nal2/3 is necessary to understand OsWOX3A regulation of lateral root and root hair development.
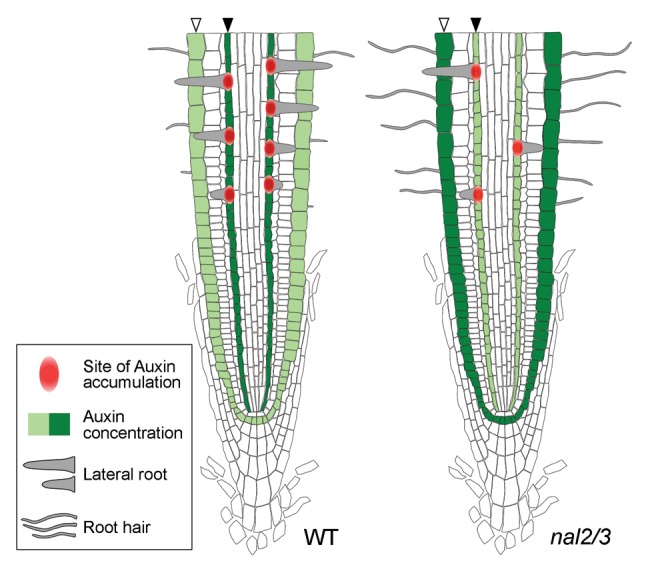
Figure 2. Tentative model for the coordinated regulation of lateral root and root hair development by OsWOX3A-mediated auxin distribution in WT and nal2/3. Altered expression of OsPIN genes compromises the establishment of auxin maxima in pericycle cells (black arrowheads) where lateral roots initiate, but increases auxin concentration in the epidermal cells (white arrowheads), leading to reduction of lateral root initiation and increase of root hair initiation.
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (Plant Molecular Breeding Center No. PJ008128), Rural Development Administration, Republic of Korea.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Petricka JJ, Winter CM, Benfey PN. Control of Arabidopsis root development. Annu Rev Plant Biol. 2012;63:563–90. doi: 10.1146/annurev-arplant-042811-105501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hochholdinger F, Park WJ, Sauer M, Woll K. From weeds to crops: genetic analysis of root development in cereals. Trends Plant Sci. 2004;9:42–8. doi: 10.1016/j.tplants.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Cho SH, Yoo SC, Zhang H, Pandeya D, Koh HJ, Hwang JY, Kim GT, Paek NC. The rice narrow leaf2 and narrow leaf3 loci encode WUSCHEL-related homeobox 3A (OsWOX3A) and function in leaf, spikelet, tiller and lateral root development. New Phytol. 2013;198:1071–84. doi: 10.1111/nph.12231. [DOI] [PubMed] [Google Scholar]
- 4.Breuninger H, Rikirsch E, Hermann M, Ueda M, Laux T. Differential expression of WOX genes mediates apical-basal axis formation in the Arabidopsis embryo. Dev Cell. 2008;14:867–76. doi: 10.1016/j.devcel.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Sarkar AK, Luijten M, Miyashima S, Lenhard M, Hashimoto T, Nakajima K, Scheres B, Heidstra R, Laux T. Conserved factors regulate signalling in Arabidopsis thaliana shoot and root stem cell organizers. Nature. 2007;446:811–4. doi: 10.1038/nature05703. [DOI] [PubMed] [Google Scholar]
- 6.Wu X, Dabi T, Weigel D. Requirement of homeobox gene STIMPY/WOX9 for Arabidopsis meristem growth and maintenance. Curr Biol. 2005;15:436–40. doi: 10.1016/j.cub.2004.12.079. [DOI] [PubMed] [Google Scholar]
- 7.Zhao Y, Hu Y, Dai M, Huang L, Zhou DX. The WUSCHEL-related homeobox gene WOX11 is required to activate shoot-borne crown root development in rice. Plant Cell. 2009;21:736–48. doi: 10.1105/tpc.108.061655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell. 2005;17:1387–96. doi: 10.1105/tpc.105.030981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu ZX, Liu Y, Liu SJ, Mao CZ, Wu YR, Wu P. A gain-of-function mutation in OsIAA11 affects lateral root development in rice. Mol Plant. 2012;5:154–61. doi: 10.1093/mp/ssr074. [DOI] [PubMed] [Google Scholar]
- 10.Friml J, Palme K. Polar auxin transport--old questions and new concepts? Plant Mol Biol. 2002;49:273–84. doi: 10.1023/A:1015248926412. [DOI] [PubMed] [Google Scholar]
- 11.Xu M, Zhu L, Shou H, Wu PA. A PIN1 family gene, OsPIN1, involved in auxin-dependent adventitious root emergence and tillering in rice. Plant Cell Physiol. 2005;46:1674–81. doi: 10.1093/pcp/pci183. [DOI] [PubMed] [Google Scholar]
- 12.Kitomi Y, Ito H, Hobo T, Aya K, Kitano H, Inukai Y. The auxin responsive AP2/ERF transcription factor CROWN ROOTLESS5 is involved in crown root initiation in rice through the induction of OsRR1, a type-A response regulator of cytokinin signaling. Plant J. 2011;67:472–84. doi: 10.1111/j.1365-313X.2011.04610.x. [DOI] [PubMed] [Google Scholar]
- 13.Liu S, Wang J, Wang L, Wang X, Xue Y, Wu P, Shou H. Adventitious root formation in rice requires OsGNOM1 and is mediated by the OsPINs family. Cell Res. 2009;19:1110–9. doi: 10.1038/cr.2009.70. [DOI] [PubMed] [Google Scholar]
- 14.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B. The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature. 2005;433:39–44. doi: 10.1038/nature03184. [DOI] [PubMed] [Google Scholar]
- 15.Fischer U, Ikeda Y, Ljung K, Serralbo O, Singh M, Heidstra R, Palme K, Scheres B, Grebe M. Vectorial information for Arabidopsis planar polarity is mediated by combined AUX1, EIN2, and GNOM activity. Curr Biol. 2006;16:2143–9. doi: 10.1016/j.cub.2006.08.091. [DOI] [PubMed] [Google Scholar]
- 16.Lee SH, Cho HT. PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell. 2006;18:1604–16. doi: 10.1105/tpc.105.035972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell. 2003;115:591–602. doi: 10.1016/S0092-8674(03)00924-3. [DOI] [PubMed] [Google Scholar]
- 18.Feraru E, Friml J. PIN polar targeting. Plant Physiol. 2008;147:1553–9. doi: 10.1104/pp.108.121756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller A, Guan C, Gälweiler L, Tänzler P, Huijser P, Marchant A, Parry G, Bennett M, Wisman E, Palme K. AtPIN2 defines a locus of Arabidopsis for root gravitropism control. EMBO J. 1998;17:6903–11. doi: 10.1093/emboj/17.23.6903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ganguly A, Lee SH, Cho M, Lee OR, Yoo H, Cho HT. Differential auxin-transporting activities of PIN-FORMED proteins in Arabidopsis root hair cells. Plant Physiol. 2010;153:1046–61. doi: 10.1104/pp.110.156505. [DOI] [PMC free article] [PubMed] [Google Scholar]


